Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista agronómica del noroeste argentino
versión impresa ISSN 0080-2069versión On-line ISSN 2314-369X
Rev. agron. noroeste arg. vol.39 no.2 San Miguel de Tucumán dic. 2019
SCIENTIFIC ARTICLE
Oogenesis and ovarian histology of the South American lizard Salvator merianae
Oogénesis e histología ovárica del lagarto sudamericano Salvator merianae
M.V. García-Valdez*; O. L. Sánchez-Loria; S. N. Chamut
Facultad de Agronomía y Zootecnia, Universidad Nacional de Tucumán. Avda. Kirchner 1900, (4000). San Miguel de Tucumán, Tucumán, Argentina. *Orcid.org/0000-0002-9596-6830. E-mail: vgarciavaldez@gmail.com
Abstract
The morphology of oogenesis in lizards has been described in few species. In this paper the ovarian histology of females of the S. merianae oviparous South American lizard is studied throughout the annual cycle. For this, ovaries were processed at different stages of the follicular cycle, using conventional histological technique. Two germinal beds were identified per ovary, from where the previtellogenic follicles emerge, present throughout the entire annual cycle. At this stage, the oocyte increases in size and the follicular epithelium change from a single layer of flat cells to a polymorphic and stratified layer, with three cell types: small, intermediate, and pyriform cells. At the beginning of the reproductive stage, vitellogenic follicles were found, showing a marked decrease in follicular epithelium, which was reduced to a single layer of flat cells. The incorporation of vitello resulted in a dramatic increase of oocyte size, reaching diameters close to 2,5 cm. After ovulation, the corpus hemorrhagicum showed the permanence of the thecal envelopes and follicular cells and the invasion of blood cells. The finding of corpus luteum with cells of steroidogenic characteristics 41 days after oviposition, leads us to propose that in this species the luteal bodies would present a functional persistence superior to that usually described for oviparous vertebrates.
Keywords: Reptile; Ovary; Follicular development; Corpus luteum; Atresia.
Resumen
La morfología de la oogénesis en lagartos ha sido descripta en pocas especies. En el presente trabajo se estudia la histología ovárica de hembras del lagarto sudamericano ovíparo Salvator merianae, a lo largo del ciclo anual. Para ello se procesaron ovarios en diferentes estadios del ciclo folicular, mediante técnica histológica convencional. Se identificaron dos nidos germinales por ovario, desde donde emergen los folículos previtelogénicos, presentes a lo largo de todo el ciclo anual. En esta etapa, el oocito aumenta de tamaño y el epitelio folicular pasa de una capa simple de células planas a polimórfica y estratificada con tres tipos celulares: células pequeñas, intermedias y piriformes. Al momento del inicio de la etapa reproductiva, se encontraron folículos vitelogénicos, los que presentaron una marcada disminución del epitelio folicular, el que quedó reducido a una única capa de células planas. La incorporación de vitelo resultó en un drástico incremento de tamaño del oocito, hasta alcanzar diámetros cercanos a los 3 cm. Luego de la ovulación, los cuerpos hemorrágicos mostraron la permanencia de las envolturas tecales y de las células foliculares y la invasión de células sanguíneas. El hallazgo de cuerpos lúteos con células de características esteroidogénicas a los 41 días de la oviposición, nos lleva a proponer que en esta especie los cuerpos lúteos presentarían una persistencia funcional superior a lo usualmente descripto para los vertebrados ovíparos.
Palabras clave: Reptil; Ovario; Desarrollo folicular; Cuerpo lúteo; Atresia.
Received 11/04/2019; Accepted 12/03/2019.
The authors declare to have no conflict of interests.
Introduction
Understanding the structure of the organs of the animal reproductive system for commercial production, allows the development of productive strategies. However, the knowledge of the dynamics of the processes of oogenesis in lizards (Lacertilia, suborder of reptiles), from the development of germ cells to ovulation, is far from complete. Despite their great diversity (more than 5000 species of lizards), most research on this topic focuses on a few species, which can be considered “model” species (Rheubert et al., 2014; Aldokhi, et al., 2019). Nevertheless, the description of oogenesis in various reptile species reveals significant morphological and physiological variations (Uribe Arazábal et al., 2010), which justifies the progress of studies in this area.
The ovary of sauropsids reptiles changes in shape and size, according to the stage of the reproductive cycle, and the number and sequence of the stage of follicular development (Lozano et al., 2014). In reptiles, as in most vertebrates, the ovaries are paired organs that contain gametes, follicles and luteal bodies embedded in a stroma of connective tissue, all surrounded by a thin epithelium (Jones, 2011).
Female gametes (oogonias) are found in one or more regions of the ovary, the germinal beds (Jones et al., 1982; Shine and Radder, 2007). These constitute sites for the multiplication of oogonies and the formation of the primordial follicles, which will emerge after the germinal beds and will be covered by the granulosa follicular cells (Ibrahim and Wilson, 1989), with which they will establish an active transfer of metabolites (Uribe Arazábal et al., 2010).
As in all vertebrates, the ovarian follicle in reptiles is composed of a central oocyte surrounded by an acellular shell, the zona pellucida, and a layer of epithelial cells called granulosa cells. Surrounding the ovarian follicle, are the internal and external theca, formed by connective tissue, blood vessels, and steroidogenic secretory cells.
The granulosa shows morphological differences between the different reptile taxa (Lozano et al., 2014; Aldokhi et al., 2019). For example, in turtles (Uribe Arazábal et al., 2010) and crocodiles (Uribe and Guillette, 2000; Calderón et al., 2004; Machado-Santos et al., 2015), granulosa consists of a single layer of cells during the whole follicular development. In lizards and snakes, the granulosa structure depends on the stage of folliculogenesis (Andreuccetti, 1992; Uribe et al., 1996; Da Silva et al., 2018). Initially, in the primary follicle, the granulosa is formed by a simple layer of small, flat or cubic cells. As previtellogenic growth progresses, some cells differentiate into intermediate cells and these into pyriform, transforming the epithelium into polymorphic and stratified. The structure and development of the follicular cells of the Squamata reptiles, with the formation of the pyriform cells, constitutes a remarkable characteristic, unique among vertebrates (Uribe Arazábal et al., 2010). These cells connect to the oocyte through intercellular bridges, where they will transfer materials that will contribute to the formation of their ooplasm (Andreuccetti et al., 1978; Motta et al., 1995; Andreuccetti et al., 2001; Aldokhi et al., 2019). At the end of the previtellogenic phase, an active sequestration of the yolk in the ooplasm begins and the intermediate and pyriform cells go into regression, leaving the granulose again composed of a simple cell layer (Jones, 2011).
In all reptiles, both oviparous and viviparous, prior to ovulation, follicular cells accumulate lipids, proliferate, and luteinize, forming the corpus luteum, which act as an endocrine secretory structure with steroidogenic capacity (Rothchild, 2003; Uribe Arazábal et al., 2010). The life of the corpus luteum is species-specific, and varies considerably among reptilian species, persisting until oviposition in oviparous squamates (Fox and Guillette, 1987).
In the ovary of reptiles, as in other vertebrate groups, not all ovarian follicles that begin their development reach maturity, some of them going into degeneration and removal processes. This follicular atresia occurs at all stages of follicular development, and it has been described in both, oviparous and viviparous reptiles (Uribe Arazábal et al., 2010)
The South American lizards of the Salvator genus (Squamata, Sauria, Teiidae), are interesting models of study from different points of view. In past decades it became one of the most commercially exploited reptiles in the world, due to the high price of its leather in international markets (Mieres and Fitzgerald, 2006), which boosted the development of sustainable management programs and breeding systems in captivity (Fitzgerald et al., 1991). In recent years, these lizards once again attracted the attention of the world scientific community, as they were reported as reptiles capable of generating body heat, raising their temperature up to 10 °C from external temperature during the reproductive period (Tattersall et al., 2016), which happened to be considered a possible model for the study of the evolution of endothermia. However, little is known about the many aspects related to the reproductive biology of these lizard.
The females of the S. merianae lizard exhibit, in our latitudes, a marked seasonality in their behavior, concentrating their reproductive functions in the spring and early summer, periods where remarkable ovarian and oviductal modifications occur (Fitzgerald et al., 1993; Manes et al., 2007; Naretto et al., 2015). The main reproductive events (courtship, mating, oviposition, and incubation) are thus limited to spring and early summer (Donadio and Gallardo 1984; Mercolli and Yanosky 1989; Noriega et al., 1996).
The reproductive rate of S. merianae is high, finding nests with 20 to 50 eggs of flexible shells (Fitzgerald et al., 1994; Porini, 2006 and personal observations), which the female incubates for approximately 70 days, displaying a series of complex maternal behaviors (Noriega et al., 1996; Manes et al., 2003). Although S. merianae’s female has annual oviposition capacity, there are usually years in which they do not reproduce, developing massive follicular atresia, both in stages of previtellogenesis and in advanced vitellogenesis (García-Valdez et al., 2011, 2016).
The present study describes the ovarian histology of the females of the S. merianae lizard, throughout the annual reproductive cycle.
Materials and methods
Morphological and histological studies were performed on five adult females of S. merianae from the experimental hatchery of the Facultad de Agronomía y Zootecnia of Universidad Nacional de Tucumán, Argentina (26º51’S and 65º17’W). The snout-vent length of the females evaluated was greater than 35 cm, and the weight exceeded 2.5 kg.
The studies were carried out at different stages of the annual reproductive cycle: 1-exit from hibernation (September), 2- period of sexual interactions, courtship (October), 3- pre-oviposition (when the eggs were in oviductal transit, November), 4 - incubation (41 days after laying the eggs, December) and 5- at the end of the reproductive cycle, in a female who performed a massive follicular atresia in the vitellogenesis stage, in the month of December. To determine the different stages of the cycle, behavioral observations and ultrasonographic examinations were performed, using a Mindray DP-6600 Vet ultrasound, with a linear transducer model 75L38EA.
To obtain the samples, the animals were anesthetized with diazepam (2.5 mg/kg) and intramuscular ketamine (25 mg/kg) and sacrificed with intracardiac injections of sodium pentobarbital (100 mg/kg) as described by Baer (2006) and HSUS (2013). In each animal both ovaries were removed, weighed and measured. For histological studies, the ovarian follicles were fixed in Dubosq-Brazil (Langeron, 1949), or in 4% formaldehyde solution. The samples were dehydrated in ethanol, cleared in xylene, embedded in paraffin and cut into sections between 5 and 7 μm thick. Sections were colored with hematoxylin-eosin, hematoxylin-eosin-floxin, Gallego trichrome and tetrachromic VOF-Type III G.S. (Sarasquete and Gutiérrez, 2005). The micrographs were taken with an AxioCam ERC5S digital camera and processed with the Microscope Imaging software ZEISS ZEN 2012-Blue edition (Germany).
All experiments performed were approved by the ethics committee of the Research Council of the National University of Tucumán (CIUNT).
Results
The ovaries of S. merianae were observed, after hibernation, as compact and elongated structures, formed by a thin wall, which involved the somatic and germ tissue structures. Both, the right and left ovaries, showed similar weights and follicles throughout the year, showing synchronous behavior during the reproductive cycle (present results and personal observations).
Among the tissue elements of the ovary of S. merianae, we found: the germinal epithelium, formed by follicles in different stages of development, depending on the moment of the female’s reproductive cycle: previtelogenic, vitellogenic follicles, corpus luteum and atretic follicles. All of them were immersed in a small amount of stroma of vascularized connective tissue, which contained fibroblasts, collagen fibers, and smooth muscle.
The ovaries showed notable differences in size and weight throughout the different stages of the annual cycle studied. At the exit of hibernation, the ovaries were observed on the ultrasound image, as small anechogenic structures, with follicles of diameters smaller than 5 mm. During the reproductive stage, the ovaries drastically increased their sizes, and the follicles reaching close to 25 mm in diameter.
The main components present in the ovary of S. merianae are described below.
Germinal beds
S. merianae presented two germinal beds for each ovary, located on both sides of the hilum, in the ovarian medulla (Figure 1A). The ovarian stroma was composed of interstitial tissue, with fibroblasts, collagen fibers, blood vessels, lymphatics, and nerves. Oogonies and primordial follicles (oocytes surrounded by a simple layer of flat cells) were found immersed in it. The oogonias were observed as spherical or oval cells with a round nucleus and homogeneous cytoplasm (Figure 1B and 1C).
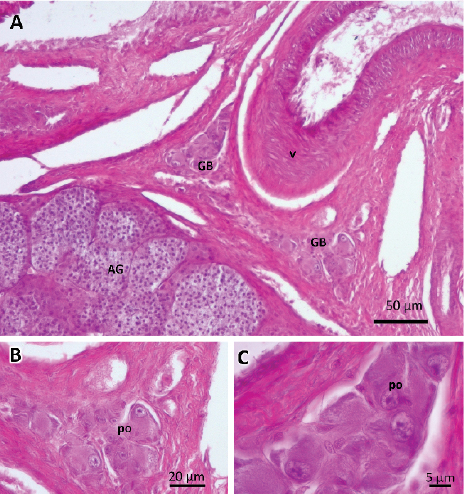
Figure 1. Photographs of germinal beds. A) Germinal beds (GB) located on both sides of the hilum. Large-sized blood vessel (v)
near the adrenal gland (AG). B and C) Detail of germinal beds with primary oocytes (po). Stained with hematoxylin-eosin-floxin.
Previtellogenic follicles
In addition to the oogonies and the primordial follicles, we found previtellogenic follicles at all stages of the annual cycle, which could be seen emerging from the germinal beds (Figure 2A). These were formed by an oocyte surrounded by epithelial cells called the granulosa layer. On the outside, and separated by a basement membrane, we find the theca.
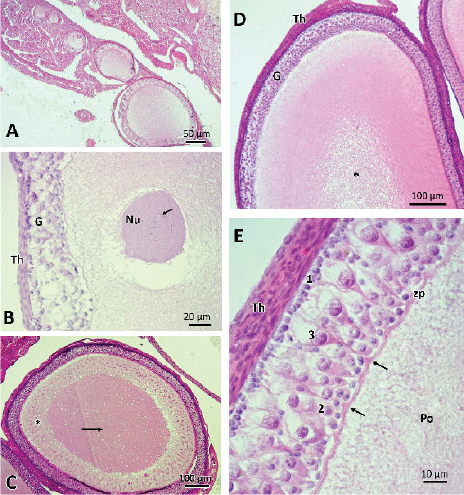
Figure 2. Photographs of previtellogenic follicles. A) Group of non-vitellogenic follicles. It is observed that the follicles present, initially, a single layer of surrounding granulosa cells that stratify as they progress in their growth. B) Detail of follicle with nucleus (Nu) with lampbrush chromosomes (→), and stratified polymorphic granulose (G) and theca layer (Th). C) Follicle with homogenous ooplasm oocyte in its central part (→) and with fibrillar material in the periphery (*). D) Later stage of previtellogenic growth where the central area of the ooplasm is vacuolized (*). E) Detail of the granulosa, with the three cell types: 1: small cells; 2: intermediate cells; 3: pyriform cells. Separating the oocyte from the granulose, a thin zona pellucida (zp) is observed with folds that mark the junction points with the pyriform cells (→). Po: previtelogenic oocyte. Stained with hematoxylin-eosin.
As previtellogenic follicular growth progressed, changes were observed in both the oocyte and granulosa cells. The oocytes increased their cytoplasmic volume and numerous nucleoli and lampbrush chromosomes were observed in the nucleus (Figure 2B). The ooplasm presented homogeneous material in the central area and material in fibrillar organization towards the periphery of the oocyte (Figure 2C). As follicular growth progressed, the ooplasm was vacuolized in its central part (Figure 2D).
The granulosa cells were initially organized in a simple and homogeneous layer of flat to cubic cells (Figure 2A). As follicular development progressed, the granular layer became polymorphic and stratified, distinguishing three types of cells: small, intermediate and pyriform (Figure 2E). The small cells were the most abundant, being in contact with the basal lamina. These cells were spherical, with a big, highly basophilic central nucleus, presenting one or two nucleoli. The intermediate cells, oval and larger than the previous ones, were located between the other two cell types. The pyriform cells, named for their pear shape, were the largest granulosa integrating cells. These presented a bulky nucleus, with heterochromatin patches and one or two nucleoli. The pyriform cells oriented their base towards the basement membrane of the epithelium, and its narrow end in contact with the oocyte membrane, which could be observed in some cuts where the plane was favorable. It was observed that the pyriform cells establish close points of contact with the oocyte pellucid zone, showing similarity between the tintorial affinities of the apical cytoplasm of the pyriform cells and the cytoplasm of the peripheral zone of the oocyte (Figure 2E) .
The zona pellucida, composed of a single layer in early previtellogenesis, changes to a double structure as previtellogenic growth progresses. In this stage, a striated layer is distinguished, in contact with the ooplasm (zona radiata) and a more external, homogeneous one, in contact with the granulose, the hyaline band (Figures 3A, B and C).
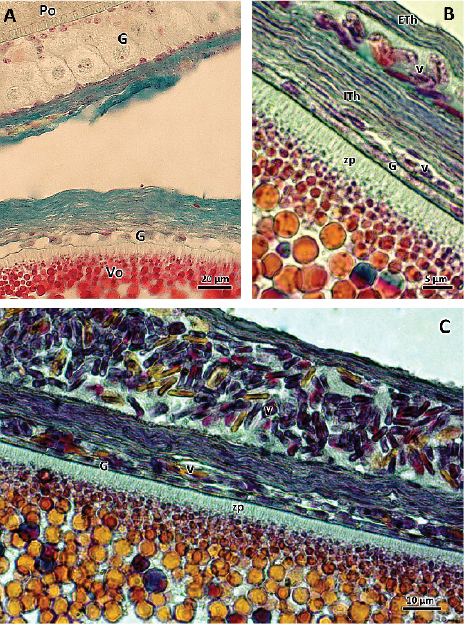
Figure 3. Photographs of vitellogenic follicles. A) Follicles with previtellogenic oocyte (Po) and vitellogenic (Vo). Changes in the granulosa layer (G) between one stage and the other are observed, which passes from stratified polymorphic (Po) to a flat cell monolayer (Vo). Stained with Gallego. B and C) Vitellogenic oocytes with abundant granules of vitello inside with thickening of the zona pellucida (zp), in which the striation of the zona radiata and the yolk granules with different tintorial affinities are easily distinguished. Internal theca (ITh) has blood vessels (v). External theca (ETh). On the outside, a blood vessel with erythrocytes is observed. G: granulosa layer. Stained with VOF tetrachromic.
Surrounding the follicles, there is a covering of connective tissue, which forms the theca (Figure 2E and 3B). It was possible to distinguish an internal vascularized theca and an external fibrous theca, with a large number of fibroblasts, collagen fibers and blood vessels. Outside the theca, larger blood vessels were observed.
Vitellogenic follicles
During the reproductive season, we found groups of follicles from both ovaries that initiated vitellogenic growth. At this stage the follicle showed remarkable morphological changes, both at the oocyte level and in the peripheral structures (Figure 3A).
At the cytoplasmic level, an accumulation of yolk vesicles was observed, which appeared, in general, as intensely acidophilic platelets, small and numerous in the periphery, and larger towards the central area of the oocyte. Among them granules with different tintorial affinity were observed (Figures 3B and 3C).
The granulosa layer was thinned until it was reduced to a single homogeneous layer of flat cells (Figure 3A). As vitellogenesis progressed, the zona pellucida thickened. Blood vessels surrounding the theca increased their caliber (Figure 3B and 3C). These follicles grew to reach diameters close to 2.5 cm, at which time they were ready to be ovulated.
Corpus hemorrhagicum and corpus luteum
After ovulation, the follicular envelopes remained in the ovary, forming first the corpus hemorrhagicum (Figure 4A and 4B) and then the corpus luteum (Figure 4C and 4D).
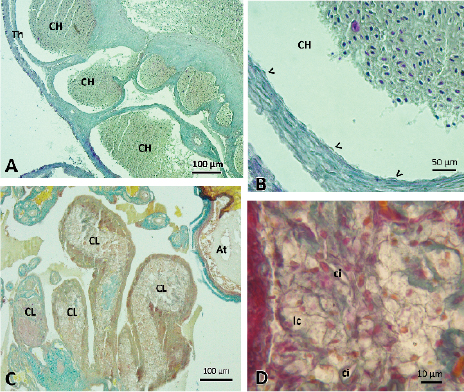
Figure 4. Photographs of corpus hemorrhagicum and corpus luteum. A and B) Corpus hemorrhagicum (CH) found in a female that presented eggs in oviductal transit. The cavities left by the oocytes that were ovulated are observed, those that are full of blood and limited by the theca (Th). The thin layer of granulosa cells (<) remains in the ovary. Stained with Gallego. C) Corpus luteum (CL) 41 days after oviposition. At: atretic follicle. Stained with Gallego. D) Detail of the cells found in the corpus luteum, lc: luteal cells, ci: cells with inclusion. Stained with VOF tetrachromic.
The study of the ovaries of a female who was with eggs in oviductal transit allowed us to observe the cavities left by the follicles, which they found full of blood, the corpus hemorrhagicum. In the ovary remained the thin layer of granulosa cells and theca, which were thickened, separating in some sections the inner and outer theca (Figure 4A and 4B).
The histological study of the ovary of a female who was incubating her eggs, showed the presence of structures with characteristics of corpus luteum in regression (Figure 4C and 4D). The luteal cavities, limited by the theca, presented hypertrophied cells with different tintorial affinities, differentiating light and dark cells. The clear cells presented vacuolated cytoplasms and small eccentric nuclei. Dark cells, irregularly distributed among light cells, showed one or two large oval cytoplasmic inclusions. Among the luteal cell mass we find connective tissue and blood vessels. In some structures the presence of scar connective tissue was observed.
Atretic follicles
The atretic follicles were found in all stages of follicular development studied (Figure 5). These were characterized by the disorganization of the structure of the follicular wall, which appeared folded, with disordered cells. Granulosa cells proliferated, invading the ooplasm (Figure 5A). The vitelline membrane was also observed folded and discontinuous, disappearing the striations of the zona radiata. The oocyte showed ripples in the periphery, losing its spherical shape and becoming irregular and with the vacuolated ooplasm (Figure 5B and 5C). In the case of the atretic vitellogenic oocytes, the vitello granules lost their individuality, forming an amorphous mass. The presence of vacuoles in the ooplasm and the invasion of blood vessels and macrophages was increased (Figures 5D, E and F).
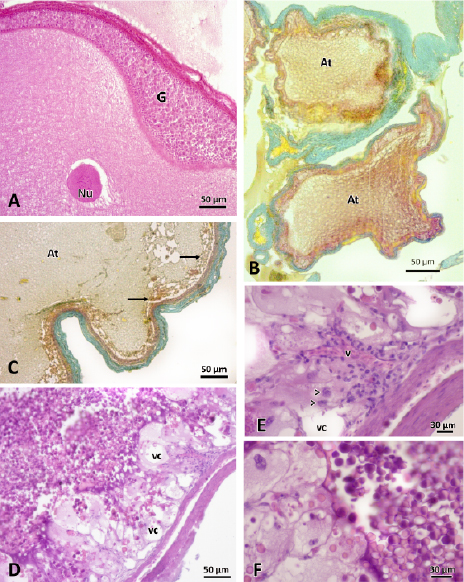
Figure 5. Photographs of atretic follicles A) Previtellogenic atretic follicles. The proliferation of cells granulosa layer (G) is observed invading the ooplasm. Nu: nucleus. Stained with hematoxylin-eosin. B) Two atretic previtellogenic follicles (At) with loss of spherical oocyte shape, and alterations at the granulose layer. Stained with Gallego. C) Atretic follicle (At) in initial vitellogenesis. Towards the periphery, granules of vitello in degradation are observed (→). Stained with Gallego. D, E and F) Vitellogenic atretic follicle. The presence of vacuoles (vc) and blood vessels (v) invading the ooplasm is observed. In the magnifications (E and F) the presence of macrophages (>) and granules of vitello in degradation are observed. Stained with hematoxylin-eosin.
Discussion
This work describes the structure of the ovaries of the females of the South American lizard S. merianae, an interesting species to be studied both from the productive point of view, due to the high quality of its skin, which are made into exotic leather accessories (Fitzgerald, 1994), as being considered a model species for the study of the evolution of endothermia (Farmer, 2016). Throughout the annual cycle, both ovaries showed remarkable morphological changes, which is consistent with that reported for squamata reptiles in temperate zones (Masson and Guillette, 1987). In the ovaries we find germ cells, follicles and corpus luteum immersed in a stroma of connective tissue.
Lizards can have one, two, or multiple germinal beds, this being a characteristic of the species (Guraya, 2013). In females of S. merianae, two germinal beds were observed per ovary, as was reported in a previous study (Manes et al., 2007), which constitute small regions that contain oogonias in division, naked oocytes and primordial follicles. The amount of germinal beds is usually related to instantaneous fertility (number of ovulations of both ovaries at the same time) and, consequently, to the size of the clutch (Jones et al., 1982). For their part, Arrieta et al. (2017) propose that fertility in lizards may not be conditioned by the amount of germinal beds, but by the mitotic activity of oogonies. This disparity of opinions is probably related to the low level of knowledge of the oogenesis process in numerous families of lizards (Rheubert et al., 2014), so it would be necessary to incorporate new species with studies of their ovarian morphophysiology to clarify this point.
Unlike mammals, where the number of oogonias is fixed during embryonic development, adult female reptiles have oogonies that show mitotic activity at the beginning of each reproductive cycle (El Mouden et al., 2001; Uribe Arazábal et al., 2010), those who later enter the meiotic prophase to form the oocytes. From these germinal beds, they will emerge surrounded by prefollicular epithelial cells, forming ovarian follicles. The present histological studies in S. merianae, allowed us to observe the oocytes emerging from the germinal beds, surrounded by a simple layer of granulosa cells, which was then stratified and complexed, as the follicular growth progressed.
During the previtellogenesis stage, the oocytes increased in size, probably as a result of an accumulation of organelles and macromolecules in the ovoplasm (e.g., enzymes, histones, tubulin, regulatory factors, mitochondria, ribosomes, and dormant mRNA), establishing the structural basis for the subsequent vitelogenesis (Rheubert et al., 2014; Uribe Arazábal et al., 2010). The finding of previtelgenic oocytes with numerous nucleoli and lampbrush chromosomes in S. merianae could be associated with the broad genetic expression of the diplotene phase of meiosis, in which the oocytes remain under arrest (Uribe Aranzábal et al., 2010).
In the initial stages, the follicles were observed surrounded by a simple layer of granulosa cells and enveloped by a thin thecal layer. With the advance of folliculogenesis, the granulose was stratified and it was possible to distinguish three types of cells: small, intermediate, and pyriform. This type of polymorphic epithelium has been described for other Squamata reptiles (Motta et al., 1995; Uribe Arazábal et al., 1996; Andreuccetti et al., 2001; Lozano et al., 2014) and presents unique characteristics among vertebrates.
Small follicular cells, adjacent to the basement membrane, would be very active in mitotic divisions and would originate the intermediate cells, which in turn would originate the pyriform cells (Uribe Arazábal et al., 2010; Aldokhi et al., 2019). These pyriform cells connect to the oocyte through intercellular bridges, where they will transfer RNA, mitochondria, ribosomes, vesicles, vacuoles containing mucopolysaccharides, nuclear constituents, and other materials (Andreuccetti et al., 1978; Motta et al., 1995; Andreuccetti et al., 2001), contributing to the formation of the oocyte ooplasm. In the present work, the active transfer of materials between the pyriform cells and the oocyte was revealed in the microscopic image, where a correspondence in the tintorial affinity between the cytoplasmic content of the follicular cells and the peripheric material of the oocytic ooplasm was observed.
Vitellogenic follicles showed remarkable morphological changes. The follicular epithelium change from stratified polymorphic to a flat cell monolayer. De Caro et al. (1998) propose that this change is due to the fact that pyriform cells undergo apoptosis, accompanying this event with an active transfer of organelles and macromolecules to the oocyte, via an intercellular bridge. This, together with the finding that mitochondria assigned to the oocyte maintain their functionality (Tammaro, 2007) would contribute to increasing the number of organelles available for the oocyte, and reinforces the idea of the “nurse” function of the pyriforms cells.
As in other sauropsid reptiles, the oocyte of S. merianae was observed surrounded by the zona pellucida, formed at the ovocyte-epithelium interface. This is composed of an amorphous gluteoproteic matrix: the hyaline band and the interdigitation of the microvilli of the oocyte, and follicular cells, the zona radiata (Uribe Arazábal et al., 1996; El Mouden, et al., 2001; Lozano et al., 2014). As the folliculogenesis progressed, the zona pellucida became thicker, particularly due to the growth of the zona radiata, which would indicate the development of abundant microvilli associated with the transport of substances from the follicular epithelium to the ooplasm.
At the cytoplasmic level, it was possible to observe the yolk deposit, associated with the telolecitic condition of S. merianae eggs. It is well established that the growth of vitellogenic oocytes occurs by accumulation of lipoprotein nutrients synthesized in the liver and released into the bloodstream (Romano et al., 2004). Once in the ovarian follicle, these nutrients (represented by vitelogenin and very low density lipoproteins) penetrate through the basement membrane, circulate between the granulosa cells, passing along the microvilli of the oocytes in the channels of the zona radiata, until reaching the oolema (Davail et al., 1998). Finally, they are incorporated into the oocyte by micropinocytosis, a mechanism of receptor-mediated endocytosis (Romano et al., 2004).
Once incorporated into the oocytes, vitelogenin and very low-density lipoproteins are cleaved into a characteristic set of polypeptides forming yolk platelets (De Stasio et al., 1999). Two types of yolk bodies can be distinguished within these: the fatty yolk and the proteid yolk globules ones, with different tintorial affinities (Guraya, 2013), which we probably see expressed in the images of the vitellogenic follicles of this work.
The finding of ovaries with vitellogenic follicles in females that have not yet completed sexual intercourse, leads us to propose that vitellogenesis in the females of S. merianae, is a process that begins independently of the copulation, and it does not require of it as a signal inductor, as proposed by Manes et al. (2007).
Maturing of the reptilian follicle shows great complexity in its vascular supply, which is limited to the thecal layers (Guraya, 2013). The finding of large blood vessels in the vitellogenic follicles would be clear indicators of the high contribution of metabolites that reach the oocyte of S. merianae during this stage of the cycle.
After ovulation, the thecal layers and granulosa cells remain in the ovary and the cavities resulting from the release of the oocytes are invaded by blood tissue, initially forming the corpus hemorrhagicum (Rothchild, 2003). These structures were detected in the ovary of the sacrificed female, in which all her eggs were found in the oviduct.
The follicular cells that remained in the ovary after ovulation proliferate and luteinize, forming the corpus luteum. The corpus luteum is a secretory structure with steroidogenic capacity, and it is considered as the main producer of progesterone in reptiles, although it is also known that it produces androgens and estrogens (Villagrán Santa Cruz and Mendez de La Cruz, 1999). While these are ubiquitous among vertebrates, both oviparous and viviparous, the half-life of the corpus luteum is considered to vary considerably between species. In the oviparous the corpus luteum maintains its secretory activity during most of the period of gravity (oviductal retention period of the egg), involving near the time of oviposition (Jones and Guillette, 1982; Fox and Guillette, 1987). An example of this group of animals is the American alligator(Alligator mississippiensis), which retains the eggs in the female ducts for approximately three weeks, during which the corpus luteum remains active until oviposition, when it enters a prolonged luteolysis (Guillette et al., 1995). Meanwhile in birds, the corpus luteum is considered to have a life span of only three or four days. Our histological studies of the ovary of a female S. merianae at 41 days posture, showed the presence of luteal cells with pycnotic nuclei and vacuolated cytoplasms, these being morphological characteristics of corpus luteum in regression (Villagrán Santa Cruz and Mendez de La Cruz, 1999). However, the discreet presence of connective scar tissue, coupled with the finding of high levels of the hormones, estradiol and progesterone found at this time of the cycle in females of Salvator rufescens (García-Valdez et al., 2016), a species phylogenetically very close to S. merianae, leads us to propose that corpus luteum of S. merianae may have a longer period of activity than the normally attributed to oviparous animals. It would be interesting to expand the studies using, for example, immunohistochemical techniques, in order to corroborate the duration of their steroidogenic functionality.
The finding of atretic follicles at different stages of growth coincides with that reported for other vertebrates (Calderón et al., 2004; Gouder et al., 2006; Uribe Arazábal et al., 2006). In general terms, the histological characteristics of the S. merianae atretic follicles were similar to those observed in other reptilian sauropsids: alterations of the ooplasm, proliferation of the follicular cells, folding and fragmentation of the zona pellucida and reduction of the follicular diameter (Uribe Arazábal et al., 2010). Although it is considered that among the lizards, atresia is more common in the stage of previtellogenesis (Jones, 2011), we have found, in S. maerianae, massive follicular atresias of large-sized vitellogenic follicles. The finding of vitello in degradation, the presence of macrophages, and the increase in vascularization of these follicles, are signs that would indicate the process of removal of the atretic follicles, in order to restore the ovary to its functional state (García-Valdez et al., 2011).
This work shows that the ovaries and follicles of S. merianae have similarities to those described in other species of lizards, with the presence of two germinal beds per ovary, previtellogenic follicles throughout the annual cycle and vitellogenic follicles during the reproductive period. The finding of corpus luteum even in regression stages more than 40 days after oviposition, leads us to propose that in this species the corpus luteum would present a functional persistence superior to that usually described for oviparous vertebrates. Also, massive follicular atresias are described in vitellogenic follicles, not reported in other species of lizards.
Acknowledgments
This study was financed by the Consejo de Investigaciones de la Universidad Nacional de Tucumán (CIUNT). We thank Verónica García-Valdez for helping with the English translation, and to the anonymous referees who contributed to improve this paper.
References
1. Aldokhi O.A., Alwasel S., Harrath A.H. (2019). Ultrastructural and histochemical study of previtellogenic oogenesis in the desert lizard Scincus mitranus. Journal of Morphology 280: 381-394. [ Links ]
2. Andreuccetti P. (1992). An ultrastructural study of differentiation of pyriform cells and their contribution to oocyte growth in representative squamata. Journal of Morphology 212: 1-11. [ Links ]
3. Andreuccetti P., Famularo C., Gualtieri R., Prisco M. (2001). Pyriform cell differentiation in Podarcis sicula is accompanied by the appearance of surface glycoproteins bearing α-GalNAc terminated chains. The Anatomical Record: An Official Publication of the American Association of Anatomists 263: 1-9.
4. Andreuccetti P., Taddei C., Filosa S. (1978). Intercellular bridges between follicle cells and oocyte during the differentiation of follicular epithelium in Lacerta sicula raf. Journal of Cell Science 33: 341-350. [ Links ]
5. Arrieta M.B., Sandova M.T., Álvarez B.B. (2017). Estructura ovárica y dinámica folicular de Liolaemus azarai (Squamata: Liolaemidae). Caldasia 39: 247-259. [ Links ]
6. Baer C.K. (2006). Guidelines on euthanasia of nondomestic animals. American Association of Zoo Veterinarians, USA. [ Links ]
7. Calderón M.L., De Pérez G.R., Ramírez-Pinilla M.P. (2004). Morphology of the ovary of Caiman crocodilus (Crocodylia: Alligatoridae). Annals of Anatomy-Anatomischer Anzeiger 186: 13-24. [ Links ]
8. Da Silva D., Cassel M., Mehanna M., Ferreira A., Dolder M.A.H. (2018). Follicular development and reproductive characteristics in four species of brazilian Tropidurus lizards. Zoological Science 35: 553-563. [ Links ]
9. Davail B., Pakdel F., Bujo H., Perazzolo L.M., Waclawek M., Schneider W.J., Le Menn F. (1998). Evolution of oogenesis: the receptor for vitellogenin from the Rainbow trout. Journal of Lipid Research 39: 1929-1937. [ Links ]
10. De Caro M.D.E., Indolfi P., Iodice C., Spagnuolo S., Tammaro S., Motta C.M. (1998). How the ovarian follicle of Podarcis sicula recycles the DNA of its nurse, regressing follicle cells. Molecular Reproduction and Development 51: 421-429. [ Links ]
11. De Stasio R.D., Borrelli L., Kille P., Parisi E., Filosa S. (1999). Genetics, gene regulation, and expression-isolation, characterization, and molecular cloning of cathepsin d from lizard ovary: changes in enzyme activity and mrna expression throughout ovarian cycle. Molecular Reproduction and Develoment 52: 126-134. [ Links ]
12. Donadio O.E., Gallardo J.M. (1984). Biología y conservación de las especies del género Tupinambis (Squamata, Sauria, Teiidae) en la República Argentina. Revista del Museo Argentino de Ciencias Naturales Bernardino Rivadavia, Zoología 13: 117-127. [ Links ]
13. El Mouden E.H., Znari M., Francillon-Vieillot H. (2001). Variations histologiques de l´ovaire au cours du cycle reproductif annuel chez Agama impalearis Boettger, 1874 (Reptilia: Agamidae). Belgian Journal of Zoology 131: 17-30. [ Links ]
14. Farmer C.G. (2016). Evolution: a lizard that generates heat. Nature 529 (7587): 470-472. [ Links ]
15. Fitzgerald L.A. (1994). Tupinambis lizards and people: a sustainable use approach to conservation and development. Conservation Biology 8 (1): 12-16. [ Links ]
16. Fitzgerald L.A., Chani J.M., Donadio O.E. (1991). Tupinambis lizards in Argentina: Implementing management of a traditionally exploited resource. In: Robinson J., Redford K. (Eds.), Neotropical Wildlife: Use and Conservation. University of Chicago Press, Texas, USA. Pp. 303-316. [ Links ]
17. Fitzgerald L.A., Cruz F.B., Perotti G. (1993). The Reproductive cycle and size at maturity of Tupinambis rufescens (Sauria: Teiidae) in the dry Chaco of Argentina. Journal of Herpetology 27: 70-78. [ Links ]
18.. Fitzgerald L.A., Porini G.M., Lichtschein V. (1994). El manejo de Tupinambis en Argentina: historia, estado actual y perspectivas futuras. Interciencia 19: 166-170. [ Links ]
19. Fox S.L., Guillette L.J. (1987). Luteal morphology, atresia, and plasma progesterone concentrations during the reproductive cycle of two oviparous lizards, Crotaphytus collaris and Eumeces obsoletus. American Journal of Anatomy 179: 324-332. [ Links ]
20. García-Valdez M.V., Chamut S., Jahn G.A., Arce O.E.A., Manes M.E. (2016). Plasmatic estradiol and progesterone variations during the reproductive cycle of captive female argentine red tegu lizards, Tupinambis rufescens. Herpetological Conservation and Biology 11: 519-526. [ Links ]
21. García-Valdez M.V., Chamut S., Valdez-Jaen G., Arce O.E.A., Manes M.E. (2011). Dynamics of ovarian follicles in Tupinambis merianae lizards. Acta Herpetológica 6: 303-313. [ Links ]
22. Gouder B.Y.M., Nadkarni V.B., Rao M.A. (2006). Histological and histochemical studies on follicular atresia in the ovary of the lizard, Calotes versicolor. Journal of Herpetology 45: 451-456. [ Links ]
24. Guillette Jr. L.J., Woodward A.R., You Xiang Q., Cox M.C., Matter J.M., Gross T.S. (1995). Formation and regression of the corpus luteum of the American alligator (Alligator mississippiensis). Journal of Morphology 224 (1): 97-110. [ Links ]
25. Guraya S.S. (2013). Ovarian follicles in reptiles and birds (Vol. 24). Springer Science and Business Media, Germany. [ Links ]
26. HSUS, Humane Society of the United States. (2013). Euthanasia Reference Manual. [ Links ]
27. Ibrahim M.M., Wilson I.B. (1989). Light and electron microscope studies on ovarian follicles in the lizard Chalcides ocellatus. Journal of Zoology 218: 187-208. [ Links ]
28. Jones R.E., Guillette J. (1982). Hormonal control of oviposition and parturition in lizards. Herpetologica 38: 80-93. [ Links ]
29. Jones R.E., Swain T., Guillette L.J., Fitzgerald K.T. (2006). The comparative anatomy of lizard ovaries, with emphasis on the number of germinal beds. Journal of Herpetology 16: 240-252. [ Links ]
30. Jones S.M. (2011). Hormonal regulation of ovarian function in reptiles. In: Hormones and reproduction of vertebrates. Reptiles. Norris D.O., Lopez K.H. (Eds.). Elsevier Academic Press, USA. Pp. 89-115. [ Links ]
31. Langeron M. (1949). Précis de Microscopie. Masson et Cie (Eds.), France. [ Links ]
32. Lozano A., Ram A., Uribe Arazábal M.C. (2014). Oogenesis and ovarian histology in two populations of the viviparous lizard Sceloporus grammicus (Squamata: Phrynosomatidae) from the central Mexican Plateau. Journal of Morphology 275: 949-960. [ Links ]
33. Machado-Santos C., Santana L.N.D.S., Vargas R.F., Abidu-Figureeiredo M., Brito-Gitirana L., Chagas M.A. (2015). Histological and immunohistochemical study of the ovaries and oviducts of the juvenile female of Caiman latirostris (Crocodilia: Alligatoridae). Zoologia (Curitiba) 32 (5): 395-402. [ Links ]
34. Manes M.E., Ibañez M.A., Manlla A. (2003). Factores físicos y conductas de nidificación de lagartos Tupinambis merianae en cautiverio. Revista Argentina de Producción Animal 23: 119-126. [ Links ]
35. Manes M.E., Noriega T., Campos Casal F., Apichela S. (2007). Ovarian changes during the reproductive cycle of the Tupinambis merianae lizard raised in a temperate environment. Cuadernos de Herpetología 21: 21-29. [ Links ]
36. Masson G.R., Guillette L.J. (1987). Changes in oviducal vascularity during the reproductive cycle of three oviparous lizards (Eumeces obsoletus, Sceloporus undulatus and Crotaphytus collaris). Journal of Reproduction and Fertility 80: 361-371. [ Links ]
37. Mercolli C., Yanosky A. (1989). Répertoire des comportements du Téju (Tupinambis teguixin). Sauria: Teiidae. Revue Française d’Aquariologie 16: 123-130.
38. Mieres M.M., Fitzgerald L.A. (2006). Monitoring and Managing the Harvest of Tegu Lizards in Paraguay. The Journal of Wildlife Management 70 (6): 1723-1734. [ Links ]
39. Motta C.M., Castriota Scanderbeg M., Filosa S., Andreuccetti P. (1995). Role of pyriform cells during the growth of oocytes in the lizard Podarcis sicula. Journal of Experimental Zoology 273: 247-256. [ Links ]
40. Naretto S., Cardozo G., Blengini C.S., Chiaraviglio M. (2015). Importance of reproductive biology of a harvest lizard, Tupinambis merianae, for the management of commercial harvesting. Wildlife Research 42: 697-704. [ Links ]
41. Noriega T., Fogliatto O., Mignola L., Manes M.E. (1996). Ciclo biológico y patrones de comportamiento en una población de iguanas overas Tupinambis teguixin (L) (Sauria, Teiidae) adaptada al cautiverio. Revista Agronómica del Noroeste Argentino 28: 109-127. [ Links ]
42. Porini G.M. (2006). Proyecto Tupinambis Una propuesta para el manejo de Tupinambis rufescens y T. merianae en la Argentina. In: Manejo Fauna Silvestre en la Argentina. Programas uso sustentable. Bolkovic M.L., Ramadori D. (Eds.). Dirección de Fauna Silvestre, Secretaría de Ambiente y Desarrollo Sustentable, Argentina, pp. 65-75. [ Links ]
43. Rheubert J.L., Siegel D.S., Trauth S.E. (2014). Reproductive biology and phylogeny of lizards and tuatara. CRC Press, Australia. [ Links ]
44. Romano M., Rosanova P., Chiara A., Limatola E. (2004). Vertebrate yolk proteins: a review. Molecular Reproduction Develoment 69: 109-116. [ Links ]
45. Rothchild I. (2003). The yolkless egg and the evolution of eutherian viviparity. Biology of Reproduction 68: 337-357. [ Links ]
46. Sarasquete C., Gutiérrez M. (2005). New tetrachromic VOF stain (Type III-G.S) for normal and pathological fish tissues. European Journal of Histochemistry 49: 211-227. [ Links ]
47. Shine R., Radder R. (2007). Germinal bed condition in a polyautochronic single-clutched lizard, Bassiana duperreyi (Scincidae). Amphibia-Reptilia 28: 159-162. [ Links ]
48. Tammaro S., Simoniello P., Filosa S., Motta C.M. (2007). Block of mitochondrial apoptotic pathways in lizard ovarian follicle cells as an adaptation to their nurse function. Cell and Tissue Research 327: 625-635. [ Links ]
49. Tattersall G.J., Cadena V., Leite C.A.C., Andrade D.V., Abe A.S., Milsom W.K., Sanders C.E. (2016). Seasonal reproductive endothermy in tegu lizards. Science Advances 2: 1-8. [ Links ]
50. Uribe Arazábal M.C., Cruz G.D.R., García Alarcón A., Guerrero Estévez S., Aguilar Morales M. (2006). Características histológicas de los estadios de atresia de folículos ováricos en dos especies de teleósteos vivíparos: Ilyodon whitei (Meek, 1904) y Goodea atripinnis (Jordan, 1880) (Goodeidae). Hidrobiológica16: 67-73. [ Links ]
51. Uribe Arazábal M.C., Guillette L.J. (2000). Oogenesis and ovarian histology of the American alligator Alligator mississippiensis. Journal of Morphology 240: 225-240. [ Links ]
52. Uribe Arazábal M.C., Hernández Franyutti A.A., Sanz Ochotorena A., González Porter G. (2010). Estructura comparada de los folículos ováricos en reptiles. In: Reproducción en reptiles: morfología, ecología y evolución. Hernández Gallegos O., Méndez de la Cruz F., Méndez Sánchez F. (Eds.). Universidad Autónoma del Estado de México, México. Pp. 167-201. [ Links ]
53. Uribe Arazábal M.C., Portale G.L., Guillett L.J. (1996). Ovarian folliculogenesis in the oviparous Mexican lizard Ctenosaura pectinata. Journal of Morphology 230: 99-112. [ Links ]
54. Villagrán Santa Cruz M., Mendez de La Cruz F. (1999). Corpus luteum through the gestation of Sceloporus palaciosi (Sauria: Phrynosomatidae). Copeia 1: 214-218. [ Links ]














