Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Agriscientia
versión On-line ISSN 1668-298X
Agriscientia vol.39 no.1 Córdoba jun. 2022
Artículos
Fractions of laurel essential oil obtained by molecular distillation with greater antioxi-dant and antimicrobial activities
Fracciones de aceite esencial de laurel obtenidas por destilación molecular con mayor actividad antioxidante y antimicrobiana.
A. J Lambir Jacobo 1 *
M. E. Carezzano 1
P R. Quiroga 1
N. R. Grosso 1
1 Lambir Jacobo, A. J. (ORCID: 0000-0002-3087-6885): Universidad Nacional de Córdoba, Facultad de Ciencias Agropecuarias. Félix Aldo Marrone 746, Ciudad Universitaria, 5000, Córdoba, Argentina. Carez-zano, M. E. (ORCID: 0000-0002-3442-996X): Universidad Nacional de Río Cuarto, Facultad de Ciencias Exactas, Fisicoquímicas y Naturales, Departamento de Microbiología e Inmunología. Ruta Nacional 36 - Km 601, 5804, Río Cuarto, Córdoba, Argentina. Quiroga, P R. (ORCID: 0000-0002-6341-5233): Universidad Nacional de Córdoba, Facultad de Ciencias Agropecuarias y Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Instituto Multidisciplinario de Biología Vegetal (IMBIV). Grosso, N R. (ORCID: 0000-0002-1709-9671): Universidad Nacional de Córdoba, Facultad de Ciencias Agropecuarias y Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Instituto Multidisciplinario de Biología Vegetal (IMBIV).
* Correspondencia a: judithlj@agro.unc.edu.ar
Fecha de recepción: 12/11/2021
fecha de aceptación: 05/04/2022
DOI: 10.31047/1668.298x.v39.n1.35407
SUMMARY
This study aimed to analyze the chemical composition and the antioxidant and antimicrobial activities of Laurus nobilis L. essential oil (LEO) and its fractions obtained by short-path molecular distillation. According to the chemical composition, it can be said that LEO and its fractions proved to have antioxidant activity since both have a high content of total phenolic content (TPC). Short-path molecular distillation was used to separate essential oil fractions with superior antioxidant activity. Laurel residue (LR) exhibited the greatest antioxidant activity, with higher values of trolox equivalent antioxidant capacity with ABTS radical cation (TEAC-ABTS) assay and TPC. In addition, LR had the lowest value of IC50-DPPH. For antimicrobial activity, all natural products tested had an effect on all foodborne pathogenic microorganisms. LEO, as well as its fractions, showed antimicrobial, bacteriostatic, or bactericidal activity against Gram-positive and Gram-negative bacteria. The LEO and its fractions obtained by molecular distillation can be used as antimicrobials and as food preservatives to prevent oxidation. Also, consumers considered the addition of LEO or its fractions in food products as positive.
Keywords: Laurus nobilis, hydrodistillation, natural products, food preservatives.
RESUMEN
Este estudio tuvo como objetivo analizar la composición química, la actividad antioxidante y antimicrobiana del aceite esencial de Laurus nobilis L. (AEL), y sus fracciones obtenidas por destilación molecular de camino corto. De acuerdo con la composición química, puede decirse que el AEL y sus fracciones tienen actividad antioxidante, ya que poseen un alto contenido de fenoles total (FT). La destilación molecular de camino corto se utiliza para separar las fracciones de aceite esencial con mayor actividad antioxidante que el original. El residuo de laurel (RL) exhibió la mayor actividad antioxidante, con valores más altos para los ensayos de la capacidad antloxldante equivalente a trolox con el radical catión ABTS (TEAC-ABTS) y FT. Además, RL tuvo el valor más bajo de IC50-DPPH. Para la actividad antimicrobiana, todos los productos naturales probados ejercieron una acción sobre todos los microorganismos patógenos utilizados. El AEL, así como sus fracciones, mostraron actividad antimicrobiana, bacteriostática o bactericida frente a bacterias Gram positivas y Gram negativas. El AEL y sus fracciones obtenidas por destilación molecular se pueden utilizar como conservantes de alimentos con funciones antimicrobianas y para prevenir oxidaciones. Asimismo, los consumidores consideraron positiva la adición de AEL y sus fracciones en productos alimenticios.
Palabras clave: Laurus nobilis, hidrodestilación, productos naturales, conservantes alimentarios.
INTRODUCTION
The use of natural products as functional ingredients in foods and beverages is increasing as consumers look for these kinds of products in order to avoid synthetic additives (Soubra et al. 2007). There is a growing interest in antioxidants and antimicrobials, particularly in those expected to prevent the alleged harmful effects of free radicals in the human body and the deterioration of fats and other food components due to spoilage microorganisms. In all cases, there is a preference for those from natural sources rather than synthetic ones (Hamdo et al. 2014). Many natural antioxidants have been proven to be effective in lipid-rich foods. Essential oils (EOs) are possible alternatives to use in foods because they can inhibit or decrease microbial contamination and oxidation processes. Attention in EOs and their components is increasing since it is believed that natural products are not dangerous to human health and have functional properties (Cohen et al., 2021). Some researchers have reported the antioxidant activity of oregano (Quiroga et al., 2013; Asensio et al., 2015) and poleo EOs (Quiroga et al., 2013) and the antimicrobial activity of thyme and suico EOs (Prieto et al., 2020).
There are few cases of direct application of EOs in food (Quiroga et al., 2011, 2013; Asensio et al., 2014; Olmedo and Grosso, 2019) showing both antioxidant and antimicrobial activities. In these reports, EOs showed a lower activity than synthetic preservatives (Quiroga et al., 2013, 2015; Asensio et al., 2015; Riveros et al., 2016). In some cases, a strong preservative activity of EOs is achieved when they are used in high concentrations compared to synthetic ones, which implies a sensory effect that is often not desired in certain products. For this reason, an EO fraction enriched in certain molecules could have greater antimicrobial activity with respect to pure EO (Rocha-Guzmán et al., 2007; Borgarello et al., 2015; Mezza et al., 2018). The use of short-path molecular distillation technology is an alternative to separate fractions and concentrate different chemical compounds of EO (Borgarello et al., 2015, Mezza et al., 2018). There is evidence that oregano EO fractions separated by short-path molecular distillation increased their antioxidant activity (Olmedo et al., 2014). A fraction enriched with molecules with greater antioxidant power can provide a greater effect, using lower doses than the EO from which it is derived. The Laurus nobllls (laurel) (LEO) has been demonstrated to have antioxidant activity (Mello da Silveira et al., 2012; Olmedo et al., 2015). The objective of this study was to separate fractions from LEO by short-path molecular distillation and to analyze their chemical composition and antioxidant and antimicrobial activities.
MATERIALS AND METHODS
Materials
Laurel essential oil (LEO) was purchased in a local market (Mendoza, Argentina). Bacterial strains Salmonella sp. (ATCC 700623), Escherichia coli O157H7 (ATCC 43895), Micrococcus luteus (ATCC 9341), Staphylococcus aureus (ATCC 25212), Enterococcus faecalis (ATCC 29212) and Bacillus cereus (ATCC 11778) ceded by the Food Microbiology and General Microbiology Laboratories, Faculty of Exact, Physicochemical and Natural Sciences, National University of Río Cuarto.
Short path molecular distillation
The LEO was distilled with a short path molecular distillation (SPMD) apparatus following the method described by Olmedo et al. (2014), then it was placed in the reception chamber (capacity, 400 mL). The distillation conditions were at 20 mbar, 26 °C for evaporation, 2.5 °C for condensation and 1.18 mL/min for flow. After this distillation, two fractions called Distillate (LD) and Residue (LR) were obtained.
Chemical analysis of the essential oil and its fractions
Gas Chromatography
The chemical composition of the LEO and its fractions were determined by CG-MS using a Perkin Elmer gas chromatograph (Clarus 600, Waltham, USA) coupled with a mass spectrometry detector (MSD) with a capillary column DB-5 (30 m, 0.25 mm i.d., 0.25 mm coating thickness). The temperature programme was the 60 °C for 0 min at 5 °C/min rate and 200 °C for final temperature. The injector was held at 250 °C. Helium was used as the carrier gas with a flow rate of 0.9 mL/min. Ionisation was induced by electron impact at 70 eV The compounds from LEO were identified by comparing their retention index, retention time and mass spectra with published data (Olmedo et al., 2015), NIST library (Shen et al., 2017) and Adams (1989). The Co-injection of Authentic standards (Sigma, St. Louis, USA) was also used for identification of the main components. The quantitative composition was obtained by peak area normalization, and the response factor for each component was considered to equal 1.
Antioxidant activity
Free-radical scavenging activity on DPPH
The free-radical scavenging activity of the LEO was determined using DPPH (Aldrich, Milwaukee, USA) according to Quiroga et al. (2011). Tested natural products solutions (100 pl) were added in 900 pl of DPPH (0.05 mM) and kept in dark for 30 min. The absorbance of the samples was measured on a spectrophotometer (Perkin Elmer, Lambda 1A, UV/VIS spectrophotometer, PerkinElmer Inc., Waltham, USA) at 517 nm. The inhibition percentage of the DPPH radical was calculated according to the following formula: % DPPH inhibition = (1-(A-Ab)/Ao)x100.
Where A is the absorbance of DPPH solution with essential oils, Ab is the absorbance of 60 % methanol with the natural products, and Ao is the absorbance of DPPH solution.
The inhibitory concentration 50 % (IC50) was calculated from the curve obtained by plotting the percentage of inhibition versus the final natural products concentrations (Quiroga et al., 2011).
Trolox equivalent antioxidant capacity (TEAC-ABTS assay)
For this assay, 10 pL LEO or their fractions were added to 990 pL of ABTS radical cation. The absorbance at 744 nm at room temperature (22 °C) was recorded after 25 min. Trolox (SIGMA ® St. Louis, USA) was used as an external standard. The antioxidant capacity of natural products was expressed as Trolox equivalent antioxidant capacity (TEAC) and TEAC was expressed as mg Trolox/mg sample (Asensio et al., 2015).
Total phenolic content (TPC)
TPC was determined by the Folin-Ciocalteu method measured at 760 nm in a spectrophotometer (Grosso et al., 2018). The concentration was calculated using gallic acid as standard. Phenolic content was expressed as milligrams of gallic acid equivalents per g sample (GAE/g sample)
Accelerated oxidation test
Canola oil (CA) (Krol, Amerika 2001 SA, Entre Ríos, Argentina) was used for an oven test experiment. The following samples were prepared: CO added with 0.02 % LEO (CO-LEO), 0.02 % LD (CO-LD) and 0.02 % LR (CO -LR) and 0.02 % Butylhydroxytoluene (CO-BHT) as the reference antioxidant. CO without antioxidants was used as control sample (CO-C). The samples were stored during 50 days at 45 °C in an oven and removed from storage at days 0, 14, 21 and 50. Two Chemical indicators of lipid oxidation were evaluated in the samples: peroxide value (PV) and conjugated dienes (CD). The PV was expressed as miliequivalents of active oxygen per kilogram of oil (mEq O2 / Kg oil) (Horwitz, 2010). Conjugated dienes were determined according to the COI method by reading the absorbance at 232 nm and the results were reported as the sample extinction coefficient E (%-1 cm-1) (COI, 2001).
Antimicrobial activity of the essential oil and its fractions
Disk diffusion technique
Bacterial inoculums were placed in petri plates containing Müeller-Hinton Agar (MHA) and incubated for 18 h. Each sample (10 pL) was impregnated in a filter paper disc placed in petri plates previously sown with microorganism. The plates were incubated at 37 °C for 24 h. Then, the inhibition halos were measured (Demo et al., 2005).
Determination of the Minimum Inhibitory Con-centration (MIC)
The antimicrobial activity of LEO and its fraction was determined using the broth microdilution method described in Mann and Markham (1998) with modifications. The EO samples were diluted in factor two with a dimethyl sulphoxide (DMSO). The inoculum used was a microbial culture in Müeller-Hinton Broth (MHB) with 0.15 % agar with a cell density required to reduce the redox resazurin indicator (Sigma-Aldrich, St. Louis, USA). The assay was performed in a sterile 96-well microtiter plate. Serial 10-fold dilutions of the overnight culture were prepared in MHB, and 170 pL of each dilution were dispensed into microtiter plates. The microtiter plates were incubated for 24 h at 37 °C and the appropriate dilution unable to reduce resazurin (blue) was chosen for the antimicrobial assays. To determine minimum inhibitory concentration (MIC), two trays were prepared for each strain and incubated at 37 °C for 3.5 h (Carezzano et al., 2017). After incubation, a 10 pL resazurin solution was added to all wells and incubated again for 2 h at 37 °C (Mann and Markham, 1998).
Minimum Bactericidal Concentration (MBC)
To perform the MBC, petri plates with MHA were used, which were inoculated with 100 pL of those dilutions that presented blue color. They were incubated at 37 °C for 24 h. The Minimum bactericidal concentration (MBC) was considered as the last dilution that did not show cellular growth (Asensio et al., 2014).
Sensory analysis
For the sensory analysis, an affective test was run. Consumer panelists residing in Córdoba (Argentina) participated for acceptance analysis. The trial was conducted with 92 panelists between 20 and 60 years old. For the evaluation, 3 mL fresh canola oil samples (CO-C; CO-LEO; CO-LD and CO-LR) were placed on a 3 x 3 cm white bread piece. The samples were coded with 3-digit numbers. A glass of water and a paper napkin in a plastic tray were also presented with the samples. Panelists were instructed to consume the entire sample and rinse their mouths with water between samples to minimize any residual effects. A nine-point hedonic scale was used where 1 = Dislike extremely, up to 9 = like extremely (Peryam and Pilgrim, 1957).
Statistical analysis
All analyses were carried out in three repetitions. Data were analyzed using the InfoStat software, version 2018 (Di Rienzo et al., 2018). Means and standard deviations were calculated. ANOVA and FisherLSD test (a = 0.05) were used to find significant differences among means.
RESULTS AND DISCUSSION
Chemical analysis of the essential oil and its fractions
Gas Chromatography
The chemical compositions of the studied
LEO and its fractions obtained by short-path molecular distillation are shown in the Table 1. Only components with concentrations > 0.5 % are reported.
The major components for LEO were 1,8 cineole (37.5 %), a-terpinyl acetate (12.37 %), linalool (8.71 %) and sabinene (7.07 %); for the LD fraction,1,8 cineole (47.56 %), linalool 9.85 %), a-terpinyl acetate (12.37 %) and sabinene (7.45 %); for the LR fraction, a-terpinyl acetate (23.2 %), 1,8 cineole 19.92 %), linalool (13.15 %) and eugenol methyl ether (8.45 %). Other authors reported 1,8 cineole as the main component of LEO. They also found a-terpinyl acetate among the first four main components. Flamini et al. (2007) observed high percentages of 1,8 cineole (37.5 %) followed by trans-sabinene hydrate (9.7 %) and a-terpinyl acetate (9.3 %). Mello da Silveira et al. (2014) found 1,8 cineole (35.50 %) and linalool (14.10 %) as major components of LEO, followed by lower proportions of a-terpinyl acetate (9.65 %) and sabinene (9.45 %). Taban et al. (2018) informed that the major components of the LEO obtained from the different extraction methods were eucalyptol (1,8 cineole) (34.37 - 50.07 %), a-terpinyl acetate (14.93 - 18.78 %), terpineol (4.72 - 6.02 %) and sabinene (4.95 - 5.93 %). Olmedo et al. (2014) reported 1,8 cineole (42.1 %) as mayor component in LEO and a-terpinyl acetate (9.3 %), they also found linalool (11.9 %) between the main components in this study. Agreeing with Taban et al. (2018), LEO and its fractions are rich in oxygenated monoterpenes (such as 1,8 cineole and a-terpinyl acetate).
The differences found between the LEO and its fractions were mainly due to the difference in the molecular weight. In the LR there was an increase in terpenes with an alcohol function, such as linalool, terpinen-4-ol, a-terpineol, eugenol methyl ether, eugenol and a-terpineol acetate. These components were present in a minor proportion in LD. There were increased concentrations of terpenes, such as a-pinene, p-pinene, eucalyptol and sabinene in LD. Differences in the compound proportions were observed in the fractions due to the molecular distillation process that increased the concentrations of compounds with low boiling points (e.g. monoterpenes) in LD. On the other hand, compounds with a high boiling point (such as molecules with functional groups and sesquiterpenes) were mainly found in LR (Olmedo et al., 2014).

Table 1: Terpenoid concentrations (relative percentage) analyzed by GC-MS analysis from laurel essential oils and its distilled and residue fractions according to their elution order
Antioxidant activity
Free-radical scavenging activity on DPPH
Essential oils from laurel have shown free-radlcal scavenging properties (Sacchetti et al., 2005; Olmedo et al., 2014, 2015; Olmedo and Grosso, 2019).
The DPPH-IC50 values of LEO and its fractions are shown in Table 2. LD and LR presented greater DPPH radical scavenging activity than the LEO. LR exhibited the best antioxidant activity with the lowest value of DPPH-IC50.
The differences in antioxidant activity and/or free-radical scavenging properties in LEO from different studies could be associated with the extraction method and chemotypes (Chizzola et al., 2008). The phenolic chemotypes express a higher antioxidant capacity than the non-phenolic chemotypes (Goudjil et al., 2015). Kaurinovic et al. (2010) found an IC50 value of 161.83 pg/mL for LEO and Fernández et al. (2019) reported a DPPH-IC50 of 257 ± 12 pg/mL for this EO.
Trolox equivalent antioxidant capacity (TEAC assay)
The mg Trolox/g EO value is shown in Table 2. LR exhibited the greatest antioxidant activity with higher values inTEAC assay. El et al. (2014) reported TEAC values of 2.4 ± 0.09 LEO obtained for hydrodistillation.
Total phenolic content (TPC)
The TPC values are shown in Table 2. LR had almost double phenolic content than LEO and LD have about half the TPC than LEO. Olmedo et al. (2015) reported a lower TPC value for LEO (10.48 mg GAE/g), while Ouchikh et al. (2011) reported a higher value of TPC in leaf laurel acetone extract (20.94 ± 0.97 mg GAE/g).
The TPC is not explainable simply based on phenol molecules as several studies have shown that other reducing compounds can also reduce Folin reagents and thus may be incorrectly considered phenolic compounds in assays of this type (Lester et al., 2012; Nielsen, 2017). TPC has been reported to be directly associated with antioxidant activity (Quiroga et al., 2011,2013). The phenolics from vegetables present molecules with reducing capacity that are probably responsible for the antioxidant capacity of this EO.
Accelerated oxidation test
The chemical indicators (PV and CD) increased during storage in all canola oil samples. These indicators are shown in Figure1. As a consequence of the accelerated oxidation condition, high peroxide values were detected in the canola oil samples. The control samples (CO-C) and CO-LEO exhibited the highest peroxide value with respect to samples with the addition of LEO fractions (LD and LR) after 50 days of storage (Figure 1a). At the end of storage, significant differences were observed between the samples. The CO-BHT showed the lowest values for PV, followed by LR and LD. The CD (%-1 cm-1) values on storage day 50 were 7.40, 4.51, 7.55, 6.73, and 5.25 in CO-C, CO-BHT, COLEO, CO-LD, and CO-LR, respectively (Figure 1b). The CO-LD and CO-LR samples presented greater antioxidant activity than CO-LEO. In general, the CO-BHT sample exhibited lower CD values on storage day 14 than the samples with LEO or its fractions. Olmedo et al. (2015) showed that the volatile composition of the EO changed during the thermal stability study. The results of this study could be due to this and to the relationships among the different components in LEO and their fractions (Olmedo et al., 2015).
Table 2: DPPH-IC50, TEAC and TPC values for laurel essential oil (LEO) and distilled (LD) and residue fractions (LR)
| DPPH-IC50 (mg/mL) | TEAC (mg Trolox/g EO) | TPC | |
| LEO | 0.43 ± 0.01 c | 2.61 ± 0.12 b | 7.46 ± 0.25 b |
| LD | 0.33 ± 0.02 b | 1.28 ± 0.12 a | 4.10 ± 0.10 a |
| LR | 0.14 ± 0.01 a | 4.46 ± 0.36 c | 15.05 ± 0.82 c |
Values with different letters in the same column are significantly different (LSD- Fisher; a = 0.05; n = 3).
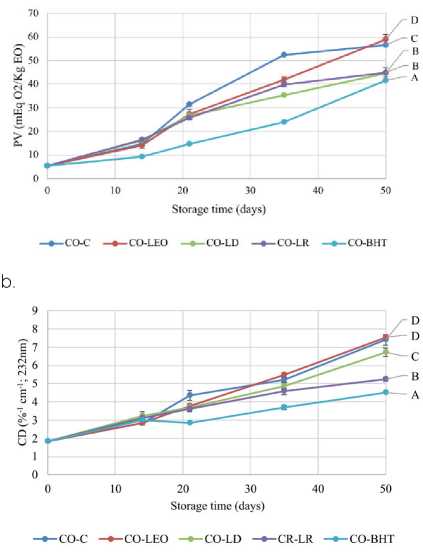
Figure 1: (a) Peroxide value (PV) and (b) conjugates dienes (CD) for canola oil control sample (CO-C) and canola oil samples added with laurel essential oil (CO-LEO) and distilled (CO-LD) and residue fractions (CO-LR) stored at 45 °C for 50 days. Different letters show statistically significant differences for the 50th day of storage (LSD- Fisher; a = 0.05; n = 3).
The antioxidant properties of EOs depend on several factors, such as the hydrophilic-lipophilic blend in a food product, the storage condition (temperature) and the chemical composition of the product. Before adding EO to a food product, it is essential to assay it to determine its real antioxidant capacity in that particular food.
Based on these results, it is observed that BHT, LEO and LEO fractions addition provides protection against the oxidation of canola oil. LR behaved better as antioxidant than LD, followed by LEO.

Table 2: DPPH-IC50, TEAC and TPC values for laurel essential oil (LEO) and distilled (LD) and residue fractions (LR)
Antimicrobial activity of the essential oil and its fractions
Disk diffusion technique
All natural products presented activity against all microorganisms assayed. LR had in general the highest values of inhibition halo, showing the best antimicrobial activity. This sample was followed by LD fraction and, finally LEO, which showed the lowest activity for this technique (Table 3). Some authors have shown the ability of laurel to inhibit the growth of microorganisms using the disk diffusion assay (Djenane et al., 2012; Zazharskyi et al., 2019).
Determination of the minimum inhibitory concentraron (MIC)
The MIC results are summarized in Table 4. LEO and these fractions showed activity not only against Gram-positive bacteria, but they also exhibited excellent activity against Gram-negative bacteria. Chmit et al. (2014) and Mello da Silveira et al. (2014) reported that LEO had inhibitory activity against these two types of bacteria.

Table 3: Inhibition halos for laurel essential oil (LEO) and distilled (LD) and residue fractions (LR)
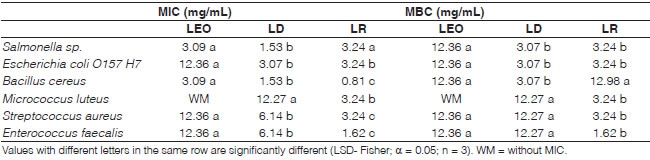
Table 4: Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) for laurel essential oil (LEO) and distilled (LD) and residue fractions (LR)
LR presented the best inhibitory activity with the lowest MIC values against B. cereus, M. luteus, S. aureus and E. faecalis and LD showed the best antimicrobial activity against Salmonella sp. and E. coli O157 H7. As it was stated above, LEO presented antimicrobial activity against all tested strains, but it was the lowest one.
Different results were reported by Chmit et al. (2014), who found that LEO has good antimicrobial activity against E. faecalis and P. aeruginosa but not against S. aureus and E. coli. According to the antimicrobial classification by Holetz et al. (2002), the results of the current study show LEO as a weak antimicrobial, LD as a moderate/high activity antimicrobial, and LR as a high activity antimicrobial.
Mínimum bactericida! concentration (MBC)
MBC results are summarized in Table 4. All natural products tested showed bactericidal activity against the assayed bacterial strains. LEO exhibited the lowest bactericide power, showing the higher MBC values for all strains tested. The LR fraction displayed the best antimicrobial activity against M. luteus, S. aureus and E. faecalis, and LD had the lowest MBC values for Salmonella sp., E. coli O157 H7 and B. cereus.
The antimicrobial or other biological activities of EOs have been thoroughly studied and there is a direct correlation between chemical composition and biological properties (Asensio et al., 2014, 2015, 2019). De Sousa et al. (2012) reported that 1,8-cinelole was effective in inhibiting Gram-positive and Gram-negative bacteria supporting the observations of other researchers about the efficacy of 1,8-cineole- in inhibiting the growth of gram-negative and gram-positive bacteria. Badr et al. (2021) found that a-terpinyl acetate and lavender EO containing a-terpinyl acetate in concentrations of 13.99 % had better antimicrobial activity against gram-positive than gram-negative bacteria; and a-terpinyl acetate had superior activity than lavender EO. Those results are in agreement with the results obtained for LR fraction, which has more quantity of a-terpinyl acetate than LEO and LD.
Sensory analyses
Acceptance and preference tests are subjective analysis concerning the degree of like or dislike of products by consumers. A consumer analysis was made in order to evaluate how the addition of LEO and its fractions on canola oil affects acceptability. For odor, flavor and overall acceptances, all canola oil samples showed means of consumer responses between 5 (neither like nor dislike) and 6.5 (like slightly) on a 9-point hedonic scale (Figure 2). Overall acceptance was high for LEO and all its fractions (hedonic scale point > 6).
The averages for flavor acceptance varied from 6.0 to 6.48 on a 9-point hedonic scale. Only between CO-LEO and CO-C there were significant statistical differences in flavor acceptances, which were higher in CO-LEO. Hence, consumers considered positive the addition of LEO. Samples added with natural products, did not exhibit significant differences in this attribute.
The means for odor acceptance varied from 5.50 to 6.22 on a 9-point hedonic scale and presented significant differences between samples. As in flavor attribute acceptance, odor acceptance of CO-LEO and CO-LR had no significant differences but, in this attribute, significant differences were found between CO-LEO and CO-LD samples. The differences in volatile composition of LEO and its fractions, when added to canola oil, resulted in variation in consumer odor acceptability. A volatile compound is seldom unique, and the common view is that characteristic odor will be represented by a blend of components, each having its own detection threshold and each present at different concentrations (Shahidi, 1998). The odor threshold is defined as the concentration where the odorant starts being perceived or as the lowest intensity at which a stimulus can be identified (Jele , 2012) and increases with increment of the volatility (Nagata, 2003; Bordiga and Nollet, 2019). In general, CO-LD had the lowest acceptance, which could be due to the fact that LD volatile composition has more volátiles compounds than LEO and LR, thus, LD odor threshold could be lower than LEO and consumers perceived LD odor stronger than LEO or LR.

Figure 2: Odor, flavor and overall consumers acceptance (9-point hedonic scale) for canola oil control sample (CO-C) and canola oil samples added with laurel essential oil (CO-LEO) and distilled (CO-LD) and residue fractions (CO-LR). Values with different letters are significantly different (LSD- Fisher; a = 0.05; n = 92).
Food sample may be considered unacceptable to the consumer when the acceptability has a value minor to 5 on a 9-point hedonic scale. Acceptability values higher than 5 mean positive acceptance for a food product. Values higher than 5 (5 = neither like nor dislike) were observed in all consumer acceptance attributes and in all samples; therefore, all samples with or without natural products addition were positively accepted by consumers.
CONCLUSION
According to the results observed in the present research, the antimicrobial and antioxidant activities and sensory acceptance of LEO, LD and LR are directly correlated with chemical composition.
LR fraction behaved as better antioxidant than the LD and LEO, with LEO having the lowest antioxidant activity. All natural products showed antimicrobial, inhibitory, or bactericidal activity against Gram-positive and Gram-negative bacteria. The bacteriostatic capacity is of great importance in the food industry since it inhibits the development of food contaminating microorganisms.
Canola oil added with LEO, LD and LR are accepted by consumers with LEO being the most accepted. The addition of these natural products to canola oil help to slow the lipid oxidation process during storage. The use of natural additives instead of synthetic ones is convergent with the present trend in food technology; for this reason, LEO, LD and LR constitute natural sources of antioxidant additives useful in the food industry for preserving quality properties in products with high lipid contents.
ACKNOWLEDGMENTS
This study was supported by Secretaría de Ciencia y Tecnología of the Universidad Nacional de Córdoba (SECyT-UNC), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Fondo para la Investigación Científica y Tecnológica (FONCYT), Facultad de Ciencias Agropecuarias of the Universidad Nacional de Córdoba, and the Laboratorio de Microbiología of the Universidad Nacional de Río Cuarto.
REFERENCES
Adams, R. P. (1989). Identification of Essential Oils by Ion Trap Mass Spectroscopy. Academic Press.
Asensio, C. M., Gallucci, N., de las Mercedes Oliva, M., Demo, M. S. and Grosso, N. R. (2014). Sensory and bio-chemical preservation of ricotta cheese using natural products. International Journal of Food Science and Technology, 49(12), 2692-2702. https:// doi.org/10.1111/ijfs.12604
Asensio, C. M., Grosso, N. R. and Juliani, R. H. (2015). Quality characters, chemical composition and biological activities of oregano (Origanum spp.) Essential oils from Central and Southern Argentina. Industrial Crops and Products, 63, 203-213. https:// doi.org/10.1016/j.indcrop.2014.09.056
Asensio, C. M., Quiroga, P. R., Huang, Q., Nepote, V. and Grosso, N. R. (2019). Fatty acids, volatile compounds and microbial quality preservation with an oregano nanoemulsion to extend the shelf life of hake (Merluccius hubbsi) burgers. International Journal of Food Science and Technology, 54(1), 149-160. https:// doi.org/10.1111/ijfs.13919
Badr, M. M., Badawy, M. E. I. and Taktak, N. E. M. (2021). Characterization, antimicrobial activity, and antioxidant activity of the nanoemulsions of Lavandula spica essential oil and its main monoterpenes. Journal of Drug Delivery Science and Technology, 65, 102732. https://doi.org/10.1016/jjddst.2021.102732
Bordiga, M. and Nollet, L. M. L. (Eds.). (2019). Food Aroma Evolution: During Food Processing, Cooking, and Aging. Taylor & Francis Group.
Borgarello, A. V., Mezza, G. N., Pramparo, M. C. and Gayol, M. F. (2015). Thymol enrichment from oregano essential oil by molecular distillation. Separation and Purification Technology, 153, 60-66. https://doi. org/10.1016/j.seppur.2015.08.035
Carezzano, M. E., Sotelo, J. P., Primo, E., Reinoso, E. B., Paletti Rovey, M. F., Demo, M. S., Giordano, W. F. and Oliva, M. de las M. (2017). Inhibitory effect of Thymus vulgaris and Origanum vulgare essential oils on virulence factors of phytopathogenic Pseudomonas syringae strains. Plant Biology, 19(4), 599-607. https:// doi.org/10.1111/plb.12572
Chizzola R., Michitsch H. and Franz C. (2008). Antioxidative Properties of Thymus vulgaris Leaves: Comparison of Different Extracts and Essential Oil Chemotypes. Journal Agriculture Food Chemistry, 56(16), 6897-6904. https://doi.org/10.1021/jf800617g
Chmit, M., Kanaan, H., Habib, J., Abbass, M., Mcheik, A. and Chokr, A. (2014). Antibacterial and antibiofilm activities of polysaccharides, essential oil, and fatty oil extracted from Laurus nobilis growing in Lebanon. Asian Pacific Journal of Tropical Medicine, 7(S1), S546-S552. https://doi.org/10.1016/S1995-7645(14)60288-1
Cohen, S. M., Eisenbrand, G., Fukushima, S., Gooderham, N. J., Guengerich, F. P., Hecht, S. S., Rietjens, I. M. C. M., Rosol, T. J., Davidsen, J. M., Harman, C. L., Lu, V. and Taylor, S. V. (2021). FEMA GRAS assessment of natural flavor complexes: Origanum oil, thyme oil and related phenol derivative-containing flavoring ingredients. Food and Chemical Toxlcology, 155, 112378. https://doi.org/10.1016ZJ.FCT2021.112378
de Sousa, J. P., de Azeredo, G. A., de Araújo Torres, R., da Silva Vasconcelos, M. A., da Conceigáo, M. L. and de Souza, E. L. (2012). Synergies of carvacrol and 1,8-cineole to inhibit bacteria associated with minimally processed vegetables. International Journal of Food Microblology, 154(3), 145-151. https://doi. org/10.1016/j.ijfoodmicro.2011.12.026
Demo, M., Oliva, M. de las M., López, M. L., Zunino, M. P and Zygadlo, J. A. (2005). Antimicrobial Activity of Essential Oils Obtained from Aromatic Plants of Argentina. Pharmaceutical Blology, 43(2), 129-134. https://doi.org/10.1080/13880200590919438
Di Rienzo, J. A., Casanoves F, Balzarini M. G., González L., Tablada M. and Robledo C. W. InfoStat (versión 2018) [Software]. Córdoba, Argentina: Grupo InfoStat, FCA, Universidad Nacional de Córdoba. http://www. infostat.com.ar
Djenane, D., Yangüela, J., Gómez, D. and Roncalés, P (2012). Perspectives on the use of essential oils as antimicrobials against Campylobacter jejuni CECT 7572 in retail chicken meats packaged in microaerobic atmosphere. Journal of Food Safety, 32(1), 37-47. https://doi.org/10.1111/j.1745-4565.2011.00342.x
El, S. N., Karagozlu, N., Karakaya, S. and Sahin, S. (2014). Antioxidant and Antimicrobial Activities of Essential Oils Extracted from Laurus nobilis L. Leaves by Using Solvent-Free Microwave and Hydrodistillation. Food and Nutrition Sciences, 05(02), 97-106. https://doi. org/10.4236/fns.2014.52013
Fernández, N. J., Damiani, N., Podaza, E. A., Martucci, J. F., Fasce, D., Quiroz, F, Meretta, P E., Quintana, S., Eguaras, M. J. and Gende, L. B. (2019). Laurus nobilis L. Extracts against Paenibacillus larvae: Antimicrobial activity, antioxidant capacity, hygienic behavior and colony strength. Saudl Journal of Blologlcal Sciences, 26(5), 906-912. https://doi.org/10.1016/j. sjbs.2018.04.008
Flamini, G., Tebano, M., Cioni, P L., Ceccarini, L., Ricci, A. S. and Longo, I. (2007). Comparison between the conventional method of extraction of essential oil of Laurus nobilis L. and a novel method which uses microwaves applied in situ, without resorting to an oven. Journal of Chromatography A, 1143(1-2), 3640. https://doi.org/10.1016/j.chroma.2007.01.031
Goudjil, M. B., Ladjel, S., Bencheikh, S. E., Zighmi, S. and Hamada, D. (2015). Study of the chemical composition, antibacterial and antioxidant activities of the essential oil extracted from the leaves of Algerian Laurus nobilis Lauraceae. Journal of Chemical and Pharmaceutical Research, 7(1), 379-385.
Grosso, A. L., Asensio, C. M., Nepote, V. and Grosso, N. R. (2018). Antioxidant Activity Displayed by Phenolic Compounds Obtained from Walnut Oil Cake Used for Walnut Oil Preservation. Journal of the American Oil Chemists' Society, 95(11), 1409-1419. https://doi. org/10.1002/aocs.12145
Hamdo, H. H., Khayata, W. and Al-Assaf, Z. (2014). The Antioxidant Activity of Tocotrienols Compared with Some Synthetic Antioxidant. Pharmacology & Pharmacy 5(7) 612-619. http://dx.doi.org/10.4236/ pp.2014.57071
Holetz, F B., Pessini, G. L., Sanches, N. R., Cortez Garcia, D. A., Nakamura, C. V. and Dias Filho, B. P. (2002). Screening of Some Plants Used in the Brazilian Folk Medicine for the Treatment of Infectious Diseases. Memorias Do Instituto Oswaldo Cruz, 97(7), 1027-1031. https://doi.org/10.1590/S0074-02762002000700017
Horwitz, W. (Ed.). (2010). Offlclal methods of analysls of AOAC International. Agricultural chemicals, contaminants, drugs. AOAC International, 1997. https:// repositorioinstitucional.ceu.es/handle/10637/3158
Jele, H. (2012). Food flavors: Chemlcal, sensory and technologicalproperties. Taylor & Francis Group.
Kaurinovic, B., Popovic, M. and Vlaisavljevic, S. (2010). In Vitro and in Vivo Effects of Laurus nobilis L. Leaf Extracts. Molecules, 15(5), 3378-3390. https://doi. org/10.3390/molecules15053378
Lester, G. E., Lewers, K. S., Medina, M. B. and Saftner, R. A. (2012). Comparative analysis of strawberry total phenolics via Fast Blue BB vs. Folin-Ciocalteu: Assay interference by ascorbic acid. Journal of Food Composition and Analysis, 27(1), 102-107. https://doi. org/10.1016/j.jfca.2012.05.003
Mann, C. M. and Markham, J. L. (1998). A new method for determining the minimum inhibitory concentration of essential oils. Journal of Applied Microbiology, 84(4), 538-544. https://doi.org/10.1046/j.1365-2672.1998.00379.x
Mello da Silveira, S., Luciano, F. B., Fronza, N., Cunha, A., Scheuermann, G. N. and Werneck Vieira, C. R. (2014). Chemical composition and antibacterial activity of Laurus nobilis essential oil towards foodborne pathogens and its application in fresh Tuscan sausage stored at 7°C. LWT - Food Sclence and Technology, 59(1), 86-93. https://doi.org/10.1016/j. lwt.2014.05.032
Mello da Silveira, S., Cunha Júnior, A., Scheuermann, G. N., Secchi, F. L. and Werneck Vieira, C. R. (2012). Chemical composition and antimicrobial activity of essential oils from selected herbs cultivated in the South of Brazil against food spoilage and foodborne pathogens. Ciencia Rural, 42(7), 1300-1306. https:// doi.org/10.1590/S0103-84782012000700026
Mezza, G. N., Borgarello, A. V., Grosso, N. R., Fernandez, H. , Pramparo, M. C. and Gayol, M. F. (2018). Antioxidant activity of rosemary essential oil fractions obtained by molecular distillation and their effect on oxidative stability of sunflower oil. Food Chemistry, 242, 9-15. https://doi.org/10.1016/J.F00DCHEM.2017.09.042
Nagata, Y. (2003). Odor measurement review, Measurement of Odor Threshold by Triangle Odor Bag Method. Ministery of Environmental Government of Japan, 18, 118-127. https://www.env.go.jp/en/air/odor/ measure/02_3_2.pdf
Nielsen, S. S. (2017). Food Analysis Laboratory Manual (5th ed.). Springer Nature.
Olmedo, R. H. and Grosso, N. R. (2019). Oxidative Stability, Affective and Descriptive Sensory Properties of Roasted Peanut Flavored with Oregano, Laurel, and Rosemary Essential Oils as Natural Preservatives of Food Lipids. European Journal of Lipid Science and Technology, 121(5), 1800428. https://doi.org/10.1002/ ejlt.201800428
Olmedo, R. H., Asensio, C. M. and Grosso, N. R. (2015). Thermal stability and antioxidant activity of essential oils from aromatic plants farmed in Argentina. Industrial Crops and Products, 69, 21-28. https://doi. org/10.1016/j.indcrop.2015.02.005
Olmedo, R., Nepote, V. and Grosso, N. R. (2014). Antioxidant activity of fractions from oregano essential oils obtained by molecular distillation. Food Chemistry, 156, 212-219. https://doi.org/10.1016/j. foodchem.2014.01.087
Ouchikh, O., Chahed, T., Ksouri, R., Taarit, M. Ben, Faleh, H., Abdelly, C., Kchouk, M. E. and Marzouk, B. (2011). The effects of extraction method on the measured tocopherol level and antioxidant activity of L. nobilis vegetative organs. Journal of Food Composition and Analysis, 24(1), 103-110. https://doi.org/10.1016/j. jfca.2010.04.006
Peryam, D. R. and Pilgrim, F. J. (1957). Hedonic scale method of measuring food preferences. Food Technology, 11, Suppl., 9-14. https://psycnet.apa.org/ record/1959-02766-001
Prieto, M. C., Lapaz, M. I., Lucini, E. I., Pianzzola, M. J., Grosso, N. R. and Asensio, C. M. (2020). Thyme and suico essential oils: promising natural tools for potato common scab control. Plant Biology, 22(1), 81-89. https://doi.org/10.1111/plb.13048
Quiroga, P. R., Asensio, C. M. and Nepote, V. (2015). Antioxidant effects of the monoterpenescarvacrol, thymol and sabinene hydrate on chemical and sensory stability of roasted sunflower seeds. Journal of the Science of Food and Agriculture, 95(3), 471-479. https://doi.org/10.1002/jsfa.6744 Quiroga, P R., Grosso, N. R and Nepote, V. (2013). Antioxidant Effect of Poleo and Oregano Essential Oil on Roasted Sunflower Seeds. Journal of Food Science, 78(12), S1904-S1012. https://doi.org/10.1111/1750-3841.12306
Quiroga, P R., Riveros, C. G., Zygadlo, J. A., Grosso, N. R. and Nepote, V. (2011). Antioxidant activity of essential oil of oregano species from Argentina in relation to their chemical composition. International Journal of Food Science and Technology, 46(12), 2648-2655. https://doi.org/10.1111/j.1365-2621.2011.02796.x Riveros, C. G., Nepote, V. and Grosso, N. R. (2016). Thyme and basil essential oils included in edible coatings as a natural preserving method of oilseed kernels. Journal of the Science of Food and Agriculture, 96(1), 183-191. https://doi.org/10.1002/jsfa.7080 Rocha-Guzmán, N. E., Gallegos-Infante, J. A., González-Laredo, R. F., Ramos-Gómez, M., Rodríguez-Muñoz, M. E., Reynoso-Camacho, R., Rocha-Uribe, A. and Roque-Rosales, M. R. (2007). Antioxidant effect of oregano (Lippiaberlandieri v. Shauer) essential oil and mother liquors. Food Chemistry, 102(1), 330-335. https://doi.org/10.1016/j.foodchem.2006.05.024 Sacchetti, G., Maietti, S., Muzzoli, M., Scaglianti, M., Manfredini, S., Radice, M. and Bruni, R. (2005). Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chemistry, 91(4), 621-632. https://doi.org/10.1016/J. FOODCHEM.2004.06.031
Shen, V. K., Siderius, D. W., Krekelberg, W. P, and Hatch,H. W. (Eds.) (2017) NIST Standard Reference Simulation Website, NIST Standard Reference Database Number 173, National Institute of Standards and Technology, Gaithersburg MD, 20899. http://doi. org/10.18434/T4M88Q
Shahidi, F. (1998). Indicators for evaluation of lipid oxidation and off-flavor development in food. Developments in Food Science, 40(C), 55-68. https:// doi.org/10.1016/S0167-4501(98)80032-0
Soubra, L., Sarkis, D., Hilan, C. and Verger, P (2007). Dietary exposure of children and teenagers to benzoates, sulphites, butylhydroxyanisol (BHA) and butylhydroxytoluen (BHT) in Beirut (Lebanon). Regulatory Toxicology and Pharmacology, 47(1), 6877. https://doi.org/10.1016Zj.yrtph.2006.07.005 Taban, A., Saharkhiz, M. J. and Niakousari, M. (2018). Sweet bay (Laurus nobilis L.) essential oil and its chemical composition, antioxidant activity and leaf micromorphology under different extraction methods. Sustainable Chemistry and Pharmacy, 9, 12-18. https://doi.Org/10.1016/j.scp.2018.05.001
Zazharskyi, V. V., Davydenko, P. O, Kulishenko, O. M, Borovik, I. V. and Brygadyrenko, V. V. (2019). Antimicrobial activity of 50 plant extracts. Biosystems Diversity, 27(2), 163-169. https://doi. org/10.15421/011922














