Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de Ciencia y Tecnología
versión On-line ISSN 1851-7587
Rev. cienc. tecnol. no.18 Posadas jul./dic. 2012
SALUD
Antifungal Formulation Based on Saturated Sucrose Solution: Evaluation of its potential genotoxic activity using the Allium cepa test
Formulación antifúngica basada en solución saturada de sacarosa: evaluación de su potencial actividad genotóxica empleando la prueba de Allium cepa
Gustavo A. Bich1, María C. Vedoya2, Martha G. Medvedeff1,2
1 Laboratorio de Microbiología e Inmunología
2 Laboratorio de Micología Universidad Nacional de Misiones UNaM - Av. Mariano Moreno 1375 (N3300LQH ) Posadas - Misiones - Argentina
. Gustavo Ángel Bich1: Licenciado en Genética, FCEQyN, UNaM. Becario doctoral CONICET-CEDIT. Auxiliar de primera Ad-Honorem cátedra de Microbiología e Inmunología, FCEQyN, UNaM.
. María Celina Vedoya2: Bioquímica. Laboratorista Químico Industrial. Mgter en Tecnología de los alimentos. Jefe de Trabajos Prácticos Cátedra de Micología (Departamento Microbiología) y Química Biológica (Departamento Química). Categoría III en Programa Nacional de Incentivos. Director y Codirector de Proyectos incentivados. Co director de proyectos de extensión FCEQyN (UNaM). Secretaria de la Asociación Argentina de Micología
. Martha Gladys Medvedeff: Bioquímica1,2. Especialista en Microbiología Clínica. Doctor en Ciencias Técnicas. Profesor adjunto regular Cátedra de Micología (Departamento Microbiología-Bioquímica) y Microbiología e Inmunología (Departamento Genética). Categoría II Programa Nacional de Incentivos. Director de Proyectos de investigación, Extensión y Servicios. Director Programa de Micología. Director y evaluador de tesis de grado y posgrado. Evaluador carreras de posgrado CONEAU. Presidente Asociación Argentina de Micología.
Abstract
In the present study, the potential genotoxicity effects of the antifungal formulation based on saturated sucrose solution were evaluated employing the Allium cepa test. Five exposure times were evaluated, mineral water was used as negative control, and experiments were carried out in duplicate. For statistical analysis, one-way analysis of variance (ANOVA) and Tukey HSD post-test were used (statistical significance a = 0.05). Frequencies of chromosomal abnormalities ranged from 0% to 0.24%. We conclude that the antifungal formulation based on sucrose saturated solution did not present a significant genotoxic activity, considering the results of the present study involving the Allium cepa test.
Key words: Saturated sucrose solution; Allium cepa test; Genotoxic activity.
Resumen
En el presente trabajo, los potenciales efectos genotóxicos de la formulación antifúngica basada en la solución saturada de sacarosa fueron evaluados utilizando la Prueba de Allium cepa. Se evaluaron por duplicado cinco tiempos de exposición empleando agua mineral como control. Para el análisis estadístico, se empleó un análisis de varianza (ANOVA) y una prueba a-posteriori de Tukey HSD (significación estadística a = 0,05). Las frecuencias de las anomalías cromosómicas observadas partieron desde 0% a 0,24% y el análisis estadístico de las frecuencias de anomalías cromosómicas reveló que no se detectaron diferencias estadísticamente significativas en relación con los controles. Se concluye que la formulación antifúngica en base a solución saturada de sacarosa no evidenció una actividad genotóxica al evaluarse los resultados del presente trabajo empleando la prueba de Allium cepa.
Palabras clave: Solución saturada de sacarosa; Prueba de Allium cepa; Actividad genotóxica.
1. Introduction
Superficial fungal infections are among the world's most common diseases [1]. However, it is important to call attention to the limited number of effective antifungal compounds available. The latter is because the toxicity of the available antifungal agents, the expression of resistance to commonly used antifungals by the microorganism and the high cost of antifungal agents [2, 3]. For these reasons, there is a constant need for new secure effective and not expensive antifungal agents.
The antifungal formulation based on saturated sucrose solution, additioned with polyethilenglycol 400 and eugenol it is a practical, cheap, and effective alternative drug to manage topically fungal opportunistic infections. Actually, this antifungal formulation has a broad-spectrum activity assayed against many pathogenic fungi in dermatomycosis [4, 5, 6, 7, 8]. While a wide number of studies on its in-vitro antifungal activity have been conducted, its safety related to potential genotoxic effects have not been tested yet. Besides genotoxicity tests are recommended to be conducted as part of product safety assessment.
Most of the toxicity testing systems rely on small animals, hence, are time consuming, very expensive, and attract considerable ethical criticism [9, 10]. Nevertheless, among the bioassays developed for detection of genotoxicity and cytotoxicity, plant systems have proven to be sensitive, cheap and effective [11, 12].
The use of plants for the toxicity assessment procedures is attributed mainly to the fact that these tests are relatively simple to perform, inexpensive, biologically sensitive, and rapid [9, 13]. In particular, the species Allium cepa presents several advantages, including low raising costs, easy handling, and suitable chromosomal features. This plant bears large and few chromosomes (2n = 16), which facilitates the evaluation of chromosome damages and/or disturbances in cell division cycle [14].
The aim of the present study was to evaluate the genotoxicity of the antifungal formulation based on sucrose saturated solution with the Allium cepa root tips test.
2. Materials and methods
2.1 Allium cepa test
The A. cepa genotoxicity test was carried out according to the method described by Fiskesjö [15], with slight modifications, for instance, approximately 12 cm diameter onion bulbs were used in this study, and the incubation temperature was 22ºC
White onion bulbs were obtained at a local market and chosen according to their size and appearance. The outer scales and old roots were removed carefully, and the bulbs were washed and kept in a refrigerator at 4 ºC until the start of the experiment. For each treatment, including the negative control (mineral water), five onion bulbs were used. The onion roots were left to grow to 1.5-2 cm in mineral water and then exposed to each treatment. They were placed in flasks filled with respective solution as far as the root growth region, and kept under laboratory conditions. Five different exposure times were evaluated (1, 4, 8, 12 and 24 hours). The onion bulbs of each treatment were subject to its respective exposure time.
At the end of each exposure time, root tips from these bulbs were cut and fixed in ethanol: glacial acetic acid (3:1, v/v) overnight and then stored in ethanol 70% at 4?C until use. Then the root tips were subjected to the Feulgen reaction. They were hydrolyzed in 1N HCL at 60?C for 10 minutes after that, they were washed in distilled water and stained in Schiff's reagent for 20 minutes. Two root tips were then squashed on each slide, stained with acetoorceine and put the coverslips carefully lowered on to exclude air bubble. These slides were analyzed at 1000x magnification. For each slide, a minimum of 100 cells in mitosis were examined for aberrations, and the frequencies of chromosomal abnormalities were determined. The chromosomal aberrations scored were stickiness and fragmentation of chromosomes, as well as anaphasic bridges.
2.2 Antifungal formulation based on saturated sucrose solution
The formulation under evaluation was made with a saturated sucrose solution (250 g of table sugar in 100 ml of mineral water), polyethilenglycol 400 (0,4 % v/v) (Merk - Merk Química Argentina) and eugenol (0,4 % v/v) (Dickinson-Lab. Dr Preston SRL, Argentina).
2.3 Statistical analysis
The root length data are given as the mean ± S.D. The mitotic index (%) was calculated as the number of dividing cells per 1000 observed cells at each treatment. The data were analyzed by a one-way ANOVA followed by the Tukey HSD (honestly significant difference) post-test. In all cases, selected threshold of statistical significance was p < 0.05.
Experiments were carried out in duplicate . And the analyses were computed using the statistical software program Statgraphics Centurion XV (StatPoint, Inc., 2006).
3. Results
In the present study, the genotoxicity of an antifungal formulation based on sucrose saturated solution was evaluated. The genotoxicity was estimated by the observation of changes in the percentage of chromosomal abnormalities of A. cepa L. meristematic root cells.
All the types of chromosomal aberrations considered for analysis, were observed in the treated root tip cells and record. Aberrant mitotic cells were counted and their ratio was expressed as a percentage. Table 1 shows the rate of mitotic abnormalities.
Table 1: Frequencies of chromosomal abnormalities for Allium cepa roots treated with the antifungal formulation.

Although we observed Chromosomal abnormalities almost in every treatment evaluated (always under 0,24%), we didn't observed a significant increase in the total number of abnormalities for all treatments compared with the controls (p > 0.05). The negative control also showed some mitotic abnormalities.
In Figure 1 are represented some anaphasic bridges, laggards and stickiness chromosomes found in our study.
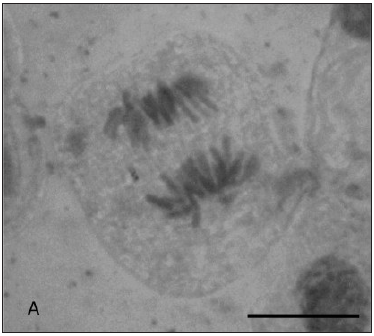
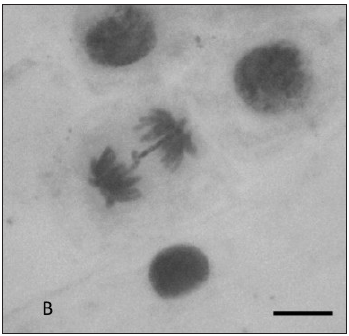
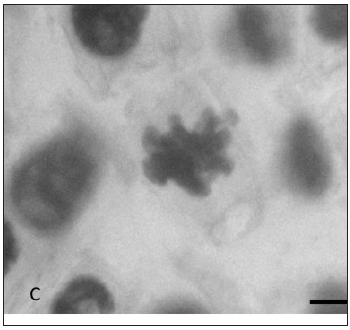
Fig. 1: Types of chromosomal aberrations seen in Allium cepa root tips. (A) Lagging chromosome at anaphase; (B) Anaphasic bridge; (C) Stickiness chromosomes; Magnification: A 1000x, B and C 400X. Bar = 10 µm.
4. Discussion
Many types of assays for evaluation of genotoxicity can be used for monitoring the toxicity of complex mixtures or environmental water samples. Plant assays and the Allium cepa test, particularly, have some advantages because they are highly sensitive to many pollutants [15]. Moreover, some higher plants are suitable for cytological analysis because of the large size of their chromosomes, thus making plant tests also good candidates for evaluating the genotoxicity of samples [12, 15, 16].
The root tips of Allium cepa have often been used for the determination of cytotoxic and/or genotoxic effects of many substances [14, 17, 18]. Furthermore, root tips of A. cepa, have been recommended as a standard for cytogenetic assay in environmental monitoring due to the correlation of these plants with mammalian and nonmammalian test systems [14].
A significant high frequency of mitotic aberrations, product of several abnormalities like micronuclei, laggard, stickiness and bridge chromosomes, indicates high toxic effect [14]. About the latter, for example authors like Monte et al. [16] in their study of genotoxicity of water samples, observed percentages of chromosomal abnormalities in the samples under evaluation starting in 0 % and as far as 5,25 %, but just samples with percentages of chromosomal abnormalities higher than 3,75% were statistically significant.
In our study, the proportions of cells with chromosomal abnormalities were low, always under 0,24% (see table 1). And these abnormalities were seen even in the negative controls. Statistical analysis of the results showed that the percentage of aberrant chromosomes, for all tested treatments, didn't expressed a significant difference related to negative controls (p > 0,05).
Owing to the low frequencies of chromosomal abnormalities observed and to the presence of these chromosomal abnormalities even in the controls, it could be suggest that clastogenic effects seen in our study might be random events.
Therefore, the antifungal formulation based on sucrose saturated solution didn't induce a significant increase in chromosomal abnormalities in all exposition times evaluated. Furthermore, potential cytotoxicity of the antifungal formulation based on sucrose saturated solution was evaluated previously by our group, and no cytotoxic effects were detected (unpublished data).
On the other hand, it is very important to establish the efficacy of plant bioassays in establishing good correlation with other bioassays. The extrapolation of the results obtained with plant cells to another species (and finally human cells) is probably the most important problem discussed in the context of biological testing. About the latter, Gentile et al. [19] and Menn [20] have pointed out that biotransformations are generally qualitatively similar in plants and in other animal systems. And according to Morais & Marin-Morales [13] the A. cepa test has shown high sensitivity and good correlation when compared with other test systems, e.g. mammals.
About the latter, Fiskesjö [15] commented that an extrapolation of results from one test system to another and, eventually, to human beings, should preferably be based on results from a battery of test systems covering various metabolic pathways for the tested substance, but results in Allium test should be regarded. Besides, the importance of genotoxicity tests based on plants should not be underestimated since any damage in the plant chromosomes by the chemical is also expected in the chromosomes of other organisms [10, 21, 22].
So, further and supplementary studies on mutagenicity and genotoxicity evaluation of the sucrose saturated solution using other biological systems will be important.
5. Conclusions
We conclude that the antifungal formulation based on sucrose saturated solution didn't present a significant genotoxic activity considering the results of the present study involving the Allium cepa test. But, other studies on mutagenicity and genotoxicity evaluation of the sucrose saturated solution using other biological systems will be important.
6. Acknowledgments
We would like to thank to the Comité Ejecutivo de Desarrollo e Innovación Tecnológica (CEDIT) - Misiones, Argentina for the scholarship fund, year 2010 (Res. 31/10, Dec. Nº 1984/05).
1. Brennan, B. and James, J., Overview of topical therapy for common superficial fungal infections and the role of new topical agents, J. Amer Acad Dermat 36: p S3-8. 1997. [ Links ]
2. Sangeorzan, J.; Bradley, S., He, X.; Zarins, L.; Ridenour, G.; Tiballi, R and Kauffman, C., Epidemiology of Oral Candidiasis in HIV-Infected Patients: Colonization, Infection, Treatment, and Emergence of Fluconazole Resistance. Amer J. Medicine 97: p 339-346. 1994. [ Links ]
3. Moshi, M.; van den Beukelb, C.; Hamzac, O.; Mbwamboa, Z.; Nondoa, R.; Masimbaa, P.; Mateed, M.; Kapingua, M.; Mikxf, F.; Verweije, P. and van der Venb, A., Brine Shrimp Toxicity Evaluation of Some Tanzanian Plants Used Traditionally for the Treatment of fungal infections. African J. Tradit 4: p 219-225. 2007. [ Links ]
4. Reca, M; Medvedeff, M.; Vedoya, M. and Herszage, L., Estudio in vitro de la inhibición del crecimiento de hongos por solución sobresaturada de azúcar. VI Cong Arg Mic 6: 1-76. 1993 [ Links ]
5. Medvedeff, M.; Lloret, M.; Vedoya, M.; Reca, M. and Herszage, L., Estudio in vitro de la acción fungicida del eugenol en solución sobresaturada de azúcar. Rev Arg Mic 20: p. 46-52. 1997. [ Links ]
6. Medvedeff, M.; Lloret, M.; Vedoya, M.; Zanek, M. and Herszage, L., Efecto fungicida de la solución saturada de azúcar, eugenol y polietilenglicol 400 sobre Candida albicans. Rev Arg Mic 21: p 14-17. 1998. [ Links ]
7. Medvedeff, M.; Vedoya, M.; Lloret, M.; Espinola, M. and Herszage, L., Saturated saccharose solution (SSS), its in vitro and in vivo action on Paecilomyces lilacinus. Rev Iberoamer Mic 17: S160-161. 2000a. [ Links ]
8. Medvedeff, M.; Vedoya, M.; Lloret, M.; Espinola, M. and Herszage, L., Bioactividad de la solución saturada de sacarosa sobre Sporothrix schenkii. Rev Iberoamer Mic 17: p. 146-148. 2000b [ Links ]
9. Fatima, R. and Ahmad, M., Genotoxicity of industrial wastewaters obtained from two different pollution sources in northern India: A comparison of three bioassays. Mutat Res 609: p 81-91. 2006. [ Links ]
10. Siddiqui, A.; Tabresz, S. and Ahmad, M., Validation of plant based bioassays for the toxicity testing of Indian waters. Environ Monitoring Assessm 179: p 241-253. 2011. [ Links ]
11. Ma, T., The international Program on Plant Bioassays and the Report of The Follow-up Study After the Hands-on Workshop in China. Mutat Res 426: p 103- 106. 1999. [ Links ]
12. Barbério, A.; Barrios, L.; Voltolini, J. and Mello, M., Evaluation of the cytotoxic and genotoxic potential of water from the River Paraíba do Sul, in Brazil, with the Allium cepa L. test. Braz. J. Biol. 69: p 837-842. 2009. [ Links ]
13. Morais, D. and Marin-Morales, M., Allium cepa test in environmental monitoring: A review on its application. Mutat Res 682: p 71-81. 2009. [ Links ]
14. De Rainho, C.; Kaezer, A.; Aiub, C. and Felzenszwalb, I., Ability of Allim cepa L. root tips and Tradescantia pallida var. purpurea in N-nitrosodiethylamine genotoxicity and mutagenicity evaluation. Anais Acad Bras Cien 82: p 925-932. 2010. [ Links ]
15. Fiskesjö, G. The Allium test as a standard in environmental monitoring. Hered 102: p 99-112. 1985. [ Links ]
16. Monte, L.; Medeiros, M.; Batistuzzo, S. and Agnez-Lima L., Cytotoxic and genotoxic potential of surface water from the Pitimbu river, northeastern/RN Brazil. Genetics Mol Biol 30: p 435-441. 2007. [ Links ]
17. Grant, W. Chromosome aberration assays in Allium: A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat Res 99: p 273-291. 1982. [ Links ]
18. Aydemir, N.; Celikler, S.; Summak, S.; Yilmaz, D. and Ozer, O. Evaluation of Clastogenicity of 4,6-Dinitro-o-cresol (DNOC) in Allium Root tip Test. J. Biol. Environ. Sci. 2: p 59-63. 2008. [ Links ]
19. Gentile, J.; Wagner, E. and Plewa, M., The Detection Of Weak Recombinogenic Activities In The Herbicides Alachlor And Propachlor Using A Plant-Activation Bioassay. Mutat Res 48: p 113-116. 1977. [ Links ]
20. Menn, J., Comparative Aspects of pesticide Metabolism in plants and animals. Environ Health Perspec 27: p 113-124. 1978. [ Links ]
21. Fiskesjö, G., The Allim test - an alternative in environmental studies: the relative toxicity of metal ions. Mutat Res 197: p 243-260. 1988. [ Links ]
22. Rank, J. and Nielsen, M., Allium cepa anaphase-telophase root tip chromosome aberration assay on N-methyl-N-nitrosourea, malei hydrazide, sodium azide, and ethyl methanesulfonate. Mutat Res 390: p 121-127. 1997. [ Links ]
Recibido: 12/01/2012
Aprobado: 09/07/2012














