KEY POINTS
• The HC-Ag method proved its usefulness in the diagnosis of progressive and disse minated HP, and it can be stated that its use and its complementation with conven tional and molecular biology methods in mycology laboratories is essential to know the real incidence and prevalence of this pathology, reduce diagnosis times, improve targeted treatment and propose follow-up, prophylaxis and prevention strategies, in order to reduce the lethality rate in HIV+ patients or those with other immunosup pressive pathologies.
• This is the first study published in Argenti na where a commercial EIA HGM 201 with monoclonal antibodies is used and compa red with other methods, and we believe that allows us to know the usefulness of this technique so that its implementation can be considered in other institutions in Argentina and Latin America as proposed in the goals set in Manaus for 202527.
Histoplasmosis (HP) is a cosmopolitan my cosis, endemic in the American continent, but with cases in the five continents. It is caused by the dimorphic fungus Histoplasma capsulatum var. capsulatum1. The areas of highest incidence lie along the valleys of the Ohio and Missouri- Mississippi rivers in North America, in several countries of Central America as Guatemala, El Salvador and Panama1,2 and in the basins of the Orinoco, Magdalena, Amazonas, San Francisco, Paraná and La Plata rivers in South America3,4. In Argentina, the main endemic area is the geo graphic region known as the Pampas and it is estimated that there are approximately seven million individuals infected with H. capsulatum5. HIV/AIDS associated histoplasmosis presents a prevalence of 2.5-5%6-8 and it is the second po tentially fatal systemic fungal infection in fre quency8.
Histoplasmosis can affect both immunocom petent and immunocompromised individuals. Clinical manifestations depend on the infecting strain9,10, the fungal load and the immune status of the patient6,7. A high risk of developing disseminated and progressive histoplasmosis ex ists in HIV/AIDS patients11. In Latin America the fatality rate ranges from 20 to 40%2,12,13, higher than that of the United States, which ranges from 4 to 8%14,15 and it is an AIDS marker disease in 30 to 75% of cases7,8,16-18.
Diverse studies have estimated that the inci dence of histoplasmosis in Latin America could be 1.5 cases per 100 HIV+ per year; which would add about 22 000 cases and produce about 9000 deaths annually, this would be higher than an nual deaths from tuberculosis19,20. Anyway, the real prevalence in our region remains unknown because it is not a communicable disease, and on the other hand in many countries diagnosis is deficient due to the lack of diagnostic tools3,12,19.
Laboratory diagnosis of histoplasmosis is made through different techniques that can be classified into: (a) Conventional, (b) Molecular and (c) Immunological methods.
Conventional methods include microscopic observation and culture of various clinical sam ples. When patients present mucocutaneous manifestations, the diagnosis can be quickly performed by microscopic observation of H. cap sulatum yeasts in Giemsa stained smears from scarifications or biopsies of such lesions; this technique has a variable sensitivity of 9-43% depending on the kind of lesion, the time of evolution and the operator expertise21,22. If the patients have no skin or mucocutaneous lesions culturing different clinical samples including lysis-centrifugation blood culture can be made. This technique can take up to 21 days for fun gal growth and has about 70% sensitivity in HIV positive patients21,23.
Molecular methods are based on different PCR techniques and have high sensitivity and specificity24-26. However, its main disadvantage is that there are still no validated and easily accessible commercial kits and reproducibility of home techniques is highly variable among labo ratories and there is a lack of consensus for their application27.
Antibody detection is an accessible immuno logical method but depends on the clinical form and the immunological status of the host. In AIDS associated histoplasmosis less than 30% of patients present detectable specific antibodies23. In contrast, detection of H. capsulatum galacto mannan antigens in these cases is much more efficient and also allows monitoring the effec tiveness of treatment28,29. The first methods for detecting Histoplasma antigens by enzyme immunoassays (EIA) were described in 198630. This technique was later adapted to using in urine and serum samples in 199431, and currently there are commercial ELISA kits that use monoclonal antibodies such as HGM 101 (IMMY®, Norman, OK, USA)32-34. The latter gave very good results in a recent study carried out in Latin America, with very high sensitivity and specificity35. However, in our country it is still not used as a routine test due to its high cost. For this reason, its perfor mance as a diagnostic method is unknown yet.
The objective of this study was to evaluate the sensitivity (S) and specificity (E) of the HGM201 kit for detecting H. capsulatum urinary antigen (HC-Ag) by EIA, to establish its usefulness in the early diagnosis of AIDS associated HP, and to compare its results with those of blood culture (BC), antibody detection (Ac -ID) and a PCR in blood (Hcp100 nPCR). Clinical and demographic characteristics of the studied patients were also analyzed.
Materials and methods
Type of study
A retrospective longitudinal and analytical study was conducted between January 2019 and December 2020.
Demographic and clinical data as well as the results of diagnosis of proven disseminated and progressive histoplasmosis (PPDH) in HIV/AIDS patients were compared with those of HIV+ patients with other pathologies and a group of healthy volunteers, at the Mycology Unit of In fectious Diseases, F.J. Muñiz Hospital, Buenos Aires.
Inclusion criteria
• HIV + patients over 18 years old with PPDH
• HIV + patients over 18 with other infectious pathologies contemplated in the differential diagnosis, which were treated at the Mycology Unit of F.J. Muñiz Hospital in the mentioned pe riod.
• Healthy volunteers (no apparent disease).
All patients were required to reside in the Rio de la Plata endemic area of Argentina.
Exclusion criteria
• Patients under 18 years’ old
• Patients who were not residents of the en demic area
• Patients without characteristic symptoms or clear clinical follow-up
• Patients that had proven coinfections with other etiological agents considered in this study, or treatment confirmed with amphotericin B, itraconazole or fluconazole pre-clinical sampling.
Patients and healthy volunteers
• Patients with PPDH. Fifty HIV+ patients were included in this group. A positive case of PPDH was defined as an individual with positive cul ture and/or histopathological findings of intra cellular yeasts and granuloma consistent with this fungal infection and by Gomori-Grocott stains [parameter according to recommenda tions of the EORCT/MSG Consensus Group36.
• HIV + patients with other infectious patholo gies. Fifty-one patients were included: 3 pneu mocystosis, 12 cryptococcosis, 1 leishmaniosis, 2 candidemias, 6 tuberculosis, 3 paracoccidioi domycosis, 24 patients with T CD4+ lymphocyte counts <200 cells/μl and different pathologies; with no characteristics signs or clinical symp toms, nor epidemiological history or other diag nostic elements of mycosis or tuberculosis (18 with impregnation syndrome and fever, 2 pem phigus vulgaris and 4 febrile neutropenia).
• Healthy volunteers. 15 individuals older than 18 years old without apparent diseases.
All demographic and clinical-epidemiological data of the patients were recorded and stored in a database for further analysis.
The diagnosis of HIV infection was deter mined by EIA serological test and confirmation by the real-time PCR technique to determine the viral load.
The Research Ethics Committee, Muñiz hos pital, reviewed and approved the conduct of this study
Clinical samples
The following samples from all individuals meeting the inclusion criteria were analyzed:
urine, EDTA anticoagulated peripheral blood, serum and pre-treated blood samples for lysis-centrifugation blood culture.
Tzanck cytodiagnosis was performed in scrapings in all patients who had skin or muco sal lesions.
Samples were taken on the first day and ten days after medical consultation taking into ac count that no patient had been previously treat ed with amphotericin B or itraconazole.
Urine samples were stored at -15 °C, serum and whole blood treated with EDTA at 4 °C until the time of their processing. Blood cultures and smears for cytodiagnosis were processed imme diately after being collected.
Conventional diagnostic methods
a. Blood culture by lysis-centrifugation (BC). Pe ripheral blood samples pretreated with 5% sa ponin and sodium polyanethol sulfonate were cultured on Sabouraud agar and Brain-Heart In fusion agar at 28 °C and 37 °C, according to the technique described by Bianchi et al.37.
b. Tzanck cytodiagnosis. Scarification with a sterile scalpel was performed in 50 patients who presented skin and mucosal lesions. It was pos sible to detect the presence of typical elements of the yeast phase of H. capsulatum and other etiological agents after Giemsa stain38,39.
Immunological methods
a. Serological tests. Detection of circulating anti H. capsulatum antibodies was performed by agar gel immunodiffusion techniques and counter-immunoelectrophoresis with secondary immu nodiffusion in agarose gel (Ac-ID)40, in serum samples from the 3 groups of patients. The an tigen used was an aqueous extract of the yeast phase of H. capsulatum41.
b. Antigen detection (HC-Ag). The presence of H. capsulatum galactomannan antigen was deter mined in urine samples of all patients and healthy volunteers with commercial EIA using monoclo nal antibodies HGM 201 (IMMY®, Norman, OK, USA). The used cutoff value was 0.2 ng/ml.
Molecular methods
A nested PCR designed by Bialek et al. and modified by Toranzo et al. was performed in whole blood samples anticoagulated with EDTA, from patients and healthy volunteers.
The primers used make it possible to amplify a DNA sequence that codes for the 100 kDa pro tein involved in the process of infection and sur vival of the fungus within the host cell21,25.
The external primers Hc I (5’-GCG TTC CGA GCC TTC CAC CTC ACC-3 ‘) and Hc II (5’-ATG TCC CAT CGG GCG CCG TGT AGT-3’) delimit a sequence of 391 bp. The internal primers Hc III (5’-GAC ATC TAG TCG CGG CCA GGT TCA-3 ‘) and Hc IV (5’-AGGAGA GAA CTG TAT CGG TGG CTT G-3’) delimit a 210 bp sequence.
Statistical analysis
Sensitivity (S), specificity (E), positive likeli hood coefficients (CVP), negative likelihood coef ficients (CVN) and accuracy with their respective 95% confidence intervals (CI) were evaluated for each of the diagnostic methods used. The latter were: blood culture, antibody detection, PCR in blood and determination of urinary antigen. Sta tistical parameters were evaluated individually and by combining them using contingency ta bles. The cases of PPDH were used as reference.
A ROC curve was built to identify one cutoff value according to the urinary antigen study re sults.
The variables normality was determined with the modified Shapiro-Wilk test, and the homo geneity of variances with the F and the Levene tests. We also analyzed the test of Kruskal-Wal lis and Wilcoxon signed-rank test to see if there were any significant differences in the response time (RT), which was defined as the average time between a clinical sample was taken and the re sult report was emitted by the lab. The programs InfoStat version 201842, EPIDAT 4.2 (Anón sf) and SPSS Statistics 20 were used. It was considered a p value <0.05 as statistically significant.
Results
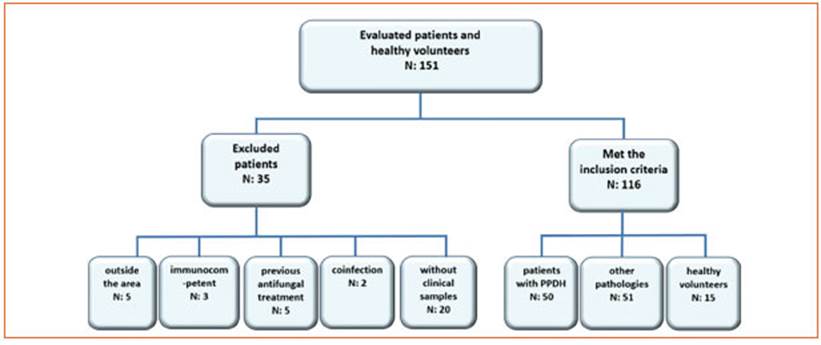
During the study period (24 months) 136 po tentially eligible patients diagnosed with PPDH or other pathologies were evaluated. On the oth er hand, 35 cases were excluded from the analy sis: 5 because of coming from outside the area of the Rio de la Plata, 3 immunocompetent, 5 with previous antifungal treatment, 2 had confirmed coinfection with Cryptococcus neoformans, and 20 patients in which clinical samples required for this study could not been analyzed. In the end, 116 cases were included. One hundred and one patients met the inclusion criteria: 50 cases were PPDH, and 51 cases suffered from other pathologies. Then, 15 apparently healthy volun teers were added (Fig. 1). Out of the 116 cases, 83 (72%) were men and 33 (28%) women, median age was 42 years (interquartile range- IQR: 33-48 years old). From these apparently healthy volun teers and patients, a total of 514 clinical sam ples were processed: 116 urine, 116 peripheral blood samples, 116 sera, 116 blood cultures and 50 mucosal or skin lesion scrapings analyzed by Tzanck cytodiagnosis.
Demographic and clinical features of HIV+ patients with PPDH
Within the 50 patients with PPDH, 37 were men (74%) and 13 women (26%) with a me dian age of 40 years (IQR: 34-48 years old). The 100% had CD4+ lymphocyte count <200 cells/μl, (median 25 cells/μl; ICR: 12-49 cells/μl). In 34% (17/50) of the cases, disseminated histoplasmo sis was the AIDS marker disease.
Definitive diagnosis was made by the growth of the fungus in blood cultures in 20 cases (40%), by the visualization of Histoplasma capsulatum yeasts in the Giemsa stain of mucocutaneous scarification sample in 15 cases (30%) and in oth er 15 cases (30%) both techniques were positive simultaneously. Regarding tegumentary mani festations, 33 patients with PPDH presented in juries that were mainly ulcerated skin papules or molluscum-like lesions. Of these, 67% (22/33) were cutaneous and 33% (11/33) mucosal. Most of the skin lesions were found on the thorax and face (90%) and predominated over injuries in the extremities 10% (χ2 = 14.73; p = 0.0001), whereas the 100% of the mucosal lesions were oral. No yeasts were observed in scarification samples of 3 (9.1%) patients.
At the time of consultation, 74% (37/50) of the patients had a pathological chest X-ray. Abnor malities were miliary 70% (n = 26), interstitial infiltrate 16% (n = 6), bilateral nodules 11% (n = 4) and peripheral pulmonary nodules 3% (n = 1). The miliary pattern appeared in a significantly greater proportion than the other lesions (χ2 = 5.44; p = 0 .02).
The established treatment was amphotericin B deoxycholate and itraconazole, depending on the magnitude of the symptoms considered by the treating physician and the general condi tion of the patients. In some cases, they received both drugs. Patients who were hospitalized at the time of histoplasmosis diagnosis were ini tially treated with amphotericin B deoxycholate at 0.7 mg/kg/day, amphotericin B lipid complex 5 mg/kg /day, or liposomal amphotericin B 3 mg/kg/day for 7, or 14 days depending on their clinical status. Only in cases of bone marrow involvement, intestinal obstruction, or menin gitis, treatment with amphotericin B was pro longed. In all cases, itraconazole 400 mg/day was indicated after amphotericin B. Treatment with itraconazole at the mentioned dose was maintained for 3 or 6 months depending on the evolution. After this period, they continued with the same medication but with 200 mg/day (sec ondary prophylaxis) until obtaining two LTCD4+ counts > 150 cells/μl with an undetectable HIV viral load.
The fatality rate in this group of patients was 14% (7/50).
Demographic and clinical characteristics of HIV+ patients with other pathologies
HIV+ patients with other diseases were 38 men (75%) and 13 women (25%), with a median age of 42 years (IQR 32-48 years old). The 97% had CD4+ counts < 200 cells/μl (median 68 cells/ μl; IQR: 36-86 cells/μl). Eleven patients had skin lesions and 6 mucosal ones. The former were lo cated on the trunk and face (82%), significantly predominating over the limb lesions which were only 18% (χ2 = 4.45; p = 0. 035), regarding the mu cosal lesions the 100% of them were of nasopha ryngeal location. Only in 10 lesions nonspecific inflammation was observed, in two Paracoccid iodes sp. yeasts, two viral syncitia, 1 Cryptococcus sp. yeasts, 1 Malassezia sp. and 1 Molluscum con tagiosum.
The fatality rate in this group of patients was 26% (13/51), the percentage was higher than in the group of patients with PPDH, although with out significant differences (χ2 = 2.101; p = 0.147)
Sensitivity (S), specificity (E), positive likelihood coefficients (CVP), negative likelihood coefficients (CVN), accuracy and response time of the different methods employed for the diagnosis of PPDH.
The results of S, E, CVP, CVN and accuracy of each of the methods can be observed and com pared in Table 1.

Table 1 Evaluation of the analytical parameters of each of the methods used in this study. E, S and accuracy values are percentages
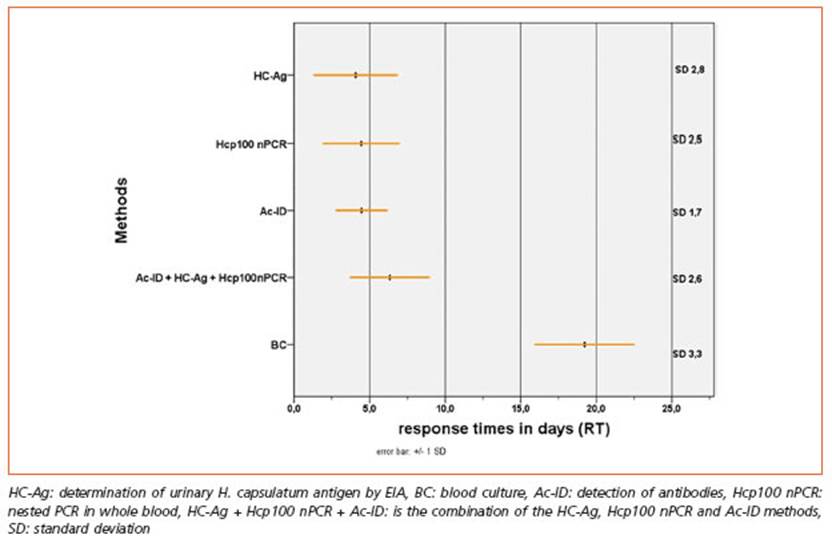
The RT of the various methods was 19 days for the BC, 4 days HC-Ag, 5 days for Hcp100 nPCR and 5 days for Ac-ID. Significant dif ferences were observed in RT as the statisti cal H calculated by the Kruskal-Wallis test was 320.97 (p < 0.05). This difference in RT is mainly due to the blood culture as when com pared with HC-Ag, Hcp100 nPCR and Ac-ID. By the Wilcoxon signed rank test were obtained the following values of the statistic Z: 9.44; 9.37 and 9.40 (p < 0.05) in the 3 comparisons of means (Fig. 2).
ROC curve
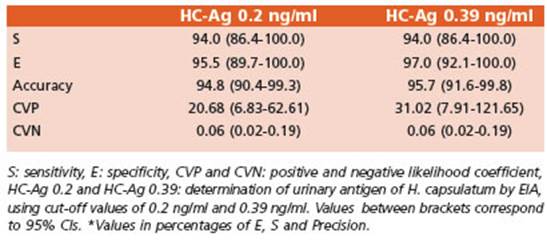
It was calculated one cutoff for the HC-Ag technique by a ROC curve that was of 0.39 ng/ ml, with S 94% (86% to 100%) and E 97% (92% to 100%). Area under the curve and other param eters were determined for this new cutoff value and can be seen in Fig. 3.
The statistical parameters of this method for the different cutoff values were compared in Fig. 4 and Table 2.
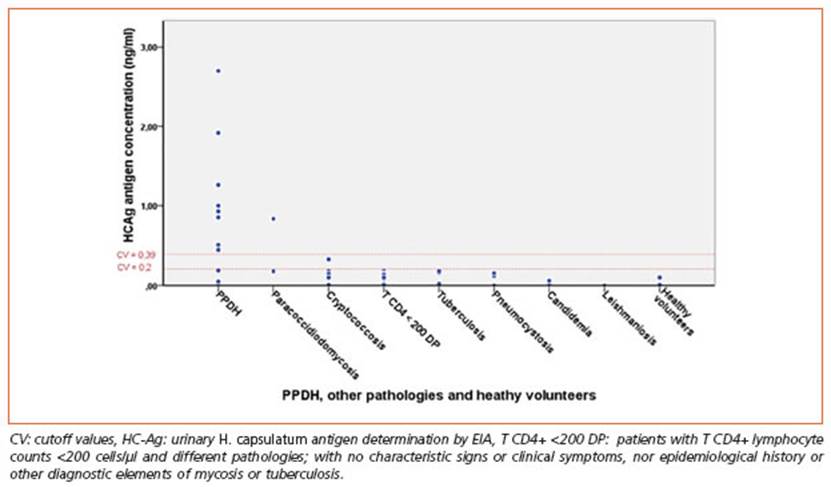
Figure 4 Urinary antigen concentration in ng/ml (HC-Ag) in patients with PPDH, other pathologies and healthy volunteers
Clinical characteristics of patients with PPDH and false negative results of HC-Ag
6 percent (3/50) of patients with PPDH had false negative results on the HC-Ag. Diagnosis was confirmed through positive BC, in 100% of these cases; H. capsulatum grew in an aver age of 19 days. In all patients of this group the Hcp100 nPCR was negative (0%) and the Ac-ID determination was positive (100%). Only one had lesions in the oral mucosa, but no intracel lular yeasts characteristic of H. capsulatum were observed by scraping and subsequent Giemsa stain. Central nervous system histoplasmosis was seen in one patient and H. capsulatum was obtained in CSF culture but both HC-Ag deter mination and Hcp100 nPCR gave negative re sults for this clinical sample. The median age of this group of patients was 34 years old (IQR: 28-51 years old), all patients were male. All had CD4+ counts < 200 cells/μl and a median of 74.00 cells/μl (IQR: 49.00-77.00 cells/μl). They were initially treated with amphotericin B dc 0.5 mg/kg/day followed by itraconazole 400 mg/ day. Lethality rate was 33.3%, higher than that in the group of patients with PPDH with HC-Ag positive results, but without significant differ ences (χ2 = 0.991; p = 0.320).
Clinical characteristics of patients with pathologies other than PPDH and healthy volunteers with false positive results of HC-Ag
Five percent (3/66) of patients without PPDH and healthy volunteers had a positive result of HC-Ag. Two had paracoccidioidomycosis and one cryptococcosis. The Hcp100 nPCR, BC and Ac-ID methods were negative in all these pa tients. One patient with paracoccidioidomycosis was the only one in this group who presented mucocutaneous lesions, on the oral mucosa. Multi-budding yeasts compatible with Paracoc cidioides spp. were found in in Giemsa-stained smears from the scarification. The median age in this group was of 42 years old (IQR: 38-74), all three patients were male. Sixty-six percent had CD4+ counts <200 cells/μl, median 70 cells/μl (IQR: 46-686 cells/μl).
None of the patients in this group died, there fore the lethality rate was 0%.
Discussion
Histoplasmosis is the endemic mycosis of greater impact on public health in Latin Ameri ca43, although its true impact is still not known and it is considered a neglected disease by various authors13,44. PPDH is associated with HIV infection in 90 to 95% of cases4,13 and oc curs mainly in patients who are not receiving antiretroviral treatment and with a CD4+ cell count <100 cells/μl8,12,16,45-47. Eighty to 95 % of patients may have a favorable evolution with an early diagnosis and adequate treatment47. The case fatality rate in different endemic ar eas of America averages 30%12, due to several factors that must be considered in order to de sign strategies that contribute to reducing the impact of this scourge. Among them: unknown actual incidence as it is not a compulsory noti fication disease, there is little information re lated to this mycosis and the scarce number of trained professionals. Clinical manifestations are not very specific and often wrongly diag nosed as tuberculosis or other diseases. Treat ment is not always accurate because of eco nomic crisis in many countries and consequent lack of access to liposomal amphotericin B or even itraconazole. Low socio-economic status of Latin American patients and their cultural differences from those of other countries con tribute to the poor adherence to antiretroviral and antifungal treatment and, finally delay in diagnosis when rapid and simple methods such as urinary antigen detection, which be gan to be developed in the US in 198648, are not available. These issues were analyzed in The Manaos Declaration in 201927 where partici pated 24 countries and whose main objective was to achieve access to rapid diagnostic meth ods and to treatments with amphotericin B and itraconazole in 100% of Latin American coun tries by 2025.
Argentina is not the exception to this problem since antifungals are difficult to obtain in most institutions and the HC-Ag kit is too expensive in our country, so only hospitals and mycology reference centers, where there is a high preva lence of histoplasmosis can have access to this method, as is the case in our hospital. Moreover, the situation is more serious because 70% of HIV+ patients are living in the endemic area8,47. Considering that the prevalence of asymptom atic histoplasmosis infections is close to 30% in our country, we can estimate that approximate ly 41,000 HIV+ individuals could be infected with this fungus and suffer a reactivation with the progression of the HIV/AIDS disease49,50. Accord ing to the Official Bulletin, there was an average of 5,800 new annual cases of HIV infection in the period 2011-201949. Therefore, an incidence of 100 cases/year of PPDH and consequently about 20 deaths can be estimated, as the prevalence of HIV in this endemic area is about 2.5% with a case fatality rate close to 20%7,8,23,47.
This estimated number may be even higher since there are 17% HIV+ people who are un aware of their diagnosis and there are also some authors who consider that the prevalence of HP in HIV+ is even higher8,18,19,47,51,52 and similar to the number of annual deaths caused by tubercu losis in those patients (close to 76 cases in 2019)53; and coincident with Adenis et al. publication20.
In 2007, a HC-Ag urinary kit using polyclonal antibodies developed by the CDC54 was evalu ated first in Guatemala and then in Colombia, on patients with PPDH in HIV+ patients. Its S was 81% in Guatemala32 and 86% in Colombia28 and its global E was 95% and 94% respectively. After that, a multicenter study using the HGM, IMMY (with monoclonal antibodies) to evalu ate the performance of this kit demonstrated a 98% S with the quantitative assay and 95% in the semiquantitative one, on 589 urine samples from patients with PPDH from Colombia and Guatemala35. In our research, the HC-Ag with the same kit had a S of 94% (with both 0.2 ng/ml and 0.39 ng/ml cutoff values) and its E reached 97% when 0.39 ng/ml cutoff value established in this research, was used.
False positive HC-Ag results were observed mainly with other mycoses (all cases of paracoc cidiodomycosis and cryptococcosis), as it was seen in the mentioned multicenter study35. The cut off value established in our research was useful to reduce false positives and improve E, without changing the other parameters.
The HC-Ag test had the highest S and the low est CVN compared to the other diagnostic meth ods. In all proven cases where urinary antigen could not be detected (false negatives), the pa tients had demonstrable antibodies. Moreover, when different rapid diagnostic methods were combined (HC-Ag, Hcp100 nPCR and Ac-ID) S reached a performance of 100%.
The time to reach diagnosis has a fundamen tal impact on patient outcome and survival. Sa mayoa et al., were able to determine that the average survival time of PPDH patients without treatment was 19 days2.
In this study, 66% of patients with PPDH had mucocutaneous manifestations, which is con sistent with data from Latin America8,16, and differs from those observed in the US where only 6% of patients present this type of lesions4. When lesions are accessible, a diagnosis can be obtained in less than 2 hours by Tzanck cytodiagnosis55.
However, about 30-40% of patients do not have tegumentary manifestations43, and in these cas es the use of rapid diagnostic methods would be highly recommended since cultures take more than two weeks. When the diagnostic times of the tests were compared by means of the RTs, it was possible to determine that those of HC-Ag, Hcp100 nPCR and Ac-ID were significantly low er than those of BC whose average time was 19 days, coinciding with the average survival time estimated by Samayoa2.