Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Anales de la Asociación Química Argentina
versión impresa ISSN 0365-0375
An. Asoc. Quím. Argent. v.92 n.4-6 Buenos Aires ago./dic. 2004
REGULAR PAPERS
Spectral And Thermal Studies Of Saccharinato Complexes
Teleb, S.M.1
1Department of Chemistry, Faculty of Science, Zagazig University, Zagazig, Egypt
Fax: +20663341303, Email: said_teleb@yahoo.com
Received May 24, 2004. In final form June 12, 2004.
Abstract
The saccharinato complexes of Au(III), ZrO(II), VO(II) and UO2(II) metal ions have been prepared and the coordination of saccharin in these complexes has been investigated through their 1HNMR and IR spectra as well as by thermal analysis. It was found that saccharin interacts with all of these metal ions in the anionic form and coordinates in a monodentate fashion through its nitrogen to Au(III), ZrO(II) and VO(II) ions, whereas it coordinates to UO2(II) ion as a bidentate ligand using its carbonyl and sulphonyl groups. A square structure has been proposed for Au(III) complexes, polymeric chain structures for ZrO(II) and VO(II) complexes and an octahedral structure for UO2-saccharin complex. The thermal properties of these complexes were shown to be consistent with the proposed structures and indicate that metallic gold, ZrO2, V2O5 and UO2SO4 are obtained as final thermal decomposition products of these complexes.
Resumen
Se sintetizaron complejos de sacarinatos de Au(III), ZrO(II), VO(II) y UO2(II). Mediante sus espectros 1HNMR e IR y análisis térmico, se determinó la coordinación de la sacarina en los complejos. La sacarina interactúa con estos iones metálicos en su forma aniónica, coordinándose en forma monodentada a través de su nitrógeno a los iones Au(III), ZrO(II) y VO(II). Sin embargo la coordinación bidentada se observa para ell ion UO2(II), a través de sus grupos carbonilo y sulfonilo. Se propone una estructura cuadrada para los complejos de Au(III), estructuras en forma de cadena polimérica para los complejos ZrO(II) y VO(II) y una forma octahédrica para el complejo UO2-sacarina. Las propiedades térmicas de estos complejos ha mostrado ser consistentes con las estructuras propuestas e indican que el oro metálico, y las especies ZrO2, V2O5 y UO2SO4 resultan ser los productos finales de la descomposición térmica de estos complejos.
Introduction
Saccharin (or o-sulphobenzoimide) is widely used as an artificial sweetener. The chemistry of saccharin has attracted attention because of its suspected carcinogenous nature [1,2] and the potential use of saccharin as an antidote for metal poisoning [3]. Metal complexes of saccharin may also have relevance to the understanding of its human metabolism [4].
The saccharin molecule contains a set of donor atoms that are able to bind metal ions. Studying the coordination nature of saccharin and determining the binding site(s) to metal ions are perhaps a key to understand the bioinorganic chemistry of saccharin. A lot of saccharin binary and ternary complexes have been reported and investigated [5-11]. The data obtained indicate that saccharin acts either as a monodentate anion, coordinating via the nitrogen or carbonyl oxygen atoms, or as a bidentate ligand using both donor atoms. A different mode of coordination has also been reported for saccharin[12] in the complexes [M(sac)2L2]xH2O (M = Cu(II) or Co(II), L = H2O or pyridine, X = 1, 2 or 4). The octahedral coordination sphere associated with these complexes contains two carbonyl groups of two saccharin molecules and two sulphonyl groups of two other saccharin molecules. The remaining sulphonyl and carbonyl groups of each molecule coordinate with other metal ions to give a polymeric structure with a molar, metal: saccharin ratio of 1 : 2. The remaining two octahedral positions are occupied by two pyridine molecules or two N-H groups of two saccharin molecules.
The present investigation deals with the preparation, spectroscopic studies and thermal analysis of the complexes obtained during the reaction of saccharin with Au(III), VO(II), ZrO(II) and UO2(II) ions with the aim of investigating the coordination mode of saccharin in these complexes.
Experimental
All of the chemical used throughout this investigation were extra pure grade.
[Au(sac)2(H2O)2]Cl and [Au(sac)3H2O]: Gold(III) chloride (0.5 g., 1.65 mmole) was dissolved in 50 ml water and added to 50 ml of a water solution containing 0.677 g (3.3 mmole) or 1.015 g (4.95 mmole) of the sodium salt of saccharin. The reaction mixtures of metal : saccharin molar ratios of 1 : 2 and 1 : 3 were heated gently at about 70oC with constant stirring for about 3 hrs and then left overnight. The formed yellow precipitates were filtered out, washed several times with hot water and dried in an oven at 50oC and then over silica gel.
[ZrO(sac)2]7H2O, [VO(sac)2]H2O and [UO2(sac)2]3H2O: ZrO(NO3)2, VOSO4 and UO2(CH3COO)2.2H2O were used to obtain the saccharinate of these ions using the same method as above with a metal: NaSac molar ratio of 1 : 2. It should be mentioned here that, we have obtained the same complexes on using saccharin, Hsac instead of its sodium salt, but the precipitates were a little bit contaminated by the undissolved saccharin.
Microanalysis (C, H, N, S) were performed using CHNS-932 (LECO) and Vario EL elemental analyzers (Martin Luther University, Germany). Metal contents were determined with an atomic absorption spectrometer PYE-UNICAM SP 1900. The percentage of water in the formed complexes was determined by thermogravimetric techniques. Chlorine was determined by burning the substance in oxygen with a platinum contact followed by titration with mercuric nitrate towards diphenyl carbazide. The results obtained are in good agreement with those calculated for the proposed complex formulas, as shown in Table 1. Infrared spectra of the reactants and the obtained complexes were recorded from KBr discs (4000 – 400 cm-1) using a Genesis II FT-IR. 1H NMR spectra of Au(III) complexes were recorded on a Varian Spectrophotometer Gemini 200 (Martin-Luther University) using dimethylsulphoxide-d6 as a solvent and TMS as an internal reference. Thermogravimetric analysis, TGA, and differential TG were carried out under a N2-atmosphere (30 ml/min) using a detector model Shimadzu TG-50 H. The heating rate used was 10°C/min on sample masses ranging from 2.75 to 3.74 mg.
Table 1. Analytical data* and yields for various saccharinates.
| Compound | C | H | N | S | Cl | M | Yield% |
| [Au(sac)2(H2O)2]Cl
| 26.48 (26.56) | 1.87 (1.89) | 3.81 (3.79) | 10.26 (10.11) | 5.63 (5.60) | 31.19 (31.14) | 90 |
| [Au(sac)3H2O]
| 33.17 (33.11) | 1.85 (1.83) | 5.54 (5.51) | 12.67 (12.61) |
| 25.92 (25.88) | 86 |
| [ZrO(sac)2]7H2O
| 28.13 (28.14) | 3.71 (3.68) | 4.67 (4.69) | 10.75 (10.72) |
| (15.28 (15.24) | 78 |
| [VO(sac)2]H2O
| 37.38 (37.41) | 2.24 (2.22) | 6.25 (6.23) | 14.21 (14.25) |
| 11.39 (11.35) | 72 |
| [UO2(sac)2]3H2O
| 24.44 (24.41) | 2.06 (2.03) | 4.10 (4.06) | 9.36 (9.30) |
| 34.63 (34.59) | 82 |
* The calculated values are shown in parentheses.
Results and discussion
The infrared spectra of the obtained Au(III), ZrO, VO and UO2-saccharinato complexes are shown in Figure 1 and their band assignments are given in Table 2. The infrared spectra of all of these complexes show a set of weak to medium bands in the region above 3400 cm-1. This set of bands is similar to that observed in the spectrum of sodium salt of saccharin and should be due to the n(OH) stretching motions of H2O molecules [13]. However, the spectrum of free saccharin (Hsac) exhibits a weak band at 3215 cm-1 due to the n(N-H) vibration [14]. This band is not present neither in the spectra of its sodium salt nor of the saccharinato complexes. This observation suggests that saccharin reacted with these metal ions in the anionic form. This was also confirmed by measuring the 1H NMR spectra of Au(III)-saccarinato complexes, Figure 2. The 1H NMR spectra of the two gold complexes are almost the same and show only a broad singlet at 4.4 ppm due to H2O protons and a multiplet signal around 8 ppm due to the aromatic protons [15]. The infrared spectra of the complexes show a group of bands lying in the region of 3100 - 2940 cm-1 similar to those observed in the spectra of saccharin and its sodium salt. This group of bands are accordingly attributed to the n(C-H) stretching vibrations of the aromatic ring [14,16].
Based on spectral investigations for a series of metal saccarinates [17,18], the wavenumber of n(CO) mode can be used to make certain predictions on the type of metal-saccharin bonding (through nitrogen). It was found that, the lowering of the n(CO) wavenumber in metal saccharinates compared to saccharin itself is more pronounced in the case of the ionic saccharinates than in the saccharinates where the metal-saccharinato bonds mainly covalent.
Table 2. Characteristic infrared frequencies* (cm-1) and their assignments for [Au(sac)2(H2O)2]Cl, [Au(sac)3(H2O)], [ZrO(sac)2]7H2O, [VO(sac)2]H2O and [UO2(sac)2]3H2O.
| [Au(sac)2(H2O)2]+ | [Au(sac)3(H2O)] | [ZrO(sac)2]7H2O | [VO(sac)2]H2O | [UO2(sac)2]3H2O | Assignments** |
| 3585 m | 3537 m |
| 3538 m | 3540 m |
|
| 3472 w | 3480 w |
| 3476 w | 3472 w | n(OH); H2O |
| 3416 m | 3425 w | 3437 br | 3417 w | 3416 m |
|
| 3089 m | 3082 m | 3105 m | 3090 m | 3109 w |
|
| 3050 w | 3048 w | 3027 w | 3034 w | 3054 vw | n(C-H) |
| 2958 w | 2962 w | 2979 w | 2958 m | 2945 vw |
|
| 1722 vs | 1725 vs | 1732 vs | 1722 ns | 1658 vs | n(C=O) |
| 1640 s | 1635 s | 1625 s | 1631 m | 1622 s | d(H2O) |
| 1583 ms | 1583 ms | 1583 s | 1583 m | 1583 m | n (C=C) |
| -- | -- | -- | -- | 1336 ms | n (C-O) |
| 1332 vs | 1334 vs | 1356 vs | 1334 vs | 1250 vs | nas(SO2) |
| 1167 vs | 1167 vs | 1167 vs | 1167 vs | 1153 vs | ns(SO2) |
| -- | -- | 972 m | 935 ms | 917 s | n (M=O) |
| 850 m | 854 m | -- | -- | -- | dr(H2O) |
| 632 w | 635 w | 638 w | 631 w | 541 vw | n (M-N)+ |
|
|
|
|
| 465 m | n (M-O) |
*br, broad; m, medium; s, strong; v, very; w, weak.
**n, stretch; d and dr correspond to bending and rocking motions, respectively.
The very strong absorption band around 1725 cm-1 in the spectra of Au, ZrO and VO-saccharinates can undoubtedly be assigned to the n(CO) stretching mode [14,19]. The corresponding vibration is observed at the same wavenumber in the spectrum of free saccharin (Hsac) [18] and at 1675 cm-1 in the spectrum of sodium saccharinate [3,4,6]. This might be an indication that the Au(III), Zr(IV) and V(IV) ions form metal-saccharinato bonds with high covalent character.
The wavenumbers of the sulphonyl stretching, n(SO2) mode in the saccharinates of gold, zirconyl and vanadyl are observed as very strong bands around 1340 and 1167 cm-1 for the asymmetric and symmetric modes, respectively. These values are relatively low compared to the corresponding wavenumbers in the spectrum of free saccharin itself (1360 and 1180 cm-1) [20-22]. This seems to be common for the previously studied N-bonded saccharinates [22] and is probably due to the charge redistribution within the saccarinato ligand [23].
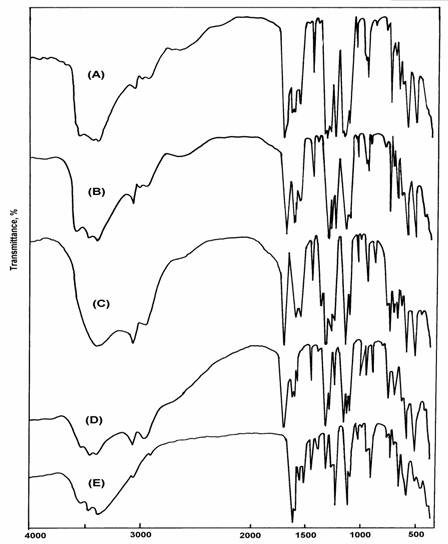
Fig. 1: Infrared spectra of (A) [Au(sac)2(H2O)2]Cl, (B) [Au(sac)3(H2O)],
(C) [ZrO(sac)2]7H2O, (D) [VO(sac)2]H2O, (E) [UO2(sac)2]3H2O
The coordination mode of saccharin in the uranyl complex seems to be different. The carbonyl stretching vibration, n(CO) in this complex spectrum is observed at 1658 cm-1. This band is shifted to lower wavenumbers by 67 cm-1 (free saccharin) and 17 cm-1(sodium saccharinate). On the other hand, the sulphonyl stretching vibrations are observed at 1250 and 1153 cm-1 for nas(SO2) and ns (SO2), respectively. The shift to lower wavenumbers in the sulphonyl stretching modes is more dramatic compared with their values in free saccharin, sodium saccharinate or even in the other metal saccharinates. The shifts to lower wavenumbers of the n(CO) and n(SO2) in the uranyl complex can be attributed to the effect of coordination through the oxygen atoms of these groups to the metal. Accordingly, the saccharinato ligand in this complex behaves as a bidentate ligand in the anionic form and the carbonyl and sulphonyl groups are involved in the coordination process.

Fig. 2: 1H-NMR spectra of [Au(sac)2(H2O)2]Cl complex.
This coordination mode could be supported by the appearance of a band of medium-strong intensity corresponding to the n(C-O) stretching vibration at 1336 cm-1 [13]. This band is not observed in the spectrum of free saccharin nor in those of the other complexes. The infrared spectra of all of these complexes exhibit bands due to n(O-H) and d(H2O) modes in the regions above 3400 cm-1 and around 1630 cm-1, respectively, indicating the presence of water molecules either inside or outside the coordination sphere of the complexes. In addition to these two modes only gold complexes show a new band of medium intensity at 850 cm-1 due to the rocking mode, dr(H2O) of coordinated H2O molecules. The appearance of this band suggests that H2O molecules in the gold complexes are inside the coordination sphere, while for the other complexes are outside. This observation was also confirmed by thermal analysis. Furthermore, qualitative analysis reveals ionic chloride in the complex [Au(sac)2(H2O)2]Cl. Therefore a square geometry is proposed for [Au(sac)2(H2O)2]Cl and [Au(sac)3H2O]. The coordination sphere of the gold atom contains two or three nitrogen atoms of two or three saccharin molecules and two or one oxygen atoms of two or one H2O molecule, respectively.
The infrared spectra of zirconyl and vanadyl complexes show a strong absorption band at 972 and 935 cm-1 due to n(Zr=O) and n(V=O), respectively, lower than expected [24]. The large shift of n(Zr=O) and n(V=O) to lower wavenumber may be explained in terms of an oxygen bridging M-O-M polymeric chain formation [25]. However, the shift of n(Zr=O) and n(V=O) values in the proposed polymeric structure, compared with that of the monomer, could be attributed to the expected increase of the reduced mass values of both metal and oxygen upon polymerization that leads to the decrease of the n(M=O) value. The n(U=O) vibration in the uranyl complex is observed as expected [26] as a strong band at 917 cm-1. Accordingly, octahedral geometry is proposed for the uranyl complex and the coordination sphere of this complex contains in addition to the two oxygen atoms of UO2 group, two oxygen and two sulphur atoms of two saccharinato ligands. Thermogravimetric (TG) and differential TG analysis were carried out under nitrogen flow. TG and DTG curves obtained for the complexes are shown in Figure 3. Table 3 gives the maximum temperature values for the decomposition along with the species lost in each step of the reaction. The data obtained indicate that, the decomposition modes of all of these complexes occur as expected in two main degradation steps (except, the [Au(sac)2(H2O)]Cl complex that shows three steps).
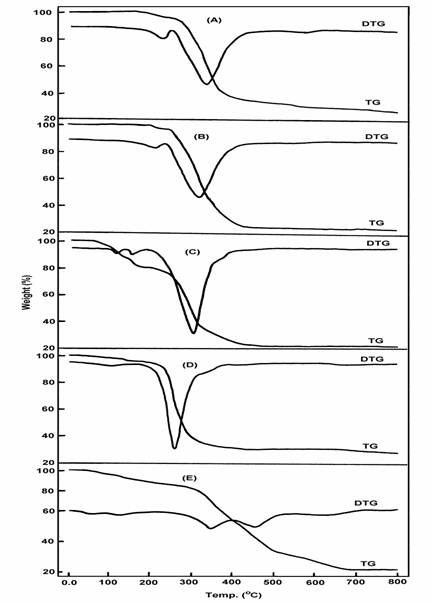
Fig. 3: TG and DTG for (A) [Au(sac)2(H2O)2]Cl, (B) [Au(sac)3(H2O)], (C) [ZrO(sac)2]7H2O,
(D) [VO(sac)2]H2O, (E) [UO2(sac)2]3H2O
Table 3. The maximum temperature values for the decomposition along with the species lost in each step of the decomposition reactions of the obtained saccharinates.
| Complex | Decomposition | Tmax/oC | Species lost | % Weight loss | |
| Found | Calc. | ||||
| [Au(sac)2(H2O)2]Cl | 1st | 186 | 2H2O | 5.43 | 5.69 |
|
| 2ed | 271 | 2Sac | 56.89 | 57.55 |
|
| 3ed | 498 | Cl | 5.80 | 5.60 |
|
| Residue | Metallic Au | 31.88 | 31.14 | |
| [Au(sac)3(H2O)] | 1st | 184 | H2O | 2.28 | 2.36 |
|
| 2ed | 276 | 3 Sac | 72.10 | 71.34 |
|
| Residue |
| Metallic Au | 25.62 | 25.88 |
| [ZrO(sac)2]7H2O | 1st | 70, 105 | 7 H2O | 20.84 | 21.10 |
|
| 2ed | 263 | 2 Sac | 58.16 | 63.08 |
|
| Residue |
| ZrO2 | 21.13 | 20.60 |
| [VO(sac)2]H2O | 1st | 70 | H2O | 4.12 | 4.00 |
|
| 2ed | 248 | 2 Sac. | 75.38 | 81.06 |
|
| Residue | VO2-5 | 20.50 | 20.26 | |
| [UO2(sac)2]3H2O | 1st | 60, 120 | 3 H2O | 7.92 | 7.84 |
|
| 2ed | 351, 456 | 2 Sac. | 40.18 | 52.90 |
|
| Residue |
| UO2SO4 | 51.90 | 53.19 |
The first stage of decomposition clearly corresponds to the total dehydration of the complexes. For zirconyl, vanadyl and uranyl complexes, this stage occurs at a relatively lower temperature lying in the range of 60 - 120oC. The observed weight loss associated with this step agrees quite well with the calculated weight loss due to the loss of seven, one and three lattice water molecules in zirconyl, vanadyl and uranyl complexes, respectively, see Table 3. For the two gold(III) complexes this stage occurs at a higher temperature around 185oC supporting the suggestion that these complexes contain coordinated rather than lattice water molecules. The weight loss values associated with this step are in good agreement with the theoretical values arising from the loss of one and two water molecules in the complexes [Au(sac)3H2O] and [Au(sac)2(H2O)2]Cl, respectively.
The second stage of decomposition occurs for all of this complexes in the temperature range of 248 - 351oC and should be attributed to the loss of the saccharinato ligand. The saccharinato ligand in the two gold complexes is completely lost during this stage of decomposition. The weight loss found for these two complexes are consistent with the calculated values, the residues left after this stage being metallic gold and AuCl for [Au(sac)3H2O] and [Au(sac)2(H2O)2]Cl, respectively. The latter complex was further decomposed at 498oC into metallic gold (Table 3). For zirconyl and vanadyl complexes, the percentages of residue after this stage of decomposition are in good agreement with ZrO2 and VO2-5 (V2O5) being the final thermal decomposition products. The infrared spectra obtained for these products support this conclusion. For uranyl complex the data obtained agree quite well with UO2SO4 being the final thermal decomposition product. The infrared spectrum obtained for this product clearly shows a set of bands characteristic for the ionic sulphate group, Figure 4.
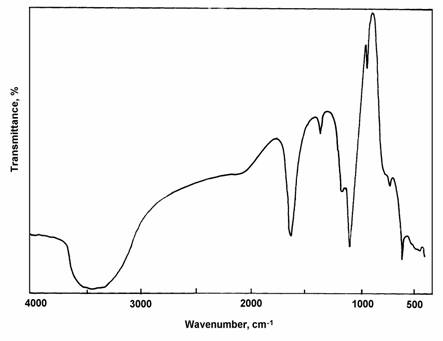
Fig. 4: Infrared spectra of UO2SO4 as a final thermal product.
Finally, from the thermal data, it is possible to conclude that the coordination mode of the saccharinato ligand in the [UO2(sac)2]3H2O complex is different from its mode in the other complexes. The presence of UO2SO4 as a final decomposition product supports the suggestion that the saccharinato ligand in this complex coordinates through its sulphonyl and carbonyl groups, in good agreement with the infrared spectrum of this compound.
References
[1] Coon, M.J., Proc. Int. Congr. Pharmacol., 1975, 6, 117. [ Links ]
[2] Munro, I.C.; Modie, C.A.; Krewski, D.; Grice, H.C., Toxicol. Appl. Pharmacol., 1975, 32(3), 513. [ Links ]
[3] Malic, K.M.A.; Haider, S.Z.; Hossain, M.A.; Hursthouse, M., Acta Crystallogr., Sect. C, 1984, 40, 1695. [ Links ]
[4] Haider, S.Z.; Malic, K.M.A.; Ahmed, K.J., Inorg. Synth., 1985, 23, 47. [ Links ]
[5] Jovanovski, G.; Hergold-Brundic, A.; Kamena, B., Acta Crystallogr., Sect. C, 1988, 44, 63. [ Links ]
[6] Haider, S.Z.; Malic, K.M.A.; Ahmed, K.J.; Hess, H.; Riffel, H.; Hursthouse, M.B., Inorg. Chim. Acta, 1983, 72, 21. [ Links ]
[7] Ainscough, B.W.; Baker, E.N.; Brodie, A.M.; Cresswell, R.J.; Ranford, J.D., Inorg. Chimica Acta, 1990, 192, 185. [ Links ]
[8] Cotton, F.A.; Favello, L.R.; Llusar R.; Libby, E.; Murillo, C.A.; Schwotzer, W., Inorg. Chem., 1986, 25, 3423. [ Links ]
[9] Zhang, Y.; Li, J.; Lin, W.; Liu, S.; Huang, J., J. Cryst. Spec. Res., 1992, 22, 433. [ Links ]
[10] Liu, S.; Huang, J.; Li, J.; Lin, W., Acta Crystallogr., C, 1991,47, 41. [ Links ]
[11] Li, J.; Ke, Y.; Wang, Q.; Wu, X., Cryst. Res. Technol., 1997, 32, 481. [ Links ]
[12] Magri, A.D.; Dascenzo, G.; Cesaro, S.N.; Chiacchierini, E., Thermochim. Acta., 1980, 36, 279. [ Links ]
[13] Nakamoto, K., Infrared and Raman Spectra of Inorganic and Coordination Compounds 4th Ed., J. Wiley & Sons, New York, 1986. [ Links ]
[14] Günzler, H.; Gremlich, H., IR Spectroscopy An Introduction Wiley-VCH Verlag GmbH 69469 Weinheim (Germany), 2002. [ Links ]
[15] Günther, H., NMR Spectroscopy An Introduction, John Wiley & Sons, New York, 1980. [ Links ]
[16] Bellamy, L.J., The Infrared Spectra of Complex Molecules, Chapman and Hall, London, 1975. [ Links ]
[17] Jovanovski, G.; optrajanov, B., J., Mol. Struct., 1988, 174, 467. [ Links ]
[18] Jovanovski, G.; optrajanov, B.; Kamenar, B., Bull. Chem. Technol. Macedonia, 1990, 8, 47. [ Links ]
[19] Naumov, P.; Jovanovski, G., Acta Chem. Slov., 1999, 46, 389. [ Links ]
[20] Hase, I., J. Mol. Struct., 1978, 48, 33. [ Links ]
[21] Hase, I., J. Mol. Struct., 1979, 52, 163. [ Links ]
[22] Jovanovski, G.; Tanceva S.; optrajanov, B., Spectrosc. Lett., 1995, 28, 1095. [ Links ]
[23] Binev, G., Stamboliyska, A.B.; Velcheva, E.A., Spectrochim. Acta, 1996, A52, 1135. [ Links ]
[24] Selbin, J., Chem. Rev., 1965, 65, 153. [ Links ]
[25] Kasuga, K.; Nagahara, T.; Yamamoto, Y., Bull. Chem. Soc. Jap., 1982, 55, 2665. [ Links ]
[26] McGlynn, S.P.; Smith, J.K.; Neely W.C., J. Chem. Phys. (USA), 1961, 35, 105. [ Links ]














