Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Latin American applied research
Print version ISSN 0327-0793
Lat. Am. appl. res. vol.33 no.2 Bahía Blanca Apr./June 2003
Optimal solvent cycle design in supercritical fluid processes
M. S. Diaz, S. Espinosa and E. A. Brignole
Planta Piloto de Ingenieria Quimica - PLAPIQUI (UNS-CONICET)
Camino La Carrindanga Km 7 - 8000 Bahia Blanca – Argentina
{ebrignole, sdiaz, espinosa}@plapiqui.edu.ar.
Abstract - This work addresses the determination of optimal solvent cycle scheme for supercritical fluid processes. The optimization model, which includes reliable thermodynamic predictions and rigorous process models, has been extended to include potential units that constitute the solvent recovery cycle. Capital cost functions, based on graphical correlations from the literature, have been derived for each unit in the superstructure. A mixed integer nonlinear programming model has been formulated, where the objective function is net profit maximization. Process and solvent cycle design has been performed for the deterpenation of lemon peel oil.
Keywords - Optimal Solvent Cycle. Supercritical Fluid Processes. Optimization.
I. INTRODUCTION
In this paper solvent cycle design in supercritical fluid deterpenation processes is addressed. Citrus peel oils are mainly composed of hydrocarbon terpenes and oxygenated compounds. The last ones, which constitute the valuable aroma fraction, can be obtained from citrus peel oil by extraction with supercritical carbon dioxide (Budich et al., 1999; Espinosa et al., 2000). In previous work, simulation and optimization models have been developed to minimize solvent-to-feed ratio in several supercritical extraction processes, as operating costs mainly depend on this ratio (Diaz et al., 2000). However, to evaluate economic feasibility of supercritical deterpenation against conventional processes, capital and operating costs of process and solvent recovery units must be taken into account and the optimal solvent cycle scheme must be determined.
As high-pressure processes are energy intensive, they must be highly integrated to be economically feasible. Special attention must be devoted to the solvent cycle scheme (Brunner, 2000). There are different alternatives for solvent recycling in supercritical fluid processes. They depend on operating conditions of the main process, the nature of the solvent and the scale of the process unit. The solvent can be driven either by a compressor or by a pump and it can be recycled either in supercritical or in liquid state. In the case of a pump cycle, lower capital costs are associated to pumps as compared to compressors and energy consumption is lower than the compressor process for pressures higher than 300 bar (Brunner, 1984). As a disadvantage, the pump cycle requires several heat exchangers and condensers and additional heat energy at low extraction pressures. The compressor cycle needs only one heat exchanger and it shows low heat energy consumption but it has higher investment costs for the compressor and higher electrical energy consumption compared to the pump cycle for pressures lower than 300 bar. Solvent cycle selection constitutes a significant step in the design problem of supercritical fluid processes.
In this paper, a mixed integer nonlinear model has been formulated to represent both lemon peel oil deterpenation process and its solvent recovery system, embedding potential units that constitute the solvent cycle. The objective is net profit maximization while rendering specified aroma fraction recovery and purity. Rigorous simulation models for process units have been formulated and a Group Contribution Equation of State (GC-EOS, Skjold-Jorgensen, 1984) has been used to provide phase equilibrium and solubility predictions. Pure group and binary interaction parameters have been determined for GC-EOS aldehyde group and a comparison of predictions with experimental data is presented. Investment costs correlations from Ulrich (1984) and Institut Français du Pétrole (1981) have been associated to each process unit.
II. THERMODYNAMIC MODELING
Lemon peel oil is composed of several compounds, but it can be modeled as a mixture of two key components: limonene and citral (Kalra et al., 1987). Limonene is the main hydrocarbon terpene in all peel oils, with concentrations ranging between 30 and 95 % weight. Citral is the most representative oxygenated aroma in lemon peel oil. Figure 1 shows their chemical structures.
 |  |
| citral | limonene |
Carbon dioxide supercritical extraction provides low operating temperatures (that avoid product thermal degradation) and no solvent residue. The terpeneless oxygenated aroma, of high commercial value, is obtained as the raffinate. Equilibrium predictions have been performed with the Group Contribution Equation of State (GC-EOS, Skjold-Jorgensen, 1984; Brignole et al., 1984). In this work, aldehyde pure group parameters have been calculated for GC-EOS, together with binary interaction parameters between this new group and paraffins, carbon dioxide and aromatics. Pure group and interaction parameters for olefin groups (Pusch and Schmelzer, 1993) and carbon dioxide and aromatics binary interaction groups (Bamberger et al., 1994) have been added to original GC-EOS parameters. Figure 2 shows binary phase equilibrium predictions for the system citral-carbon dioxide, with GC-EOS, which are in good agreement with experimental data (Marteau et al., 1995).
Figure 2. Vapor-liquid equilibria for CO2 – Citral mixture
The quality of ternary predictions has also been tested; Fig. 3 shows a pressure-composition diagram for a model lemon oil mixture (75% limonene and 25% citral), prepared by Kalra et al. (1987), with CO2, and our predictions at three isotherms.

Figure 3. Pressure-composition diagram for the ternary mixture CO2-Limonene-Citral
Figure 4 shows a comparison between GC-EOS vapor - liquid equilibrium predictions and the same experimental data for the ternary. Finally, solubility data for an actual lemon oil-CO2 mixture (Kalra et al., 1987) have been compared to GC-EOS predictions for a 75% limonene-25% citral (weight) mixture in CO2, as it is shown in Fig. 5, at three different temperatures. Lemon peel oil can be accurately modeled as a binary mixture composed of limonene-citral (75-25 weight %) and process simulation and optimization have been performed for this lemon oil feed composition.
Figure 4.Vapor-liquid ternary equilibrium diagram for CO2-Citral-Limonene mixture. (symbols: experimental data; lines: GC-EOS predictions)

Figure 5. Predictions and experimental data for lemon oil solubility in CO2
III. OPTIMIZATION MODEL
The determination of optimal solvent cycle scheme and operating conditions for the supercritical deterpenation of lemon oil has been formulated as the following Mixed Integer Nonlinear Programming (MINLP) model:

where vector x corresponds to continuous optimization variables: extraction pressure, solvent recovery unit pressure, solvent flowrate and reflux ratio. Binary variables y have been associated to potential units in the process and the solvent recovery system. Equality constraints, h, represent the process mathematical model. It is solved within a sequential process simulator which includes rigorous models for a high-pressure multistage extractor (Brignole et al., 1987) and a multiphase flash (Michelsen, 1982). The GC-EOS has been integrated as thermodynamic support for these model unit simulation routines. Additional equality constraints c correspond to reflux recycle, as the nonlinear optimization subproblem has been solved in an infeasible-path way. Inequality constraints, g, include product purity and recovery specifications, solvent purity and operating bounds.
Figure 6 shows the deterpenation process together with the solvent recovery system superstructure. In the extraction column (E), lemon oil is fed at the sixth stage in countercurrent with supercritical carbon dioxide; citral is the raffinate. Limonene and carbon dioxide constitute the extract, which may be heated (y5=1) and/or expanded through a Joule-Thomson valve (y6=1) to reduce solute solubility in supercritical carbon dioxide. This stream is then sent to a solvent recovery unit (S1); the vapor is the recovered solvent and the liquid is partly returned to the extractor as reflux and partly recovered as limonene product after a further expansion to ambient pressure. Other alternative to separate the extract from the solvent is to change temperature in order to reduce solute solubility in carbon dioxide at constant pressure, so the valve (V1, y6=1) is also optional; however solvent purity for reuse cannot be achieved in this option. In compression mode, the recovered carbon dioxide is compressed (C1, centrifugal, y1=1 or C2, reciprocal, y2=1) to extraction pressure and then it is cooled to extraction temperature (HE1, y3=1). In pumping mode (y4=1), the recovered solvent is condensed (HE2), pumped (P1) and heated (HE3) to extraction pressure and temperature respectively. In pumping mode, a propane refrigeration cycle has been considered to condense carbon dioxide; the solvent is heated in HE3 with low-pressure steam to extractor temperature. In compressor mode, the solvent is cooled (HE1) to extractor temperature with cooling water. The objective is to maximize net profit.

Figure 6. Supercritical deterpenation scheme with solvent cycle superstructure
IV. DISCUSSION
Optimal solvent recovery scheme for the deterpenation of lemon peel oil has been determined for supercritical extraction with carbon dioxide together with optimal operating conditions. The plant processes 20 kg/h feed oil, which is composed of 75% limonene and 25% citral (weight basis), a mixture that closely represents natural lemon oil. A twenty-stage column has been selected. Nonlinear constraints, which correspond to process specifications, are shown in Table 1.
Table 1. Nonlinear constraints

A simple countercurrent scheme has been studied as a first step, but none of the nonlinear constraints could be fulfilled with this process scheme. An 89 % product purity (carbon dioxide free) with 66.50% citral recovery could be obtained, together with 97.4% and 89.70% limonene recovery and purity in top product, respectively. Extractor operating conditions were 93.22 bar and 345 K. The addition of external reflux to the extraction step, as it is shown in Fig. 6, increases both product recovery and purity. The objective function, net profit maximization, has been calculated as :
Net profit = (price * production)terpeneless lemon oil + (price * production)limonene – (cost * consumption)lemon oil – (Operating Costs + Capital Costs),
where operating costs include electrical motor consumption, either as pump or compressor driver, cooling water and steam consumption. Capital cost models are of the general form: Ci = ai yi + bi wihi, where ai, bi and hi are cost parameters. These functions are defined over the entire domain of the design variable wi (which stands for heat exchanger area, power consumption in pumps and compressors, etc.) and they have been derived for each unit in the superstructure based on graphical correlations from Ulrich (1984) and Institut Français du Pétrole (1981). In the MILP master problem, these functions have been replaced with linear underestimators. Also, for a variable wi , associated to unit or path i, the following inequality constraints have been written:
wi - Mi yi £ 0,
wi ³ 0,
where Mi is a known upper bound on wi ; therefore, for a non-existing unit i (yi =0), the associated variable wi must be zero. If yi =1, the upper bound holds on wi .
The MINLP problem has been solved with an implementation of the Outer Approximation algorithm (Duran and Grossmann, 1986) that can interface a process simulator (Diaz and Bandoni, 1996); NLP subproblems have been solved with an SQP algorithm (Biegler and Cuthrell, 1985) and MILP problems, with LINDO (Schrage, 1987).
Lemon oil is corrosive so stainless steel process units are required. The extraction column is a structured packed column with Sulzer packing (Mellapack 250). Shell and tube heat exchangers, compressors and reciprocating pump costs curves have been obtained from Ulrich (1984). Capital cost curves for the extraction column have been obtained from Institut Français du Pétrole (1981). Investment cost has been annualized considering a project life of three years. Raw material cost is 28 $/kg (Chemical Market Reporter, Apr/01) and product prices strongly depend on product purity. Terpeneless lemon oil (citral, in this work) price is 847 $/kg (TGSC, www.execpc.com/~goodscnt/, Apr/01). Limonene price has been considered as 1.44 $/kg (Chemical Market Reporter, Apr/01).
The MINLP optimum corresponds to a solvent recovery system in compression mode. Numerical results, which are reported in Table 2, show that a compressor cycle is the best option because this process operates at pressure below 300 bar (Brunner, 1994). Terpeneless lemon oil high-commercial value justifies working with larger solvent-to-feed ratio. Figure 7 shows a comparison between solvent cycle consumption in compressor mode and in pump mode for the optimal determined conditions, in a T-S diagram. It can be clearly seen that a compressor is the option with lower energy consumption at this pressure level.
Table 2. Continuous optimization variables and main costs for the countercurrent extraction with reflux at solvent cycle MINLP optimum

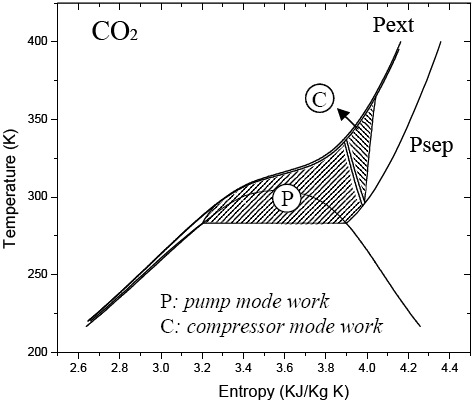
Figure 7. T-S diagram for CO2 recycle.
V. CONCLUSIONS
The problem of optimal solvent cycle design has been addressed as an MINLP problem for the supercritical deterpenation of lemon oil. Argentina is lemon first world producer and a nontoxic technology for the production of a high commercial value aroma, as citral seems to be of great interest. The MINLP model includes reliable thermodynamic predictions with a Group Contribution Equation of State and rigorous unit models. The close agreement between experimental equilibrium and solubility data for the ternary system limonene-citral-CO2 and thermodynamic predictions, together with rigorous process simulation models guarantee optimal process schemes and operating conditions. A better insight has been obtained as regard process feasibility, operating conditions and solvent cycle design when performing economic evaluation through a detailed cost analysis.
Acknowledgements
The authors gratefully acknowledge financial support from CONICET, ANPCYT and Universidad Nacional del Sur, Argentina.
REFERENCES
Bamberger, A., J. Schmelzer, D. Walther and G. Maurer, "High-Pressure Vapour-Liquid Equilibria in Binary Mixtures of Carbon Dioxide and Benzene Compounds and their Correlation with the Generalized Bender and Skjold-Jorgensen's Group Contribution Equation of State", Fluid Phase Equilibria, 97, 167-189 (1994). [ Links ]
Biegler, L. And J. Cuthrell, "Improved Infeasible Path Optimization for Sequential Modular Simulators.II: The Optimization Algorithm", Comp. Chem. Eng. 9, 257-267 (1985). [ Links ]
Brignole, E.A., P.M. Andersen and Aa. Fredenslund, "Supercritical Fluid Extraction of Alcohols from Water", Ind. Eng. Chem. Res. , 26, 254-261 (1987). [ Links ]
Brignole, E.A., S. Skjold-Jorgensen and Aa. Fredenslund, "Application of a Local Composition Equation of State to Supercritical Fluid Phase Equilibrium Problems", Ber. Bunsen-Ges. Phys. Chem., 88, 801-806 (1984). [ Links ]
Brunner, G., "Design and Scale-up of Processes with Supercritical Fluids: The Solvent Cycle", 5th International Symposium on Supercritical Fluids Westin Atlanta North Atlanta, USA, April 8-12 (2000). [ Links ]
Brunner, G., Gas Extraction. An Introduction to Fundamentals of Supercritical Fluids and the Application to Separation Processes, Springer, NY (1994) [ Links ]
Budich, M., S. Heilig, T. Wesse, V. Leibkuchler and G. Brunner, "Countercurrent Deterpenation of Citrus Oil with Supercritical CO2", J. Supercritical Fluids, 14, 105-114 (1999). [ Links ]
Diaz, S. and J.A. Bandoni, "A Mixed Integer Optimization Strategy for a Large Scale Plant in Operation", Comp. Chem. Eng., 20, 5, 531-545 (1996). [ Links ]
Diaz, S., S. Espinosa and E.A. Brignole,. "Modeling and Simulation Tools for Supercritical Fluid Processes", Computer Aided Process Eng., 8, 319-324 (2000). [ Links ]
Duran, M. and I.E. Grossmann, "A Mixed-Integer Nonlinear Programming Approach for Process Systems Synthesis", AIChE J., 32, 592-606 (1986). [ Links ]
Espinosa, S., S. Diaz and E.A. Brignole, "Optimal Design of Supercritical Fluid Processes Comp. Chem. Eng., 24, 2/7, 1301-1307 (2000). [ Links ]
Institut Français du Pétrole, Manual of Economic Analysis of Chemical Processes. McGraw-Hill Book Company, France (1981). [ Links ]
Kalra, H., S.Y.-K Chung, and C.-J. Chen, "Phase Equilibrium Data for Supercritical Extraction of Lemon Flavors and Palm Oils with Carbon Dioxide", Fluid Phase Equilibria, 36, 263-278 (1987). [ Links ]
Marteau, Ph., J. Obriot and R. Tufeu, "Experimental Determination of Vapor-Liquid Equilibria of CO2 + Limonene and CO2 + Citral Mixtures", J. Supercritical Fluids, 8, 20-24 (1995). [ Links ]
Michelsen, M.L., "The Isothermal Flash Problem. Part II. Phase Split Calculation", Fluid Phase Equilibria, 9, 21-40 (1982). [ Links ]
Pusch, J. and J. Schmelzer, "Extension of the Group-Contribution Equation of State Parameter Matrix for the Prediction of Phase Equilibria Containing Argon, Ammonia, Propene and other Alkenes", Ber. Bunsenges. Phys. Chem., 97, 597-603 (1993). [ Links ]
Schrage, L. Linear, Integer and Quadratic Programming With LINDO. The Scientific Press, Palo Alto (1987). [ Links ]
Skjold-Jørgensen, S., "Gas Solubility Calculations. II. Application of a New Group-Contribution Eqn. of State", Fluid Phase Equilibria, 16, 317-351 (1984) [ Links ]
Ulrich G.D., A Guide to Chemical Engineering Process Design and Economics, John Wiley & Sons, (1984). [ Links ]
Received: September 16, 2001.
Accepted for publication: November 06, 2002.
Recommended by Guest Editors: J. Cerdá and A. Bandoni.














