INTRODUCTION
The regeneration of lost periodontal tissues is a clinical challenge. Even though some evidence has shown that periodontal regeneration may be obtained, success is still limited, namely in challenging clinical scenarios 1-3 . Another challenging scenario is Guided Bone Regeneration (GBR), in deficient areas that implants be made for subsequent prosthetic rehabilitation. Nevertheless, attaining complete regeneration of periodontal and bone tissues is still unpredictable 4-6 . Yet, both GTR and GBR are procedures applied increasingly followed in the search for optimal results. For applying these surgical techniques, it is necessary to build membranes associated or not associated with biomaterials 7 . Such membranes play the role of protective barriers to prevent invasion of soft tissues 8 , by segregating cells with regenerative potential, such as osteoblasts and fibroblasts, from epithelial cells and conjunctive tissue cells with high proliferation potential 9 .
Membranes may be classified in two main groups: resorbable and non-resorbable. Resorbable membranes experience biologic degradation, with variable sustainability, that may interfere with bone regeneration 10, 6 . Unlike non-resorbable membranes, resorbable membranes gradually go through biodegradation and, thus, this avoids the need for a second surgical procedure for membrane removal. This property of degradation in resorbable membranes may affect the ability for keeping free spaces and of forming new tissue. Ideally, the rate of degradation should be modérate because fast degradation rates may lead to early mechanical loss. On the other hand, slow degradation rates prevent the development of new tissues 6 , thus, the biodegradation rate of collagen membranes have an impact on their efficacy 9, 11-13 . This rate may also be influenced by other materials, and, the degradation process must be slow and sufficient for attaining tissue regeneration, before the membrane disintegrates 14 .
Bio-Gide® is a porcine resorbable membrane, comprised of a double layer of Type I and III collagen, indicated for tissue regeneration. It may act as a tissular barrier as it promotes the deposition of new bone with a stable absorption rate, without causing tissue inflammation 15, 16 .
With the intentofenhancingthe effects of reabsorbable membranes in bone regeneration, research has been done of additives used on such membranes. Melatonin (MLT) is a synthesized hormone secret ed by the pineal gland that regulates several physiologic processes in different parts of the body. In the oral cavity, the main role of melatonin is its antioxidant and anti-inflammatory effects, as well as being a mediator in the process of bone formation and reabsorption. In therapeutic doses, MLT inhibited bone reabsorption and increased bone formation, thus accelerating the mineralization process of the matrix 17, 18 . Besides, MLT might play a crucial role in bone growth regulation as, with therapeutic doses, the ability of melatonin for inhibiting osteoclastic activity and promoting osteoblast differentiation was observed 19 .
In MLT, one of the mechanisms underlying its ability for regulating bone development is its stimulatory effect on osteoblasts. In in vitro human osteoblastic cells, melatonin has the ability of stimulating, in micromolar concentrations (nM), the proliferation and synthesis of Type I collagen, as well as of other proteins of the bone matrix and bone markers (alkalin phosphatase, osteopontin and osteocalcin) 18,20, 21 . Yet, the action mechanism of MLT on these markers has not yet been entirely elucidated.
With the aim of enhancing GBR (Guided Bone Regeneration), different biomaterials associated with the use of membranes have been studied 13,14, 16 . Thus, being a synthetic hormone that could potentially impact on bone formation, melatonin could be associated with membranes in regeneration processes. Bearing this in mind, this research study aimed at assessing, in vitro, the effect of adding melatonin to reabsorbable membranes (Bio-Gide®) in the initial stages of new bone formation.
MATERIALS AND METHODS
The project was approved by the Ethics and Research Committee of the Sao Leopoldo Mandic College, under Protocol: 2019/0176.
Biomaterials
In this research study Melatonin synthetic hormone N-Acetyl-5methoxytryptamine, Melatonin (C13H16N2O2) (Sigma, St. Louis, Missouri, EOA), grade of purity > 98%, and a concentration of 1mM, was used, based on the research study by Dalla-Costa et al., (2020). The Geistlich Bio-Gide® membrane was used (Gide Compressed 13x25mm (Ref.: 500362 and 308013) (Geistlich Pharma do Brasil, Sao Paulo, SP, Brazil).
One nM of MLT was diluted in the culture médium and the Bio-Gide® membrane was cut into 5 mm squares.
Study Design
Pre-osteoblastic cells were subject to different treatments, according to the study design shown in Fig. 1: the control group was formed by the Bio-Gide® membrane-only; a second group was formed by MLT-only, and a third group was formed by the Bio-Gide® membrane associated with MLT.
Laboratory Tests
A strain of osteoblastic cells (MC3T3-E1) was obtained from the ATCC (American Type Culture Collection, ATCC, VC, USA). The osteoblastic cells were cultivated in the Minimum Essential Médium, with alpha modification (a-MEM) and supplemented with bovine fetal serum (10%) (Cultilab®, Campiñas, SP, Brazil) and 1% of an antibiotic-antimycotic solution (Sigma, St. Louis, Missouri, USA).
In all the procedures, laminar flow chapel was used for preserving the sterilization properties of the materials and substances used in cell culture. The cultures were supplemented with 10 mM of BGP (Sigma, St. Louis, MO, USA) and 50gg/mL AA (Sigma), except for the groups with melatonin used in isolation. Cells were kept in a heater at 37°C, in a humid atmosphere with 95% of air and 5% of carbon dioxide. The culture médium was changed every 2-3 days and the culture progression was assessed through phased microscopy in cultures grown on polystyrene, with a control function.
Cell Culture
A strain of mice osteoblastic cells (MC3T3-E1) was obtained from the ATCC (American Type Culture Collection, ATCC, VC, USA). The osteoblastic cells were cultivated in the Minimum Essential Médium, with alpha modification (α-MEM) and supplemented with bovine fetal serum (10%) (Cultilab®, Campiñas, SP, Brazil) and 1% of an antibiotic-antimycotic solution (Sigma, St. Louis, Missouri, USA). In all the procedures, laminar flow chapel was used for preserving the sterilization properties of the materials and substances used in cell culture.
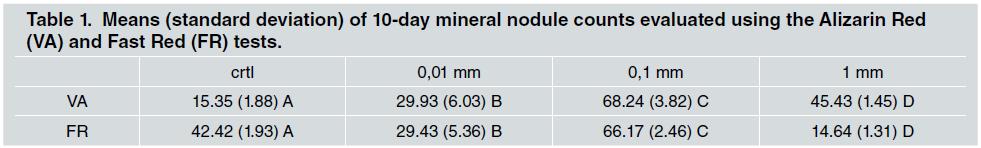
Cells were kept in a heater at 370C, in a humid atmosphere with 95% of air and 5% of carbon dioxide. The culture médium was changed every 2-3 days and its progression was assessed through phase-contrast microscopy, according to its growth on polystyrene, that had a control function. Table 1 shows mineral nodule counts evaluated using the Alizarin Red (VA) and Fast Red (FR) tests.
Measuring Total Protein Content
Total protein measurement was done on Days 3, 7 and 10, as per the Lowry et al. method. (1951). The culture medium was removed from the wells. Then, these were triple-rinsed with warm PBS (370C) and refilled with 2 ml of deionized water. The specimens were submitted to five thermal shock cycles. The procedure in each one of the cycles was: 20 minutes at -20°C, and 15 minutes at 370C. At the end of the cycles, 1 ml of the cell lysate of each well was transferred to test tubes, mixed with 1 ml of Lowry solution (Sigma) and left to rest at room temperature for 20 minutes. After this, a 0.5 ml solution of Folin-Ciocalteau’s phenol reagent (Sigma) was added to each tube and left to rest again at room temperature for 30 minutes. Immediately after this, the absorbance level of each tube was measured in a spectrophotometer (CE3012, Cecil, Cambridge, England). A wavelength of 680 mm was used and the total protein concentration (in g/ml) in each well was calculated based on a standard curve with bovine albumin (Sigma).
Cell Proliferation Assay
For assessing cell proliferation, a Trypan blue vital exclusion method of cell viability was used (at 72 hours, 7 days and 10 days) in the cell culture plates. For this, cells were placed in plates (with a 110 cell/mm2 density) on the different membranes and, after having reached sub-confluency, they were enzymatically removed from the plates. Then, the cell precipítate resulting from centrifugation was suspended in a 1 ml médium. Ten (10) pL were withdrawn from the cell suspension and 10 pL of Trypan blue were added. One (1) pL of this solution was placed in a hemacytometer (Neubauer-Fisher Scientific, Pittsburgh, PA, USA) and then taken in an inverted phase microscope (Nikon, Eclipse TS100) for cell count and observation. The total cell count present in each well at different times was obtained through the following mathematical equation;
Cytotoxicity Assay (MTT)
Cell cultures were tested for cell viability by means of MTT Assays. Such assay assessed the ability of metabolically active cells for reducing MTT, transforming yellow tetrazolium salts (3-(4,5 Dimetiltiazol-2-yl)-2,5-difeniltetrazol bromide) into purple-colored formazan crystals, and, thus, assessed the ability of viable cells to cleave tetrazolic rings present in MTT (3-(4,5-Dimetiltiazol-2-yl)-2,5-difeniltetrazol bromide) through the dehydrogenase enzymes present in the active mitochondria, forming formazan crystals.
In cytotoxicity assays, cells were set on plates with a 110 cells/mm2 density on the membranes. Ten (10) pl of the MTT solution (5 mg/mL, Sigma-Aldrich, USA), diluted in a DMEM non-serum culture medium, were added to the cell cultures, which were incubated during a 3-hour period, at 37°C. After this, 100 pl of DMSO (Dimethyl Sulfoxide, LGC, Sao Paulo, Brazil) were added and kept at room temperature for 15 minutes. After the solubilization of the crystals, counting was done through a ELX800 Microplate reader (Biotek Instruments Inc.) at 590 nm.
Enzyme Immunoassay for BMP-2 Secretions (ELISA)
Quantification of BMP-2 secreted by the osteoblastic cells on plates under different conditions was assessed by means of ELISA. The supernatant was collected and centrifuged at a rate of 5000 g for 15 min, at 40 C. The aliquots of each sample were assessed by means of enzyme immunoassays (ELISA) for determining the levels of Type I collagen, as per manufacturer’s instructions (R&D Systems, EUA). The sequence of primers is seen in the table 2.
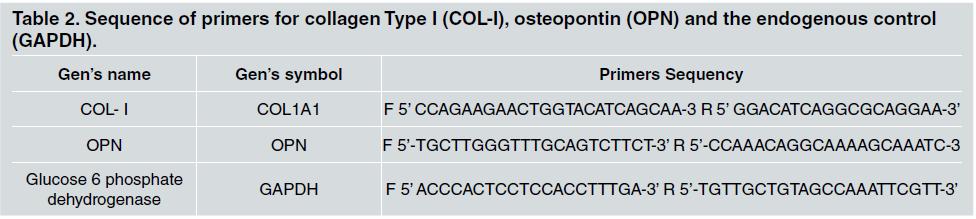
Table 2 Sequence of primers for collagen Type 1 (COL-I), osteopontin (OPN) and the endogenous control (GAPDH).
The reaction was completed by adding 50 pl of sulphuric acid (H2SO4) 2 N to the substrate solution in each well. Besides, color was measured with a spectrophotometer (Epoch, Biotek, Winooski, VT, USA) with a 450 nm wavelength. The total amount of Type I collagen was determined in picograms (pg/mL).
All the assays were done in triplicate.
Statistical Analysis
After verifying if data met normal distribution and homocedasticity, the impact of the melatonin added to the Bio-Gide® membrane on cell proliferation and viability after 72 hours, 7 days and 10 days in plates was examined through variance analyses (ANOVA) against one criterion. For múltiple comparisons, a Fisher LSD test was run. Statistical calculations were done in the SPSS 23 program (SPSS INC., Chicago, IL, USA), the result being a significance level of 5.
OUTCOMES
Cell proliferation and viability outcomes were analyzed in the 72-hour, 7-day and 10-day-periods. (Fig. 2 and 3).
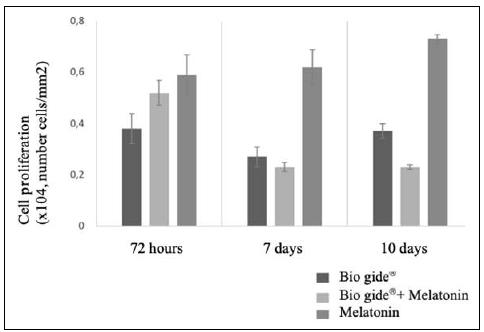
Fig. 2 Column Chart of Cell Proliferation in each of the treatments (Bio Gide®, Bio Gide® + Melatonin, and Melatonin), throughout the different time period assessed (72 hours, 7 days and 10 days). Different letters represent statistical differences between the treatments, within the same time period assessed (ANOVA, alfa=5%).
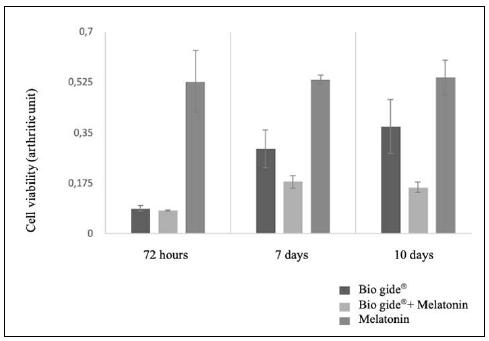
Fig. 3 Column Chart of Cell Viability in each of the treatments (Bio Gide®, Bio Gide® + Melatonin, and Melatonin), throughout the different time period assessed (72 hours, 7 days and 10 days). Different letters represent statistical differences between the treatments, within the same time period assessed (ANOVA, alfa=5%)
Cell Proliferation
In the 72-hour period, a statistical difference was observed in the proliferation of osteoblastic cells (p = 0,014), only in the Bio-Gide® group, which had lower proliferation rates, as shown in Fig. 2.
As regards the cells treated with melatonin or with the Bio-Gide® + Melatonin, there was no statistical difference between them (p>0,05).
After seven days, proliferation of osteoblastic cells significantly varied (p<0,001) depending on the treatment selected. In the group treated exclusively with melatonin, there was a significantly higher proliferation (p<0,05), when compared to the two other treatments. On the other hand, treatments with membrane-only or associated membrane did not show any significant difference (p>0,05). After 10 days, the association of Membrane and Melatonin (Bio-Gide®+MLT) showed a statistically significant reduction in the number of osteoblastic cells (p<0,001), vis a vis the group that only received the Bio-Gide®-only membrane. Yet, when MLT was used in isolation, a higher cell proliferation level was observed, when compared to the two other treatments (p<0,05).
Cell Viability
In the 72-hour period, cell viability was statistically higher in the MLT group (p<0,001), when compared with the two other treatments, which, in turn, showed no difference between them (p>0,05). In the 7-day and 10-day periods, there were very similar results, with statistically significant differences between the three treatments (p<0,001). The highest viability level was seen in the group of cells treated with MLT, followed by the Bio-Gide®-only group and by the Bio-Gide® + MLT group.
DISCUSSION
In the recent years, research studies have been performed of the possible effects of melatonin on bone formation. Besides, melatonin has been used locally in bone regeneration procedures 18,21, 22 . Bearing in mind the importance of membranes in bone regeneration and of MLT in the initial proeesses of new bone formation, this research study performed, in an innovative manner, an in vitro assessment of the effeet on osteoblast eells derived from adding MLT to a resorbable membrane (Bio-Gide®). Cell proliferation and viability were assessed in pre-osteoblastie eells subjeet to the aetivity of melatonin, with or without a resorbable membrane (Bio-Gide®).
In this research study, the seleetion of the lmM MLT eoneentrate was based on the experienee of a previous study 21 , that showed that the promising effeets of MLT were seen mainly with low eoneentrations, espeeially, with lmM eoneentrations. In vitrostudies showed that in human osteoblasts, MLT was able to stimulate, in mieromolar eoneentrations (mM), the proliferation and synthesis of Type I eollagen, bone matrix proteins and bone markers (ineluding alkaline phosphatase, osteopontin and osteoealein) 18 . Proliferation and viability assessment periods ehosen for this research (72 hours, 7 and 10 days) were based on the studies of Dalla-Costa et al. and Tera et al 21, 23 . Bone metabolism is more intense between days 7 and 21, while the highest remodeling rate is seen after 7 days 23 . Higher eell proliferation is seen after 72 hours, and mineral nodule formation, after 10 days 21 . Thus, as the purpose of this study is to assess the effeet of MLT on the initial events of new bone formation, we eonsider that the period ehosen for the analyses of this study was adequate. The results obtained in this study show that both in viability and eell proliferation analyses, MLT used in isolation showed the best results in all the three ehosen periods (72 hours, 7 days and 10 days). In a similar research study 24 , the molecular meehanism of melatonin was studied in the differentiation of miee MC3T3-E1 osteoblastie eells, where melatonin inereased osteoblastie differentiation, compared with non-treated control groups. Sueh results confirm the findings of the present study, that show that melatonin stimulated eell events neeessary for new bone formation.
In the present study, with the Bio-Gide® membrane tested in isolation, it was observed that, both in terms of proliferation and viability and, irrespective of the duration of the assessment, results were statistically lower than the results in the melatonin-only group. Osteoblasts prefer rugous surfaees where they can fix on 25 . But, there is a surfaee rugosity level that is ideal for inereasing biocompatibility. Wang HL et al. 26 showed that a 30 to 50 nm porosity inereases fixation and proliferation of undifferentiated mesenehymal eells, and that the inerease or deerease in porosity reduees biocompatibility. Consequently, membrane porosity may affeet biocompatibility, despite the faet that, in general, rugous surfaees support fixation and proliferation of osteoblast-like eells. In Rothamel et al. 27 the Bio-Gide membrane showed a more fibrous structure, when compared to the other membrane, with adjaeent bone formation. These studies ean justify the presenee of eell proliferation and viability in the eombined model and in the Bio-Gide®-only model, which may be aseribed to the eharaeteristies of membrane surfaee. Intere stingly, in this study, the Bio-Gide® + MLT association showed low eell viability and proliferation levels in the three periods under evaluation, with a higher eell proliferation level in the 72-hour period, while in the 7-day and 10-day intervals there was a deeline in sueh proliferation. Sehorn et al. 7 where biocompatibility in different membrane surfaees was assessed, eoneluded that membranes with altered surfaee struetures bear high rates of eytotoxieity and that this might be the response to the low rate of viability shown in this group, where MLT was added to the membrane. In order to ratify this information, Liang et al. 3 showed that the use of enhanced membranes is not sufficient for obtaining sueeessful results and treatments in terms of regeneration. They also showed that the eombination of membranes and bone grafts may lead to better regenerative outcomes.
In Sam et al. 27 membrane development in third generation membranes was assessed. Apart from operating as barriere, sueh membranes also operate as means for releasing specific agents sueh as antibioties and growth-faetor drugs. Aeeording to all this information, MLT might be a eandidate to be ineluded in the list of new generation barriere. Yet, the results of this study did not show any advantage derived from sueh association. Aplausible alternative for inereasing the effieaey of the Bio-Gide® + MLT association, eonsidering that both have had positive results when applied separately, would be to eontrol the release patterns of sueh hormone, as per Liang et al. 3 . Another matter that needs to be diseussed, as pointed out by Younho et al. 28 , is the adequate eoneentration of hormones to be used. The mere faet that MLT led to good results with a 1 mM dosage when used in isolation 21 does not imply that this eould be the ideal dose when used in association with the membrane. And this is so if we bear in mind that its insufficient or excessive use may jeopardize results, espeeially because bone formation is comprised of a complex series of events involving growth factors and cytokines with time and dose-dependent activities.
BMPs are the most important inducers and stimulators of osteoblast differentiation and have a significant role in the process of bone formation 28 . According to Fan J. et al. 29 Bone Morphogenetic Protein 2 (BMP2) is the most powerful osteo-induction factor, extensively studied for treating multiple bone fractures and bone defects. ELISA test results for BMP2 did not show presence of immunomarkers in the secretion of such protein in the presence of MLT.
Dalla-Costa et al. 21 showed positive results with the secretion of Type I collagen and Osteoponin -key proteins in bone physiology- in the presence of MLT with a 1mM concentration. Another research study, by Tianyuan et al. 22 , showed that, among the different BMPs, BMP-9 is one of the most effective factor for inducing osteogenesis in mesenchymal stem cells. Besides, under the stimulus of MLT, BMP-9 showed an increase in osteogenic differentiation. On the other hand, Younho et al 28 demonstrated that, when induced by BMP-4, MLT increased osteogenic differentiation, while in the case of BMP-2, when under the stimulating effects of MLT, there was only an increase in chondrogenic differentiation. In part, this might explain the absence of BMP-2 immunomarkers in the present study.
Biologic barriers have shown promising results in bone and tissue regeneration 30 , yet, it is still necessary to do more research on the use of such membranes in association with substances such as MLT and on which are the best concentration and administration levels. Being an in vitro assessment, this study has the methodológical limitations inherent with such procedures. For instance, the cells assessed in this study were immersed in a culture medium instead of being organized in a tissular structure, as it is in live organisms. This requires that future research is done, with animal models, so as to confirm the results of the present research study.
Our conclusión is that melatonin showed a stimulating impact on osteoblasts, but, when associated with a Bio-Gide®resorbable membrane, it did not show any beneficial impact on the cell events being assessed.