INTRODUCTION
Chaetarthria Stephens is a water scavenger beetle genus included in the subfamily Chaetarthriinae, tribe Chaetarthriini (Short & Fikácek, 2013). Formally the tribe includes four genera in the Neotropical region: Apurebium García, Chaetarthria, Guyanobius Spangler and Venezuelobium García (Spangler, 1986; García, 2002; Gustafson & Short, 2010). Nonetheless, it has been suggested by Short (2009) that the genera Apurebium and Venezuelobium include species that seem to be variants of Chaetarthria, in which case the tribe would have only two genera in the Neotropical region, Guyanobius and Chaetarthria (Clarkson et al., 2018). The other two genera belonging to this tribe,
Hemisphaera Pandellé and Thysanarthria Orchymont, are restricted to the Ethiopian, Palearctic and Oriental regions. This tribe is easily recognized by a unique characteristic of the adults that consists of a fringe of setae arising at the anterior margin of the first abdominal ventrite that covers a large depression usually filled with a hyaline substance of unknown function.
Chaetarthria has a worldwide distribution, comprising 49 species (52 species if Apurebium and Venezuelobium are considered synonyms of Chaetarthria), most of which are restricted to the New World. Larval knowledge of the New World Chaetarthriini is better than that of other regions since larvae of both Chaetarthria and Guyanobius have been described (Spangler, 1986; Archangelsky, 1997, 2002).
Nonetheless, the original descriptions only deal with the general morphology of these larvae, and no attempts were made to include chaetotaxic and morphometric characters. In this contribution, the primary chaetotaxy of the first instar larva of Chaetarthria bruchi Balfour-Browne is described in detail and morphometric characters are also included. The chaetotaxy of C. bruchi is compared to that of the European species C. seminulum (Herbst) presented by Fikácek (2006). Larvae of Hemisphaera and Thysanarthria remain unknown.
MATERIAL AND METHODS
Source of material. Two first instar larvae of C. bruchi were studied for the descriptions. P. N. Talampaya, arroyo Shimpa, 29° 44’ 43” S, 67° 44’ 52” W, 9-XI-1999. The material studied is kept in the larval collection of the author and will be deposited in the larval collection of the Laboratory of Entomology, Buenos Aires University, Argentina.
Methods. Larval specimens were cleared in warm lactic acid, dissected, and mounted on glass slides with Hoyer’s medium. Observations (up to 1000x), photographs and drawings were done with a Leica S6D dissecting microscope and Leica DMLB compound microscope both with camera lucida and a photographic camera attached.
Morphometry. Different measurements of the head capsule and head appendages were taken with a micrometer. Measurements were used to calculate ratios, which are practical to characterize shapes. Measured structures were adjusted as parallel as possible to the plane of the objective. The following measurements were taken: TL: total body length; MW: maximum body width, measured at level of prothorax; HL: head length, measured medially along epicranial stem from anterior margin of frontoclypeus to occipital foramen; HW: maximum head width; AL: length of antenna, derived by adding the lengths of the first (A1L), second (A2L) and third (A3L) antennomeres; SeL: length of antennal sensorium; SL: length of stipes; MPL: length of maxillary palpus, obtained by adding the lengths of the first (MP1L), second (MP2L), third (MP3L) and fourth (MP4L) palpomeres; ML: length of maxilla, derived by adding SL and MPL; cardo omitted; LPL: length of labial palpus, obtained by adding the lengths of the first (LP1L) and second (LP2L) palpomeres; LigL: length of ligula; MtW: maximum width of mentum; PrmtL: length of prementum, measured from its base to the base of LP1; PrmtW: maximum width of prementum.
Chaetotaxy. Primary (present in first instar larva) setae and pores were identified in the cephalic capsule and head appendages following system developed by Fikácek et al. (2008) and Byttebier & Torres (2009). Homologies were established using the criterion of similarity of position (Wiley, 1981). Sensilla were coded with a number and two capital letters, usually corresponding to the first two letters of the name of the structure on which they are located. The following abbreviations were used: AN: antenna; FR: frontale; LA: labium; MN: mandible; MX: maxilla; PA: parietale; gAN: group of antennal sensilla; gAPP: group of sensilla on the inner appendage of the maxilla; gFR1, gFR2: groups of sensilla on the frontale; gLA: group of sensilla on the labial palp; gMX: group of sensilla on the maxillary palp.
RESULTS
Description of the first instar larva of C. bruchi
Diagnosis of Chaetarthria larvae (first instar). The following combination of characters distinguish larvae of Chaetarthria from other known Hydrophilidae larvae.
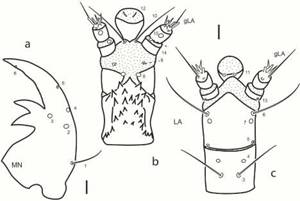
Larval morphology (Figs. 1-4). Head capsule subquadrate; frontal lines subparallel, reaching occipital foramen widely apart, coronal line absent; clypeolabrum symmetrical, nasale bearing three sharp teeth, middle one larger than lateral ones; lateral lobes of epistome symmetrical, not projected farther than nasale, bearing a few sharp spines projecting mesally; posterior tentorial grooves close to midline, subapical. Stemmata closely aggregated, difficult to count. Cervical sclerites present, narrow. Antenna short, first and second antennomeres subequal in length, basal one slightly wider. Mandibles symmetrical, with two inner teeth similar in size. Maxilla with stout stipes, longer than palpus, with sharp cuticular spines along inner face dorsally; first palpomere wider than long, incompletely sclerotized dorsally, smooth, second and third palpomeres short, fourth palpomere slightly longer than first. Labium stout, submentum wide, subpentagonal; mentum and prementum closely united, subequal in width; mentum narrow, subquadrate, with strong cuticular projections on dorsal face; prementum incompletely sclerotized dorsally; palpi smooth, basal palpomere the shortest; ligula as long as palpi, spatulate. Prothoracic plate large, covering most of pronotum, with sagittal line, prosternal sclerite poorly sclerotized, mostly membranous; meso- and metathorax with pleural areas slightly lobed, bearing one pair of narrow subrectangular tergites on anterior margin, those of mesothorax larger. Legs short, reduced, three-segmented. Abdominal segments poorly sclerotized, with one pair of small oval sclerites dorsally and pleural areas slightly lobed; segment eight bearing two longitudinal plates; meso-, metathorax and abdominal segments I-VII bearing small membranous oval tubercles covered by microtrichia. Chaetotaxy (Figs. 2-4). Frons with gFR1 bearing six setae; gFR2 with three or four setae; pores FR15 closely aggregated; setae FR5 and FR6 stout; seta FR3 minute; pore FR2 and seta FR3 closely aggregated; seta FR1 long. Parietale with PA6 sub-basal, not touching frontal lines; PA1-PA5 arranged in a zigzag; setae PA13 and PA14 closely aggregated; setae PA16 and PA18 long, closely aggregated; pore PA29 posterior to pore PA30. Antenna with AN9 absent; SE1 as long as or slightly longer than A3. Mandible with seta MN1 long, sub-basal; seta MN5 closer to pore MN4 than to apex. Maxilla with seta MX1 very long; setae MX8-11 simple distally. Labium with LA10 close to base of ligula; LA11 subapical or sub-basal. Morphometric measures are detailed in Table I.
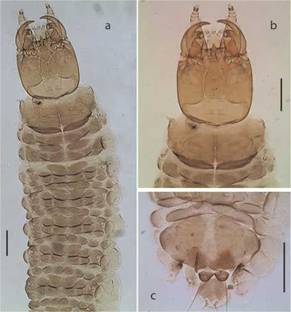
Fig. 1: Chaetarthria bruchi, first instar larva. a. habitus, dorsal view. b. detail of head capsule and prothorax, dorsal view. c. detail of abdominal segment VIII, dorsal view. Scale bars = 0.1 mm.
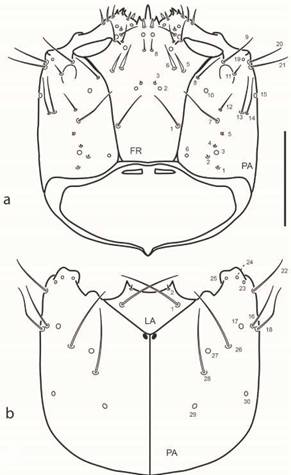
Fig. 2: Chaetarthria bruchi, first instar larva, head capsule. a. dorsal view. b. ventral view. Scale bar = 0.1 mm.
Chaetotaxy (Figs. 2-4). Head capsule (Figs. 2, 3a). Frontale with 42 sensilla: two long setae at basal fourth close to frontal lines (FR1); two pores (FR2) and two minute setae (FR3) closer to midline at midlength; two pairs of stout setae (FR5 and FR6) and one pore (FR4) behind inner margin of antennal socket; one rather long seta (FR7) on inner margin of antennal socket; distal area of frontale with three setae (FR9 and FR10 rather long, FR12 minute) and three pores (FR11, FR13 and FR14); central area behind nasale with one pair of pores (FR15) and one pair of short setae (FR8); nasale dorsally with six short and stout setae (gFR1); each epistomal lobe with three rather stout setae (gFR2). Each parietale with 30 sensilla. Dorsal surface with a basal zigzag row of four minute setae (PA1, PA2, PA4, PA5) and one pore (PA3); one sub-basal pore (PA6) close to but not touching frontal line; four setae arranged in a transverse row behind stemmata (PA7 rather long and slender, closer to frontal line, PA12 short, PA13 and PA14 long and slender on outer face); one long seta (PA8) close to frontal line on distal third of parietale; one pore (PA10) between setae PA8 and PA12; three long setae (PA9, PA20, PA21) and one pore (PA19) distal to stemmata; one short seta (PA11) arising among stemmata. Ventral surface with three pores (PA23, PA24, PA25) and one long seta (PA22) on anterolateral corner, behind mandibular acetabulum; two long setae (PA16, PA18) and two pores (PA17, PA30) along outer margin; central area of parietale with two long setae (PA26 and PA28) and two pores, PA27 (between setae PA26 and PA28) and PA29 (behind seta PA28). Antenna (Fig. 3b). A1 with five pores, three dorsal (AN1 at midlength closer to outer margin, AN2 at center, AN4 distally on inner margin) and two ventral on apical margin (AN3 on outer corner, AN5 on inner corner). A2 with one dorsal pore (AN6) and four setae on membrane connecting with A3, two minute apical setae on outer margin (AN7, AN8) close to base of SE1, and two apical setae on inner margin (AN10 long, AN11 very short); AN9 absent. A3 bearing a group of several setae of different lengths (gAN); SE1 as long as or slightly longer than A3. Mandible (Fig. 4a). Bearing six sensilla; three dorsal pores at level on retinacula arranged in a triangle (MN2 and MN4 on outer margin, MN3 at base of distal retinaculum); one rather long seta (MN1) on outer face at basal fourth; one minute seta (MN5) on outer margin at distal third; one subapical pore (MN6) on inner margin. Maxilla (Fig. 3c-d). Cardo with one long seta (MX1) ventrally; stipes with an inner row of five short setae (MX7-11), MX7 slender, remaining ones stout and simple apically, ventrally with three pores (MX2 at basal third, MX3 at midlength close to inner margin, MX4 at distal third on outer margin) and two long setae on outer margin (MX6 apical, MX5 subapical, close to MX4). MP1 dorsally with one basal setiform seta (MX16) and one pore at base of appendage (MX17), ventrally with two long subapical setae (MX13, MX14) and two pores (MX12 close to outer margin and MX15 at base of appendage); inner appendage with two long setae and two short sensoria (gAPP). MP2 with two pores, one ventral on outer face (MX18) and one dorsal on membrane connecting with MP3 (MX19) on inner face; minute seta MX27 basal on outer margin. MP3 with two long setae (MX21 on inner margin, MX23 on outer margin) and two ventral pores (MX20, MX22). MP4 with one basal long seta (MX24) on inner margin and two subapical pores on outer face (MX25 digitiform and dorsal, MX26 ventral); a group of at least seven short sensoria constitute gMX. Labium (Figs. 2b, 4b-c). Submentum with two pairs of setae, one long (LA1), the other very short, on anterior margin (LA2). Mentum ventrally with two rather long sub-basal setae (LA3) and two pores (LA4) close to anterolateral angle. Prementum ventrally with two pairs of setae (LA6 long on anterolateral corner, LA5 short on basal corner) and one pair of pores (LA7) behind setae LA6; dorsally with one pair of pores on disc (LA8) and one pair of minute seta-like sensilla (LA9) on membrane connecting with labial palpi. Ligula with three pairs of sensilla, one pair of very long basal setae (LA10) and two pairs of pores (LA11 sub-basal and ventral, LA12 subapical and dorsal). LP1 with one minute seta (LA13, ventral) and one distal pore (LA14, dorsal) on membrane connecting with LP2; LP2 dorsally with one subapical pore on outer face (LA15); distally with a group of at least six or seven sensoria (gLA).
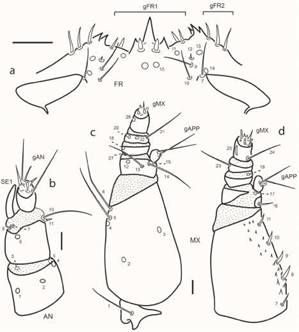
Fig. 3: Chaetarthria bruchi, first instar larva. a. detail of clipeolabrum, dorsal view. b. left antenna, dorsal view. c. left maxilla, ventral view. d. left maxilla, dorsal view. Scale bars: a = 0.025 mm, b-d = 0.01 mm.
DISCUSSION
On a worldwide basis larval knowledge of Chaetarthriini is rather limited (Short & Fikácek, 2013;Clarkson et al., 2018) since only larvae of Chaetarthria and Guyanobius are known. In the New World larvae of Guyanobius adocetus Spangler were described by Spangler (1986) and later by Archangelsky (1997); an unidentified north American larva of Chaetarthria and larvae of C. bruchi were described by Archangelsky (1997, 2002). With regards to the chaetotaxy of the tribe, the only species for which the chaetotaxy is known in detail is C. seminulum (Fikácek, 2006). Therefore, the chaetotaxy of C. bruchi can only be compared to that of C. seminulum.
Both larvae are very similar morphologically and also in their chaetotaxy, nonetheless several chaetotaxic differences can be mentioned. In the frontale, gFR2 has three setae in C. bruchi (four in C. seminulum); the distance between both pores FR2 is greater than that between both setae FR3 in C. bruchi (distance between both pores FR2 smaller than that between both setae FR3 in C. seminulum); FR5 and FR6 are short and very stout in C. bruchi (very long and rather stout in C. seminulum). The parietale also shows some differences between these two species, in C. bruchi seta FR12 is short (long in C. seminulum); pore PA17 is in line with setae PA16 and PA18 in C. bruchi (pore PA17 distal to setae PA16 and PA18 in C. seminulum); seta PA26 is placed behind pore PA17 in C. bruchi (distal to pore PA17 in C. seminulum); pore PA30 is posterior to seta PA28 in C. bruchi (pore PA30 is in line with seta PA28 in C. seminulum). The chaetotaxy of the antennae is very similar and no evident differences could be found. The mandible in C. bruchi has pore MN6, in C. seminulum this pore was not illustrated by Fikácek (2006) but later it was identified and included in a paper describing the chaetotaxic ground plan of Hydrophilidae (Fikácek et al., 2008); this pore is very small and sometimes difficult to detect. In maxillary palpomere one of C bruchi seta MX16 is short (long in C. seminulum); in palpomere two seta MX27 in C. bruchi is present, (absent in C. seminulum, probably overlooked). In the labium two evident differences could be identified, seta LA9 is minute in C. bruchi (long in C. seminulum); pore LA11 is placed at midlength of ligula in C. bruchi (subapical in C. seminulum); it can also be mentioned that seta LA5 is present in C. bruchi (absent in C. seminulum, but it could have been overlooked since these setae are very small and sometimes difficult to see).
From what has been mentioned above it seems clear that even though morphologically Chaetarthria larvae are very similar, detailed chaetotaxic studies are useful to differentiate between larvae of different species. Of all these differences the most obvious is the number of setae in gFR2; in C. bruchi, and also in an unidentified third instar larva described by Archangelsky (1997), gFR2 has 3 setae, while the European C. seminulum has four setae in gFR2 (Fikácek, 2006). It would be interesting to see if this character could help to tell apart Chaetarthria larvae of the New World from those of the Old World; that will require further larval studies on new Chaetarthria species from different regions. The information herein presented will be useful for future chaetotaxic studies of the tribe Chaetarthriini and also for phylogenetic studies using larval characters.
ACKNOWLEDGMENTS
CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas) is acknowledged for supporting systematic research. APN (Administración de Parques Nacionales) and CONICET are acknowledged for allowing fieldwork funded by the Project PIP 0568/98 (Diversidad, conservación y manejo de la fauna del Parque Talampaya, La Rioja). The anonymous reviewers are also acknowledged for their critical review.












 uBio
uBio