Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista argentina de endocrinología y metabolismo
versión On-line ISSN 1851-3034
Rev. argent. endocrinol. metab. vol.52 no.3 Ciudad Autónoma de Buenos Aires jun. 2015
TRABAJO ORIGINAL
Long-Term Follow Up of Thyrotropinomas: The Role of Radiotherapy and Long-Acting Somatostatin Analogs. Report of Two Cases
Evolución a largo plazo de tirotropinomas: papel de la radioterapia y análogos de somatostatina de acción prolongada. Reporte de dos casos
Insua A, García L
Service of Endocrinology, Department of Internal Medicine, CEMIC (Centro de Educación e Investigaciones Clínicas), Buenos Aires, Argentina
Correspondencia: Dr. Alvaro Insúa Hospital Universitario CEMIC sede Saavedra, Galván 4202, CABA, Argentina. Tel:541152990100. Email: insuaalvaro@gmail.com
Recibido: 6-01-2015
Aceptado: 28-05-2015
ABSTRACT
Thyrotropinomas or TSHomas are functioning pituitary adenomas that produce central hyperthyroidism and are often detected at macroadenoma stage, with the expected mass effects of large pituitary tumors. We report the long-term outcome of two thyrotropinomas, and discuss the treatment options adopted and available for these patients. Our first patient was seen 4 years after diagnosis of hyperthyroidism and two years after pituitary surgery, with persistent disease. The second subject was diagnosed soon after the detection of a symptomatic pituitary mass with extrasellar extension, with the corresponding clinical and laboratory workup. None of them was cured by surgery alone; both were treated with somatostatin analogs and radiotherapy with a clear improvement of their condition.
No financial conflicts of interest exist.
Key words: Thyrotropinomas; Long-term evolution; Radiotherapy; Octeotride
RESUMEN
Los tirotropinomas son raros adenomas hipofisarios funcionantes, que producen hipertiroidismo central y, como se suelen detectar en el estadio de macroadenomas, con los signos de masa correspondientes. Presentamos la evolución a largo plazo de dos tirotropinomas para analizar las opciones terapéuticas adoptadas y disponibles para estos pacientes. Nuestro primer caso fue visto 4 años después del diagnóstico de hipertiroidismo, y dos años pos cirugía pituitaria, con enfermedad persistente. El segundo sujeto fue diagnosticado poco después de la detección de una masa hipofisaria, con expansión extraselar sintomática, mediante la evaluación clínico endocrionológica y de laboratorio pertinente. Ninguno de los dos fue curado por la cirugía solamente; ambos fueron tratados con análogos de somatostatina y radioterapia, con un claro beneficio clínico, imagenológico y bioquímico.
Los autores declaran no poseer conflictos de interés.
Palabras clave: Tirotropinomas; Evolución alejada; Radioterapia; Octeotride
INTRODUCTION
Thyrotropinomas or TSHomas, are rare pituitary neoplasms(1), that comprise less than 2 % of all hypophyseal tumors.They are usually detected at the stage of macroadenomas. These pituitary tumors(2) produce central hyperthyroidism and the corresponding local manifestations of the mass. Even nowadays around 30 % of patients are treated previously as if they have had different forms of hyperthyroidism of thyroideal origin(3), which can turn their management difficult and worsen the natural course of the disease.
MATERIAL AND METHODS
The medical records of 2 patients with a diagnosis of thyrotropinoma were exhaustively reviewed with emphasis in clinical presentation, laboratory and imaging results, according to treatments employed at different periods of the evolution. The trend over time in TSH values was analysed by an ANOVA test.
RESULTS
Case 1
A 73 years old caucasian asymptomatic woman consulted because of a pituitary tumor that had been operated two years before. At age 69 a diagnosis of overt hyperthyroidism was made [T4: 19.5 ug/dl (nv: 4.5-12.5 ug/dl)] with an elevated TSH [5.63 uU/ml (nv: 0.27-4.20 uU/ml)]. Months before, she had had atrial fibrillation, along with a 10 % decrease in body weight, without headaches or ocular complaints. A 5 mCi dose of I131 had been given that resulted in hypothyroidism, so she had been placed under levothyroxine (LT4) (100 ug/day). At age 71, because a progressive increase in TSH [maximum: 33.10 uU/ml, (Table 1, period A)], in spite of normal serum T4 (Table 2,A), a pituitary MRI was performed, that depicted a macro adenoma of 2.8 cm height, with upward displacement of the pituitary stalk and chiasm, and bilateral contact with the cavernous sinuses (Figure 1, A). Serum PRL was slightly elevated, IGF1 normal, and serum cortisol decreased. Hydrocortisone (30 mg/day) was added to levothyroxine. A surgical transsphenoidal pituitary approach of the tumor was done, partially resecting an adenoma that stained positive for TSH. The procedure reduced the cephalocaudal length of the tumor to 2,2 cm (Figure 1, B). After surgery, a drop of serum TSH to 4,15 uU/ml was noted, followed by a stepwise increase up to 20.43 uU /ml [(Table 1, period B), with normal T4 (Table 2, B), during the next 20 months. At this moment she was seen by us for the first time. Physical examination showed a kyphotic woman, 1.56 mts height, with a body weight of 64 kg(BMI: 26. 30KG/M2), blood pressure 150/80 mmg/Hg, pulse rate 80 beats/min. Laboratory workup was as follows: T4: 10.7 ug/dl, free T4: 1.25 ng/dl (nv: 0.94-1.7 ng/dl), T3: 63 ng/ml (nv: 79-149) and TSH: 20.43 uU/ml (0.35-4.69). Hypopituitarism was diagnosed because of low levels of IGF1 [50 ng/ml (n: 70-350]), FSH (2.8 mU/ml) and ACTH (5.8 pg/ml). Alkaline phosphatase was high. The patient was menopaused since age 49 and had experienced a lateral neck fracture at age 66. She was aware of having had a lumbar crush fracture.
TABLE 1. Case #1: TSH evolution after 7.9 years of follow up(n being the number of observations during each period) [(ANOVA: F: 8.88; p < 0.0003). *POST-HOC ANALYSIS: P< 0.05
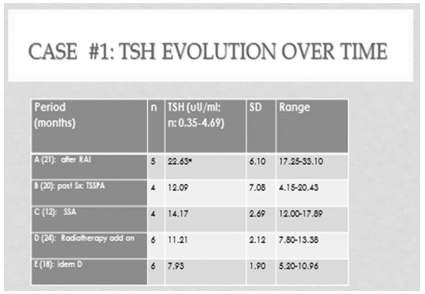
TABLE 2. Case #1: T4 evolution after 7.9 years of follow up (n being the number of observations during each period)
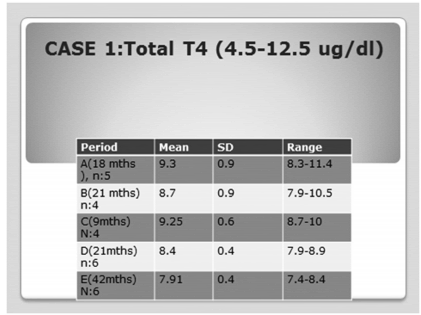
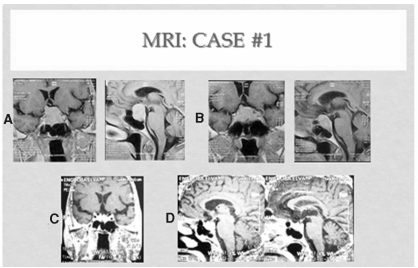
Figure 1. Case #1 A: Left upper panel: before surgery. B: right upper: postTSSPA. C: left lower panel: 1 y after radiotherapy plus octeotride. D: lower right panel; at the end of follow up(3.5 ys after radiotherapy).
Lumbar spine and femur neck BMD were reduced with a - 4.07 and -5.46 TS, respectively. Rx examination of the spine showed the known L1 crush and 2 incident fractures, at T9 and T12 levels. She had established osteoporosis.
A long acting somatostatin analogue (SSA), octeotride, was offered to her and escalated up to 30 mg IM/month. In addition, risedronate 35 mg/week and ergocalcipherol 2400 IU/week were initiated while hydrocortisone was reduced to 20 mg/day, and thyroid hormone switched to a fixe combination of T3(10 ug/d) and L T4 ( 90 ug/d). TSH remained elevated (although less fluctuating) [(Table 1, period C), (max v: of 17 uU/ml)] [and Table 2, C), by this approach, without significant changes in tumor size. One year later, radiotherapy of the pituitary was instituted, receiving 50 Gy after threedimentional virtual planning. Octeotride was maintained. During the next 2 years (Table 1, period D) TSH was slightly reduced, with a stable mean T4 (Table 2, D). Three and a half years after radiotherapy plus SSA, the mean TSH value was 7.93 uU/ml, l (Table 1, period E) & (Table 2, E]. The MRIs performed one year and 3.5 years after radiotherapy was given (Figure 1: C&D) showed a progressive decrease in the adenohypophyseal volume, accompanied by an increase in the width between the upper edge of the gland and the chiasm, so at the end of the 8 years of follow up, the height of the pituitary was 1 cm.
Overall, a sustained tendency towards lower TSH levels was obtained by the successive and additive treatments during an almost 8 years of follow up.
A rapid normalization of alkaline phosphatase was noted and bone mineral density (BMD) improved markedly at LS and FN (TS: - 3.3, at both sites), by 2 years after the implemented treatment.
The overall clinical evolution was good, the patient being asymptomatic, with a normalization of body weight and BP and a very good quality of life.
Case 2
A 25 years old man had had recent onset frontal headaches, accompanied by occasional diarrhea, heat intolerance, axilar discomfort, and fatigue. An MRI of the brain detected a pituitary macroadenoma with suprasellar extension and posterior displacement of the optic chiasm (Figure 2, A.)
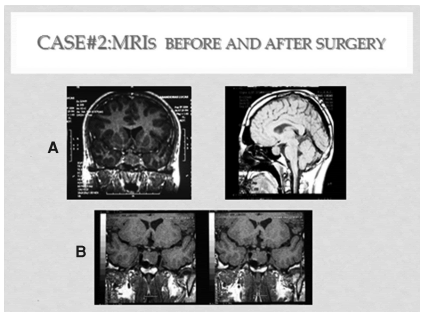
Figure 2. MRIs case #2. A: upper panel: before surgery. B: after TSSPA
Computarized visual field assessment was normal. He was referred for endocrinological evaluation, prior to eventual surgery. At physical examination, he was overweight (BMI: 29,7 kg/m2), heart rate was 120 beats/min, regular, and blood pressure 140/80 mmHg. Neither goiter nor ocular or skin abnormalities were evident, but bilateral tender gynecomastia was noticed.
Initial laboratory values were as follows: Free T4: 2.48 ng/dl(nv: 0.93-1.7); total T3: 268 (80-200) ng/ml; and TSH: 6.19 uU/ml (nv: 0.27-4.2 uU/7ml). Prolactin: 43.2 ng/ml (2-15), LH 1.5 mU/ml (nv: 2-9 mU/ml), Total To: 0.35 ng/ml (nv: 2.8-9) and Alkaline phospahtase (AP): 383 UI (nv: up to 280 IU). Basal and (ACTH) stimulated cortisol, ACTH, GH and FSH were within normal values.
Ten months earlier laboratory analysis had been performed, showing an increased TSH (5.18 uU/ml; nv: 0.27-4.2). Alkaline phoshatase was also high. However, no further tests had been ordered at that time by the interviening physician.
Since measurement of alpha subunit was not available, a TRH-TSH test was done. Basal TSH was 6.75 uU/ml and stimulated: 6.86. A diagnosis of TSH secreting pituitary adenoma was made, without concomitant secretion of GH, ACTH or gonadotrophins, accompanied with hypogonadotrophic hypogonadism and hyperprolactinemia, either secondary to stalk distortion or cosecretion by the tumor.
Prior to transsphenoidal pituitary microsurgery, the SSA octeotride was prescribed at an initial dose of 20 mg IM, followed by a second 30 mg dose, a month later. Clinical resolution of the thyrotoxicosis was obtained, with FT4 of 1.13 ng/dl, total T3 of 127 ng/ml, and TSH of 1.39 uU/ml.
An uneventual transsphenoidal surgical procedure was done, partially removing fragments of a pituitary chromophobe adenoma, with negative immnunostainning for TSH, FSH, LH, GH, ACTH and PRL. Ki 67 was < 1 %. A month later TSH was 0.87 uU/ml, PRL 22 ng/ml, ACTH stimulated cortisol was also normal, as well as T4 and T3. Cabergoline was initiated at 0.25 mg per week, with normalization of serum PRL and amelioration of gynecomastia. SSA therapy was not reinstituted.
Post surgery MRI showed a decompression of the chiasm along with central reduction of the adenoma, but significant tumor remnants with lateral contact with the cavernous sinuses, were also apparent (Figure 2,B).
Bone mineral density revealed osteopenia at femoral neck (TS: -1,9) and lumbar spine (TS:-1,4). Testosterone replacement normalized alkaline phosphatase and gynecomastia was resolved.
Mean TSH during the first year post surgery was 1.77 uU/ml (n: 4) (Table 3, B) and FT4 was 1.54 ng/dl (n:4), (Table 4, B) with 2 values slightly above the upper limit of normality. MRI did not change further.
TABLE 3. Case #2. Mean TSH trend over time. A: Before treatment. B: After surgery. C: post radiotherapy. D: SSA add on Tx. E: without medical tratment. ANOVA: F: 21.75; p < 0.0000).(post -hoc test: p < 0.03)
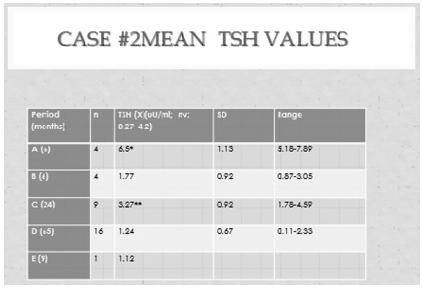
A decision was made, taking into account patient preferences, to perform pituitary radiotherapy, preceded by three-dimentional conformational planning. A total dose of 43 Gy was delivered, one and half years after surgery. During the following two years mean TSH was 3.1 uU/ml (n: 9,) (Table 3,C) and FT4 1.68 ng/ml (n: 7), with 4 out of 7 values above the upper limit of normality (1.7 ng/dl.) (Table 4,C). Basal and stimulated cortisol values were still normal and PRL was 1.1 ng/ml under cabergoline treatment.
Two years after radiotherapy, MRI depicted a superior and central cystic area with significant reduction in overall tumor volume. This was accentuated with time, showing a partial secondary empty sella appearance. At that time, and as a consequence of a trend towards central hyperthyroidism, the SSA was reinstituted (30 mg every 45 days). TSH was normalized: 1.15 uU/ml (n:4), but subclinical central hypothyroidism resulted. The octeotride dose was reduced progressively to 20 mg every 60 days. Although TSH remained normal, central hypothyroidism persisted, so levothyroxine was added with normalization of LT4, Finally, both drugs were interrupted. The duration of SSA treatment lasted 5 years and 5 months, during which mean TSH was: 1.24 uU/ml (Table 3,D), and FT4 was 0.98 ng/dl (Table 4,D). Nine months after treatment arrestment, TSH was 1.12 uU7ml and FT4 1.10 ng/dl. (Table 4,E). MRIs of the pituitary showed a progressive increase in the central cystic area of the adenohypophysis, so that at the 8 th year of follow up, a partial empty sella turcica developed, thus revealing an important reduction in the overall volume of the adenoma, without appreciable long term sequelae of radiotherapy. (Figure 3).
TABLE 4. Case #2. Free T4 trendover time. Anova F: 8.94,p<0.0001
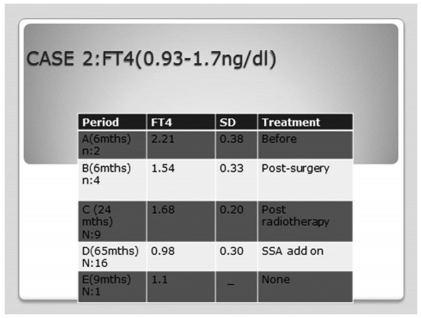
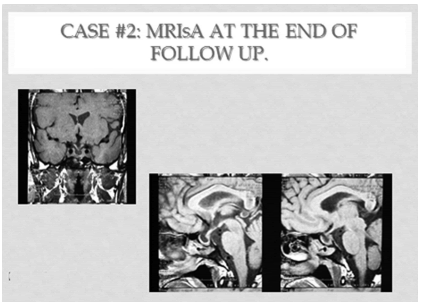
Figure 3: MRIs case #2: at the end of the observation period: 9.1 ys.
During time, laparoscopic cholecystectomy was performed, secondary adrenal failure appeared, IGF 1 still remained normal and femoral neck and lumbar spine BMD were normalized (TS: -0.6 and 0.2, respectively).
DISCUSSION
Thyrotropinomas are frecuently located centrally within the adenohypophyisis. They are usually sporadic and fibrous, probably(4) as a consequence of basic fibroblast growth factor( FGF) expression. The pathophysiology of TSHomas is unknown; however overexpression of Pit-1 factor(5), perhaps in combination with mutations of(6), may contribute to their development. A very recent series(7) of 5 patients, was published in Argentina. Most of them were followed for less than five years, with the exception of 1 subject with an observation period of 8 years. Unfortunately, the patient with the worse prognosis was lost from follow up soon after diagnosis. Nonetheless, 3 patients, with tumor´s diameter ≤ 3.0 cm were cured. No subject had been previously treated with either thyroidectomy or I131.
Thus we have presented two cases followed for 8 and 9 years, respectively, aming to contribute to the regional experience with these rare pituitary tumors.
Our first case is representative of those patients in whom a misdiagnosis of primary hyperthyroidism (probably Graves´ disease) resulted in radioiodine treatment followed by post dose hypothyroidism, two years before surgery. Her hyperthyroidism was moderate to severe and evidence of ACTH deficiency was already evident previous to TSSPA, attributable to the mass effect of a large macroadenoma with supraselar extension. In the Belgium-French series published by Socin et al(8), 13 out of 43 patients (30 %) had received a treatment targeted to the thyroid before pituitary surgery. Patients previously treated with radioiodine or thyroidectomy, are detected with greater delay from de initial symptoms of hyperthyroidism(2,9), present more frequently macroadenomas with compression signs, higher TSH levels, and experience a worse prognosis(8), most probably as a consequence of insufficient negative feedback of thyroid hormones with de tumor(6) allowing it to grow further during longer periods of time ;however, this conclusion(8), is not proven. Our first case suggests that this might be correct, as it happens in Nelson´s syndrome. Socin8 et al did not find greater tumor volumes in patients that had been previously treated with thyroidectomy or I131. However, adenomas´ diameter(8) was higher in patients treated with TT or RAI than in those that received methimazole. After Drucker Davis et al(9), severity of hyperthyroidism, duration of symptoms and tumor size and invasiveness were the main prognostic factors.
The suspicion of a TSHoma is likely(9) in a patient with thyrotoxicosis, elevated free T4 and T3, without biochemical confounders, in whom sensitive TSH is slightly elevated or even inappropiately normal, distinguishing him from primary hyperthyroidism. TSH(2,6,7,9) is frequently between 4 and 14 µU/ml. The biochemical gold standard for diagnosis(8) is still the measurement of α subunit in the appropriate context, which is overexpressed in TSHomas, resulting in an absolute increase with an elevated molar ratio ofα subunit/TSH. The most important distinction is between tumoral and non tumoral inappropriate TSH secretion(10) namely thyroid hormone resistance syndromes (THR). We could not measureα subunit in neither patient, but in case #2 we were able to demonstrate the lack of response to TRH, that can differentiate TSHomas from the normal response seen in THR(10). In 3 out of 5 patients reported in Argentina(7) TRH-TSH test was also flat. Our two patients had either osteoporosis (case 1) or osteopenia (case 2). In addition, both had high alkaline phosphatase.The two cases were hypogonadal, as the female patient was menopaused, and the male one had secondary hypogonadism from the diagnosis. It should be stressed that the combination of thyrotoxicosis with hypogonadism is particularly dangerous for the skeleton. We thus suggest that bone status and/or remodeling needs to be evaluated in patients with TSHomas, especially when there is a compromise in the gonadal axis.
As with other functional pituitary adenoma(1,8,9,11), with the exception of prolactinomas, surgery is the treatment of choice. In thyrotropinomas(8,11,12) its effectiveness is inversely related with tumor size, success being greater in microadenomas. The overall success rate seems to be around 60 %. It is generally accepted that cure should include a persistent normalization of the thyroid axis dynamics, as well as the absence of residual tumor demonstrated by MRI .Our two patients had residual disease after surgery, so they were not cured by the procedure. Nonetheless, a decompression of the optic chiasm was achieved, along with a temporal but significant reduction in TSH. In accordance with patients' preferences, we did not re-operate them. Radiotherapy, which is a second line treatment, was offered instead. Prevention of tumor growth and its consequences was the therapeutic priority in both. In case 2, although he could have been further treated by medical means, the patient' s fear of tumor expansion contributed to our decision of irradiating the adenoma relatively early in the curse of the disease. This procedure was well tolerated, without side effects and, in the long run, helped both of them to control their condition. In addition, a reduction in tumor volume was evident in both cases. Moreover, although not cured, our 2nd patient finally achieved a remission of his hyperthyroidism and tumor growth, the latter most probably as a consequence of radiotherapy without ancillary medical treatment. And our first patient remained euthyroid, albeit substituted, as a result of radiotherapy plus medical treatment, with a clear reduction of her tumor volume. TSHomas usually express somatostatin receptors. Accordingly(8,13), somatostatin analogs have been used for the treatment of TS Homas. As we did with our second patient, they are primarily indicated for the preoperative treatment of hyperthyroidism. Thyonamides are only a second option for the preoperative medical treatment of hyperthyroidism. SSA can be employed as second, or third line treatment alternatives. They were assayed in our first patient, when first seen, after faillure of surgery to remove the adenoma and uncontrolled TSH values. Octeotride alone was unable to reduce tumor size, but there was an amelioration of the visual field. TSH was slightly diminished, although not normalized. Consequently radiotherapy was implemented. The combination of both permitted a better control of hypersecretion of TSH, but the shrinkage of the tumor seems to be primarily a result of radiotherapy. Our male patient responded very well to octreotide for the treatment of hyperthyroidism. The drug could have been employed as a second line option, after the faillure of surgery as a curative treatment. As aforementioned, patient´s preferences prompted us to irradiate the macroadenoma instead. It was only after the reappearance of subclinical central hyperthyroidism that octeotride was reinstituted, with an excellent although excessive response that allowed us to stopped it. Development of central hypothyroidism(14) in a girl with a thyrotropinoma treated with a SSA alone, has already been reported.
CONCLUSIONS
We repport the long term evolution of two patients with thyrotropinomas after failure of transsphenoidal pituitary surgery to resolve their condition. Our first case is representative of a linical situation that should no longer be seen: a misdiagnosis of primary hyperthyroidism in the context of inappriopiate secretion of TSH. Her disease could be managed with a combination of radiotherapy plus octreotide. Our second subject was treated from diagnosis with an effective combination of a SSA, prior to surgery, plus the subsecuent addition of radiotherapy.
We thank Dr Alejo Florin Christensen for his kind revision of the manuscript.
1. Oruckaptan HH, Sanmevsin O, Ozcan OE, Ozgen T. Gesundheit N, Petrick PA, Nissim M, Dahlberg PA, Doppman JL, Emerson CH, Braverman LE, Oldfield EH, Weintraub BD Pituitary adenomas: results of 684 surgically treated patients and review of literature. Surg Neurol, 53:211-219, 2000 [ Links ]
2. Gesundheit N, Petrick PA, Nissim M, Dahlberg PA, Doppman JL, Emerson CH, Braverman LE, Oldfield EH, Weintraub BD. Thyrotropinsecreting pituitary adenomas: clinical and biochemical heterogeneity. Case reports and follow-up of nine patients. Ann Intern Med. Nov 15,111(10):827-3, 1989 [ Links ]
3. Kienitz T, Quinkler M, Strasburger CJ, Ventz M. Long-term management in five cases of TSH-secreting pituitary adenomas: a single center study and review of the literature. Eur J Endocrinol, Jul157(1):39-46, 2007 [ Links ]
4. Ezzat S, Horvath E, Kovacs K, Smyth HS, Singer W, Asa. Basic fibroblast growth factor expression by two prolactin and thyrotropin-producing pituitary adenomas. Endocr Pathol,6:125-3,1995 [ Links ]
5. Poncin J, Stevenaert A, Beckers. Somatic MEN-1gene mutation does not contribute significantly to sporadic pituitary tumorigenesis. Eur J Endocrinol,140:576-576, 1999 [ Links ]
6. Ando S, Sarlis NJ, Oldfield EH, Yen PM Somatic mutation of TRbeta can cause a defect in negative regulation of TSH in a TSH-secreting pituitary tumor J Clin Endocrinol Metab, 86:5572-6, 2001 [ Links ]
7. Pignatta A,Danilowicz K, Manavela M,Loto M, Gonzalez Abbati S, Toloza C,Fazio L. Adenoma hipofisario productor de TSH (tirotropinoma): presentación de cinco casos. Rev Argent Endocrinol Metab, 51: 141-150, 2014 [ Links ]
8. Socin HV, Chanson P, Delemer B, Tabarin A, Rohmer V, Mockel J, Stevenaert A, Beckers A. The changing spectrum of TSH-secreting pituitary adenomas: diagnosis and management in 43 patients. Eur J Endocrinol, 148: 433-42, 2003 [ Links ]
9. Drucker Davis F,Olfield E,Skarulis M,Doppmann JL, WeintraUB BD.Thyrotropin-secreting pituitary tumors: diagnostic criteria, thyroid hormone sensitivity, and treatment outcome in 25 patients followed at the National Institutes of Health. J Clin Endocrinol Metabol, 84(2):476-486, 1999 [ Links ]
10. Mahler GA, Bergoglio LM. Inappropiate Secretion of TSH syndrome. Rev Argent Endocrinol Metab ;5: 253-264, 2013 [ Links ]
11. Beck-PeccozP, Persani L, Thyrotropinomas, Endocrinol Met Clin N Am 37: 773-779, 2008 [ Links ]
12. Losa M, Giovanelli M, Persani L, Mortini P, Faglia G, Beck-Peccoz P. Criteria of crure and follow up of central hyperthyroidism due to thyrotropin secreting pituitary adenomas. J Clin Endocrin Metabol; 81: 3084-90, 1996 [ Links ]
13. KhunJM, Arlot S, Lfebvre H, Caron P, Corlet-Rudelli C,Archabaud F. Evaluation of the treatment of thyrotropin- secreting pituitary adenomas with a slow release formulation of somatostatin lanreotide.J Clin Endocr Metabol, 85:1487-91, 2000 [ Links ]
14. Rabbiosi S, Peroni E, Tronconi GM, Chiumello G, Losa M, Weber G. Thyrotropin -secreting pituitary macroadenoma in a 13 years old girl: successfull first line treatment with a somatostatin analog. Thyroid, Oct22(10):1076-1079, 2012 [ Links ]














