Introduction
In Argentina, Melanoplinae grasshoppers represent one of the most relevant (and numerous) subfamilies within the Acrididae family (Insecta Orthoptera). Several species in this subfamily are considered plagues 2,12. These species cause serious damage to grasslands and economically important crops such as maize, soybean, and wheat, among others 1,14. Since the mid-nineteenth century, these insects have been reported in several regions of Argentina, following the progressive agricultural development of the country. So far, synthetic insecticides are still the only alternative against grasshoppers, regardless of negative environmental consequences 5.
In this sense, entomopathogens acting as biocontrol agents have been considered excellent alternatives to chemical control. Fungi are among the most important entomopathogens, naturally regulating insect populations widely found in multiple types of environments 9,23. More than 700 species of entomopathogenic fungi have been described worldwide. Nevertheless, only a few have been found to affect grasshoppers. Beauveria bassiana (Balsamo) Vuillemin, Entomophaga grylli (Fresenius) Batko, Metarhizium anisopliae (Metsch.) Sorokin and Metarhizium flavoviridae Gams & Rozsypal are the most frequently observed fungal species infecting acrididae 10. Furthermore, B. bassiana has been reported to cause natural epizootics in grasshoppers, in different geographical regions 4. However, in Argentina, only a few records mention acridids naturally infected with B. bassiana16. This work aimed to test the efficacy of the strain B. bassiana (LPSc 1067) on six grasshopper species in Argentina.
Materials and Methods
Insect collecting
Dichroplus maculipennis (Blanchard 1851), Dichroplus elongatus (Giglio-Tos 1894), Dichroplus pratensis (Bruner 1900), Scotussa lemniscata (Stal 1861), Ronderosia bergi (Stal 1878) individuals were collected from the southern Pampas region (Laprida county, Buenos Aires province, Argentina, 37°32’60’’ S, 60°49’00’’ W). Trimerotropis pallidipennis (Burmeister 1838) individuals were sampled from the locality of Salinas de Bustos, in La Rioja province. The insects were kept in a rearing room under controlled conditions (30°C, photoperiod 14-10 h light-dark, 40% RH) as previously described 13. Different bioassays used first laboratory generations [F1].
Pathogenicity assays
B. bassiana strain LPSc 1067 (GeneBank accession number KF500409) was isolated in 2008 from a katydid (Orthoptera: Tettigoniidae), closely related to the long-horned grasshopper. The strain was collected at Salinas de Bustos, (30°18’9.4” S, 67°34’40.6” W), La Rioja province, Argentina, where high temperatures and low humidity are unfavourable for fungal development 8,23. After isolation, the strain was deposited at the Spegazzini Institute culture collection. Conidia were obtained from cultures on potato-dextrose-agar medium after incubation for 10 days at 25°C in the dark 7. They were later harvested with disposable cell scrapers (Fisherbrand®) and placed in test tubes containing 0.01% (v/v) Tween 80 (Merck). Suspensions were vortexed for 2 min, filtered through four layers of sterile muslin, and concentration was adjusted to 1 x 108 conidia/ml using a Neubauer hemocytometer according to Prior et al. (1995). Conidia viability was determined after 24 h, as described by Lane et al. (1988). This germination test was repeated for each stock suspension. Nine replicates (on different dates) of 10 third-instar nymphs of each grasshopper species, were sprayed with about 1 ml of conidial suspension using a 35-ml glass atomizer, according to Prior et al. (1995). Three additional control replicates per species, each with 10 grasshoppers, were sprayed with 1 ml 0.01% [v/v] Tween 20. Groups of 10 individuals were kept in acetate tubes of 50 x 9 cm and fed with lettuce, cabbage leaves and wheat bran 6. Treated and control insects were kept at 30°C, 60% relative humidity, and 14:10 h light:dark photoperiod. Cumulative mortality was recorded for 10 days. Dead grasshoppers with no external mycelia were surface-sterilized by successive dipping in 70% ethanol (10-15 s), 0.5% sodium hypochlorite solution (1 min), and sterile distilled water (1 min, two consecutive baths) according to Vega et al. (2012). Next, insects were placed in sterile culture chambers consisting of a Petri dish (60 mm diameter) with a filter-paper disk periodically moistened with sterile distilled water and incubated at 25°C in the dark. Mycosis was confirmed by microscopic examination of dead grasshoppers.
Statistical analysis
Mortality data were subjected to one-way ANOVA, after checking assumptions were met. Mean comparisons were assessed by the Tukey test (P = 0.05). Analyses were performed with InfoStat 2011 software 3. For mortality equal to or higher than 50%, median survival time (MST) was calculated based on the Kaplan-Meier Survival distribution function 25. Pairwise comparisons between survival curves were made by Long-rank Test (P<0.0001).
Results
Significant differences were observed when assessing pathogenicity of B. bassiana (LPSc 1067) on third-instar nymphs (DF= 5; F= 9.93; P<0.0001). The highest mortality (100%) was registered in third-stage nymphs of T. pallidipennis and D. maculipennis (Figure 1, page 101).
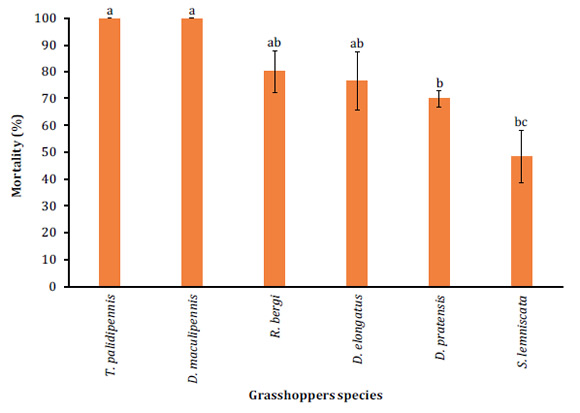
Different letters denote significant differences between treatments according to the Tukey test (P<0.05).
Letras distintas indican diferencias significativas entre tratamientos de acuerdo con el test de Tukey (P<0,05).
Figure 1: Mean mortality (percent + SD) on third-instar nymphs of different grasshopper species with 1x108 conidia/ml of B. bassiana (LPSc 1067) strain. Figura 1: Porcentaje de mortalidad + DS sobre ninfas de tercer estadio, de las diferentes especies de tucuras plagas cuando sobre ellas fue aplicada una concentración de 1x108 conidios/ml de la cepa (LPSc 1067) de B. bassiana.
The lowest mortality (50 + 3.5%) was observed in nymphs of S. lemniscata (Figure 1, page 101). Further mortalities ranged between 70 + 8.3% on D. pratensis and 80 + 5.7% on R. bergi (Figure 1, pag 101). Controls recorded no mortality. Besides, significant differences in MST were observed according to the log-rank test (P<0.0001). The lowest MST was observed on T. pallidipennis nymphs at 3.5 + 0.15 days while the highest MST was observed on D. pratensis nymphs with 7.48 + 0.28 days MST (Table 1, page 101).
Table 1: Median survival time (MST) expressed in days, on third-instar nymphs for each evaluated grasshoppers species. Tabla 1: Tiempo medio de supervivencia (MST) expresado en días, sobre ninfas de tercer estadio de cada una de las especies de tucuras evaluadas.
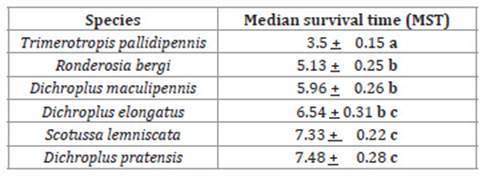
Different letters indicate significant differences according to the Long-rank Test (P<0.0001).
Letras diferentes indican diferencias significativas según Long-rank Test (P<0,0001).
Discussion
Entomopathogenic fungi comprise important pathogens of insect pests. Some advantages to consider in control programs consist of their high specificity, contact transmission, natural dispersion, safety for non-target organisms and the ability to maintain lasting control once established in the environment 24. The present study determined pathogenicity of B. bassiana (LPSc 1067) strain on six harmful grasshopper species in Argentina. T. pallidipennis and D. maculipennis resulted the most susceptible, exhibiting 100% mortality, while the least affected grasshopper species was S. lemniscata, with 50% mortality. These results agree with those obtained by Pelizza et al. (2012a), who evaluated the association between enzymatic activity and fungal virulence in 59 entomopathogenic fungal isolates native to Argentina. Isolate LPSc 1067 caused the highest mortality on Tropidacris collaris nymphs (97.7 ± 1.22%), nine isolates caused no mortality, while the remaining 49 caused mortalities ranging between 6.6 ± 0.3% (LPSc 770) to 91.06 ± 1.51% (LPSc 906). Furthermore, another study showed laboratory effectiveness of 26 fungal strains (isolated from insects and soil in Argentina) against Schistocerca cancellata (Serville) (Orthoptera: Acrididae) 18. These authors also studied the association between chitinase, protease, and lipase levels in these fungi and their insecticidal activities. They observed that B. bassiana (isolate LPSc 1067) caused the highest mortality (90 ± 1.03 %) while exhibiting the highest values of chitinolytic, proteolytic and lipolytic activity (6.13 +0.05; 2.56 + 0.11, and 2.33 + 0.47, respectively) and the lowest median survival time (MST) (5.96 days).
The study by Schaefer et al. (1936) demonstrated that, in the laboratory, B. bassiana infects grasshoppers and all locust nymphs and adults sprayed with conidia. Mortalities caused by the fungi were registered within 5-20 days. Regarding the MST, our results agree with those obtained by Roberts and Hajek (1992), who observed MST values between 4.1 and 7.9 days when applying B. bassiana conidia on Melanoplus sanguinipes (Fabricius) adults. Also, results agree with those reported by Prior et al. (1995) who during various experiments concerning inoculation protocols, observed 95% mortality within 4-5 days using conidial suspension with 1 x 107 and 1 x 108 conidia/ml concentrations. On the other hand, Uvarovistia zebra (Uvarov) (Orthoptera: Tettigoniidae) treated with 5 × 106 conidia/ ml of B. bassiana showed a cumulative mortality of 57.7% 15, while other authors evaluated the effect of B. bassiana (LPSc 1067) on nymphal developmental time, fecundity, and survival of D. maculipennis and R. bergi under laboratory conditions 19, and observed altered adult survival after infection, with a fungal concentration of 1x103 conidia/ml. Mortality of D. maculipennis during third through sixth-instar (last) was significantly higher among treated nymphs (66 + 3.8%) than in controls (15 + 1.7%). Similarly, mortality in R. bergi during third through fifth instar (last) was higher in treated nymphs (71 + 2.8%) than in controls (19 + 1.5%).














