Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell v.32 n.1 Mendoza ene./abr. 2008
A cryopreservation protocol for immature zygotic embryos of species of Ilex (Aquifoliaceae)
Luis A. Mroginski*, Pedro A. Sansberro, Adriana M. Scocchi, Claudia Luna and Hebe Y. Rey
*Instituto de Botánica del Nordeste (IBONE), Facultad de Ciencias Agrarias (UNNE), C.C. 209. (3400) Corrientes, Argentina
Address correspondence to: Luis A. Mroginski. Instituto de Botánica del Nordeste (IBONE), Facultad de Ciencias Agrarias (UNNE), C.C. 209. (3400) Corrientes, ARGENTINA. E-mail: luis@agr.unne.edu.ar
ABSTRACT: Tropical Ilex species have recalcitrant seeds. This work describes experiments demonstrating the feasibility of long-term conservation of Ilex brasiliensis, I. brevicuspis, I. dumosa, I. intergerrima, I. paraguariensis, I. pseudoboxus, I. taubertiana, and I. theezans through cryopreservation of zygotic rudimentary embryos at the heart developmental stage. The embryos were aseptically removed from the seeds and precultured (7 days) in the dark, at 27± 2ºC on solidified (0.8% agar) 1/4MS medium, [consisting of quarterstrength salts and vitamins of Murashige and Skoog (1962) medium] with 3% sucrose and 0.1 mg/l Zeatin. The embryos were then encapsulated in 3% calcium alginate beads and pretreated at 24 h intervals in liquid medium supplemented with progressively increasing sucrose concentrations (0.5, 0.75 and 1 M). Beads were dehydrated for 5 h with silicagel to 25% water content (fresh weight basis) and then placed in sterile 5 ml cryovials. Then the beads were either plunged rapidly in liquid nitrogen were they were kept for 1 h (rapid cooling) or cooled at 1ºC min-1 to -30ºC. Then the beads were immersed in liquid nitrogen for 1 h (slow cooling). The beads were rewarmed by immersion of the cryovials for 1 min in a water bath thermostated at 30ºC. Finally, beads were transferred onto culture medium (1/4MS, 3% sucrose, 0.1 mg/l zeatin, solidified with 0.8% agar) and incubated in a growth room at 27 ± 2ºC under a 14 h light (116 μmol. m-2.s-1)/ 10 h dark photoperiod. Maximum recovery percentages between 15 and 83% (depending on de the species and the treatment) were obtained with the cryopreserved embryos.
Key words: Encapsulation-dehydration; Germplasm preservation; Liquid nitrogen; Llex
Introduction
The genus Ilex belongs to the Aquifoliaceae family, which consists of about 600 species that inhabit temperate and tropical regions of the world. They are generally deciduous or evergreen trees or shrubs (Hu, 1989). Although most of them are found in Asia, approximately 220 species are found in South America (Giberti,1995; Loizeau, 1994).
From an economical point of view, the "mate tree" (Ilex paraguariensis) is a very important tree in South America, mainly because of the value of its leaves for making the stimulatory beverage "mate", a tea-like infusion with a high caffeine and theobromine content (Filip et al., 1998). I. paraguariensis grows spontaneously and is also extensively cultivated in North-Eastern Argentina, Eastern Paraguay, and South-eastern Brazil (Giberti, 1995). Other Ilex species (I. aquifolium, I. cornuta, I. opaca and I. crenata) generally named "hollies" are cultivated for producing Christmas trees or for landscaping (Hu, 1989). I. vomitoria and I. tarapotina are also used in infusions (Loizeau, 1994).
Regarding the possibilities for germplasm conservation, most of the tropical and subtropical Ilex species present two major constraints: 1) They usually have seeds with rudimentary embryos that remain at the immature heart-shaped stage for a long time after the fruits reach maturity (Hu, 1989; Niklas, 1987) and, therefore, the percentage of seed germination is very low (Fontana et al., 1990). Embryo culture has proved to be a very useful tool to solve this problem (Hu, 1975, 1976, 1989; Sansberro et al., 1998, 2001). 2) They have seeds which are highly sensitive to desiccation and cold. In other words, according to Roberts (1973), the seeds are recalcitrant and, therefore, unsuitable for the dry and/or cold seed storage procedures traditionally employed for germplasm conservation (Engelmann, 1991). Thus, the germplasm of Ilex spp. is conserved as whole plants in field collections. With this storage method, the genetic resources are exposed to diseases, pests and natural hazards such as human error, drought, and weather damage. In addition, field genebanks are costly to maintain, and trained personnel requirements are very important (Engelmann, 1991; Withers and Engelmann, 1998). This highlights the need to develop protocols for in vitro conservation. Cryopreserved storage in liquid nitrogen at -196ºC appears as a viable option for long-term germplasm conservation of recalcitrant seeds (Engelmann, 2000; Scocchi and Rey, 2004). Although research on the development of cryopreservation techniques has been conducted with various explants of numerous plant species (Engelmann, 2000), only one preliminary report exists on cryopreservation of Ilex species (Mroginski et al., 2006).
The research presented in this paper was aimed at developing a cryopreservation procedure for eight Ilex species using immature zygotic embryos and recovering plants through in vitro culture of these immature embryos.
Materials and Methods
Plant materials
Immature fruits (drupes), 2-3 months after pollination, of Ilex brasiliensis, I. brevicuspis, I. dumosa, I. intergerrima, I. paraguariensis, I, pseudoboxus, I. taubertiana, and I. theezans were collected from trees planted in the experimental station of INTA Cerro Azul (Misiones, Argentina). The fruits were cold-pretreated (two weeks at 4ºC) and afterwards they were surfacesterilized according the protocol of Sansberro et al. (2001). The protocol consisted of soaking the fruits in 70% ethanol for 5 min, followed by immersion in 1.8% sodium hypochlorite solution containing two drops of Triton X-100® (Merck, Darmstadt, Germany) for 30 min, and then washing them three times with sterilized water. Rudimentary embryos (0.18-0.26 mm in length) at the heart developmental stage (Fig. 1A) were aseptically removed under a stereomicroscope under the laminar flow and were used in the cryopreservation experiments.

FIGURE 1. Plant regeneration from cryopreserved zygotic embryos of Ilex paraguariensis. Vertical bars represent 1mm (A and B), 1 cm (C) and 10 cm (D). A. seed showing a zygotic embryo at the heart developmental stage (arrow). B. germination of an encapsulated embryo. C. regenerated seedling. D. plants transferred to soil.
Preculture
Preculture of excised embryos consisted of a 7-day culture in the dark, at 27 ± 2ºC, on solidified (0.8% agar) 1/4MS medium [consisting of quarter-strength salts and vitamins of Murashige and Skoog (1962) medium] with 3% sucrose and 0.1 mg/l zeatin.
Experiment 1
Cryopreservation protocol for Ilex paraguariensis
After preculture, embryos were encapsulated in 3% calcium alginate. For pretreatment, encapsulated embryos (beads approximately 4-5 mm in diameter) were transferred at 27 ± 2ºC with 24 h intervals in liquid medium supplemented with progressively increasing sucrose concentrations (0.5, 0.75, and 1 M). The beads were then dehydrated for 5 h with silicagel to 25% water content (fresh weight basis). Dried beads were placed in sterile 5 ml cryovials (10 beads/cryovial) and either plunged rapidly in liquid nitrogen were they were kept for 1 h (rapid cooling) or cooled at 1ºC min-1 to -30ºC (using a Controller Rate Freezing System, Gordiner Electronics, Inc., USA) and then immersed in liquid nitrogen for 1 h (slow cooling). The beads were rewarmed by immersing the cryotubes in a water bath thermostated at 30ºC for 1 min. Finally, the beads were transferred to culture medium (1/4MS, 3% sucrose, 0.1 mg/l zeatin, solidified with 0.8% agar) and incubated in a growth room at 27 ± 2ºC under a 14 h light (116 μmol. m-2.s-1)/10 h dark photoperiod.
Experiment 2
Cryopreservation protocol for Ilex spp.
Based on the results obtained in experiment 1, other experiments were performed using seven additional Ilex species. Both procedures, slow and rapid cooling, were tested.
Survival assessment and statistical analysis of results
Survival of the embryos was evaluated 60 days after freezing by counting the number of embryos that developed into plantlets. Experiments were performed with 10 embryos/treatment and replicated three times. The mean and standard error for each treatment are summarized in Tables 1 and 2. Data were analyzed by analysis of variance (ANOVA) using Tukey's Multiple Comparison test (p<0.05) for data comparison.
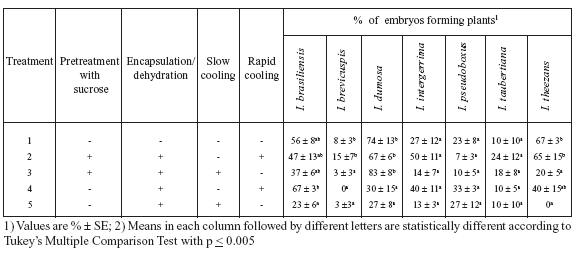
TABLE 1. Effect of cryopreservation by encapsulation/dehydration of Ilex paraguariensis rudimentary embryos on their in vitro germination after 60 days in culture on nutrient medium (1/4MS, 3% sucrose and 0.1 mg/l zeatin).

TABLE 2. Effect of cryopreservation by encapsulation/dehydration of Ilex spp. rudimentary embryos on their in vitro germination after 60 days culture on nutrient medium (1/4MS, 3% sucrose, and 0.1 mg/l zeatin)
Results
Experiment 1.
The results of cryopreservation of Ilex paraguariensis embryos (Table 1) permit the following five points to be made:
1) Excision and in vitro culture had no detrimental effect on the embryos because 50% of them germinated and developed into plants (Treatment 1). This percentage is similar to that reported by Sansberro et al. (1998) with the culture of non-treated embryos. Similarly, encapsulation of the embryos (Treatment 2) did not have inhibitory effects on the percentage of embryos that formed plants.
2) The survival of control (non-frozen) embryos ranged between 33 and 50%, while, after freezing, survival of embryos ranged between 0 and 67%.
3) After freezing, no survival (Treatments 6 and 7) or only 7% survival (Treatments 10 and 11) was achieved with nondehydrated embryos, while, with dehydrated embryos, as much as 67% of the embryos formed plants (Treatment 9).
4) Pretreatment with sucrose did not improve germination of frozen embryos.
5) If frozen embryos had been previously dehydrated, none of the cooling procedures (slow and rapid freezing, Treatments 8 and 9) induced significant differences in the percentage of embryos that formed plants.
As a consequence of the results, shown in Table 1, the procedure selected for cryopreserving Ilex paraguariensis zygotic embryos comprised: encapsulation in calcium alginate beads followed by dehydration of beads to 25% water content (fresh weight basis) and direct cooling in liquid nitrogen. With this treatment, after 1-2 weeks of reculture the cryopreserved embryos germinated (Fig. 1B) and the plants obtained (Fig. 1C) were successfully transferred to soil (Fig. 1D).
Experiment 2.
The results obtained (Table 2) with cryopreserved (following encapsulation and dehydration) embryos of seven Ilex species permit the following five points to be made:
1) Most of the treatments employed, independent of the species, permitted that plants to recover when embryos were recultured.
2) Although cryopreserved embryos of all Ilex species tested formed plants when recultured, the maximum values varied depending on the species and ranged between 15% (I. brevicuspis) and 83% (I. dumosa).
3) In all Ilex species tested, the results achieved with the best treatments were not significantly different from the results obtained with non-frozen controls.
4) The sucrose pretreatment, independent of the cooling procedure (slow or rapid), was ineffective with the exception of I .brevicuspis and I. dumosa, for which sucrose pretreatment significantly increased the percentage of embryos developing into plants.
5)With five out of the seven Ilex species tested, the results did not show significant differences when slow or rapid cooling was employed. However, with I. brasiliensis, rapid cooling of non sucrose-pretreated embryos allowed for better plant recovery than slow cooling. Similarly, in I. theezans, rapid cooling of sucrose-pretreated embryos led to the highest percentage of embryos forming into plants when compared with slow cooling.
Discussion
In this paper, we report, for the first time, the recovery of whole plants from zygotic embryos of eight subtropical Ilex species (including the economically most important Ilex paraguariensis) after cryopreservation. It is interesting to note that the percentages of embryos forming into plants that were obtained in this work were superior to the percentages obtained by Mroginski et al. (2006). In this study, zygotic embryos of eight Ilex species extracted from fruits directly frozen in liquid nitrogen (rapid cooling) did not regenerate plants. Plants were obtained only with five Ilex species (with recovery percentages between 3 to 23% depending on the species) when embryos were extracted from fruits frozen slowly in liquid nitrogen (slow cooling).
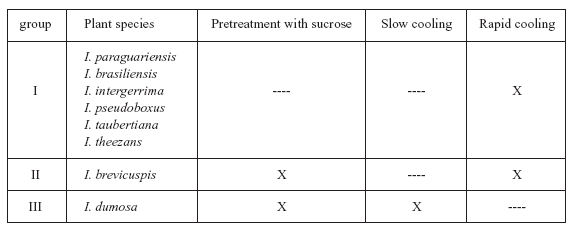
The optimal cryopreservation procedure we recommend varies depending on the species (Table 3). With Ilex paraguariensis, although the results obtained with rapid cooling did not differ significantly from those obtained with slow cooling, we recommend the use of rapid cooling because it is simpler than slow cooling. For the same reason, we recommend rapid cooling without sucrose pretreatment with for I. brasiliensis, I. pseudoboxus, I. taubertiana, I. theezans and I. intergerrima. However, for I. brevicuspis, we recommend rapid cooling with sucrose pretreatment. Finally, for I. dumosa, the best plant regeneration percentage was obtained when embryos that were pretreated with sucrose and then cooled slowly. Several reports have shown that differences are often observed between species and between varieties or clones of the same species in their behavior when different explants are cryopreserved (Reed, 1993, 2000; Lambardi et al., 2004).
TABLE 3. Procedures recommended for cryopreservation of encapsulated and dehydrated zygotic embryos of eight Ilex plant species (X= procedure recomended).
Dehydration of zygotic embryos before freezing is fundamental for successfully using this technique. These results are consistent with those obtained for other plant species such as Melia azedarach (Scocchi et al., 2004) and Oncidium bifolium (Flachsland et al., 2006). On the other hand, sucrose pretreatment, which proved effective for numerous tropical plant species (Takagi, 2000), was necessary only for I. brevicuspis and I. dumosa. These results demonstrate that immature zygotic embryos of all the Ilex species tested can still express their plant regeneration capacity after freezing in liquid nitrogen. Cryopreservation can therefore be considered a potential technique for long-term germplasm storage to complement field germplasm preservation. However, survival of some of the Ilex species tested (I. brevicuspis, I. taubertiana, and I. pseudoboxus) in this study was still very low. Higher recovery percentages after freezing should be achieved in order to envisage using cryopreservation for the long-term storage of Ilex spp. germplasm. In all the Ilex species employed in this study, the highest recovery percentages obtained with cryopreserved embryos were not significantly different from those obtained with control embryos. Improvements in the recovery percentage of in vitro cultured Ilex embryos might be achieved by selecting the optimal developmental stage of the embryos and/or by modifying the culture conditions (composition of culture medium, environmental conditions).
Acknowledgments
This work was partially supported by ANPCyT and CONICET.
References
1. Engelmann F (1991). In vitro conservation of tropical plant germplasm -a review. Euphytica 57: 227-243. [ Links ]
2. Engelmann F (2000). Importance of cryopreservation for the conservation of plant genetic resources. In: Cryopreservation of tropical plant germplasm. F. Engelmann and H. Takagi, Eds. IPGRI, Rome, pp. 8-20. [ Links ]
3. Filip R, López P, Coussio J, Ferraro G (1998). Mate substitutes or adulterants; study of xanthine contain. Phytoterapy Res. 12: 129-131. [ Links ]
4. Flachsland E, Terada G, Scocchi A, Rey H, Mroginski L, Engelmann F (2006). Cryopreservation of seeds and in vitro cultured protocorms of Oncidium bifolium Sims. (Orchidaceae) by encapsulation/dehydration. CryoLetters 27: 235-242. [ Links ]
5. Fontana HP, Prat Krikum SD, Belingheri LD (1990). Estudios sobre la germinación y conservación de semillas de yerba mate (Ilex paraguariensis St. Hil.). Informe Técnico 52, EEA INTA, Cerro Azul (Misiones), Argentina. pp. 14. [ Links ]
6. Giberti GC (1995). Ilex en Sudamérica: Florística, sistemática y potencialidades con relación a un banco de germoplasma para la yerba mate. In: Erva-Mate: Biología e Cultura no Cono Sul. H. Winge, A. G. Ferreira, J. E. de Araujo Mariíta and L. Tarasconi, Eds. UFRGS, Brazil, pp. 303-312. [ Links ]
7. Hu CY (1975). In vitro culture of rudimentary embryos of eleven Ilex species. J Amer Soc Hort Sci. 100: 221-225. [ Links ]
8. Hu CY (1976). Light-mediated inhibition of in vitro development of rudimentary embryos of Ilex opaca. Am J Bot. 63: 651-656. [ Links ]
9. Hu CY (1989). Holly ( Ilex spp.). In: Biotechnology in Agriculture and Forestry. 5, Trees II Y.P.S Bajaj, Ed. Springer, Verlag, Berlin. pp. 412-427. [ Links ]
10. Lambardi M, De Carlo A, Biricolti S, Puglia AM, Lombardo G, Siracusa M, Di Pasquale F (2004). Zygotic and nucellar embryo survival following dehydration/cryopreservation of Citrus intact seeds. CryoLetters 25: 81-90. [ Links ]
11. Loizeau PA (1994). Les Aquifoliaceaes péruviennes. Boissiera 48: 1-306. [ Links ]
12. Mroginski LA, Sansberro PA, Scocchi AM, Luna CV, Rey HY (2006). Effect of fruit cryopreservation on in vitro germination of zygotic embryos of several species of Ilex. Acta Horticulturae 725: 417-420. [ Links ]
13. Murashige T, Skoog F (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 15: 473-497. [ Links ]
14. Niklas CO (1987). Estudios embriológicos y citológicos en la yerba mate Ilex paraguariensis (Aquifoliaceae). Bonplandia 6: 45-56. [ Links ]
15. Reed BM (1993). Responses to ABA and cold acclimation are genotype dependent for cryopreserved blackberry and raspberry meristems. Cryobiology 30: 179-184. [ Links ]
16. Reed BM (2000). Genotype consideration in temperate fruit crop cryopreservation. In: Cryopreservation of tropical plant germplasm. F. Engelmann and H. Takagi, Eds. IPGRI, Rome, pp. 200-204. [ Links ]
17. Roberts EH (1973). Predicting the storage life of seeds. Seed Sci Technol. 1: 499-514. [ Links ]
18. Sansberro PA, Rey H, Mroginski LA, Collavino MM (1998). In vitro culture of rudimentary embryos of Ilex paraguariensis: Responses to exogenous cytokinins. J Plant Growth Regul. 17: 101-105. [ Links ]
19. Sansberro PA, Rey HY, Mroginski LA (2001). In vitro culture of zygotic embryos of Ilex species. HortScience 36: 351-352. [ Links ]
20. Scocchi AM, Faloci M, Medina R, Olmos S, Mroginski L (2004). Plant recovery of cryopreserved apical meristemtips of Melia azedarach L. using encapsulation/dehydration and assessment of their genetic stability. Euphytica 135: 29-38. [ Links ]
21. Scocchi AM, Rey HY (2004). Conservación de germoplasma in vitro. In: Biotecnología y Mejoramiento Vegetal. V. Echenique, C. Rubinstein and L. Mroginski, Eds. INTA, Buenos Aires, Argentina. pp. 179-185. [ Links ]
22. Takagi H (2000). Recent developments in cryopreservation of shoots apices of tropical species. In: Cryopreservation of tropical plant germplasm. F. Engelmann and H. Takagi, Eds. IPGRI, Rome. pp. 178-193. [ Links ]
23. Withers LA, Engelmann F (1998). In vitro conservation of plant genetic resources. In: Agricultural Biotechnology. A. Altman, Ed., Marcel Dekker Inc. New York. pp. 57-88. [ Links ]
Received on January 1, 2007.
Accepted on October 23, 2007.














