Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Latin American applied research
versão impressa ISSN 0327-0793
Lat. Am. appl. res. v.34 n.1 Bahía Blanca jan./mar. 2004
X-ray fluorescence analytical methodology for the determination of Nb, Ta, Fe and Mn extracted in hydrometallurgic processes
M. del C. Ruiz1,*, M. H. Rodriguez1, E. Perino2 and R. A. Olsina2
1 Instituto de Investigaciones en Tecnología Química (INTEQUI), Universidad Nacional de San Luis-CONICET, C.C. 290, 5700 San Luis, Argentina.
mruiz@unsl.edu.ar
2 Área de Química Analítica, Facultad de Química, Bioquímica y Farmacia
Universidad Nacional de San Luis, C.C. 375, 5700 San Luis, Argentina
* Author to whom correspondence should be addressed
Abstract — Analytical methodologies were developed for the quantification of niobium, tantalum, iron and manganese in samples of minerals and solid residues coming from ore pressure leaching, using different acids. The adopted instrumental technique was X-ray fluorescence on pelleted solid samples. The analysis of the experimental data obtained by the different assayed methods showed that the direct technique using calibration curves built from pure oxide patterns and the method of standard addition were those giving statistically acceptable results. Comparison of the analytical data obtained by application to ore and leaching residue samples of these methods with a reference method showed that the experimental results were satisfactory.
Keywords — X-Ray Fluorescence. Niobium. Tantalum. Iron. Manganese. Determination. Leaching Process.
I. INTRODUCTION
Niobium and tantalum compounds are used in several high technology industrial activities such as electronics, superconductors, superalloys, aerospatial, and nuclear, among others. (Tolley, 1992; Gupta and Suri, 1994). These elements are not abundant on the earth surface and the major ores containing them are pyrochloro, microlite, columbite, tantalite and tantalo-columbite (Habashi, 1997). In Argentina, there are deposits of Nb and Ta containing ores in pegmatitic rocks in the provinces of Salta, Catamarca, La Rioja, Córdoba and San Luis. Several studies of the geochemical and geological features of these deposits have been performed (Arcidiácono, 1974; Gallisky, 1983 and Angelelli, 1984). Also, various chemical treatments have been developed for Nb and Ta extraction from ores, among them reduction, chlorination, alkaline fusion and acid dissolution (Gupta and Suri, 1994; Habashi, 1997). At the same time, several methodologies have been designed for the determination of these metals in the products of the above mentioned treatments. (Habashi, 1997). Among these are gravimetry (Gibalo, 1970) together with UV-Visible absorptiometry (Charlot, 1961), inductively coupled plasma optical emission spectrometry (ICP-OES) (Kubová et al., 1993; Roychowdhury et al., 1995) and X-ray fluorescence (XRF) (Parker and Brocchi, 1981; Ruiz et al., 1993 and 1996; Balaes et al.,1987).
The determination of Nb and Ta constitutes one of the most complex tasks in analytical chemistry, since they occur together in nature and also they exhibit similar physicochemical characteristics (Gibalo, 1970). Therefore, analytical methodologies by wet processes are particularly difficult. Studies performed by Atkinson et al. (1952) have demonstrated that the results obtained in quantitative determinations of these elements may be unsatisfactory and that they not only depend on the method used but may also vary if the same method is performed by different analysts. Our research group is carrying out studies on the extraction of niobium and tantalum from local ores by means of pressure leaching in acid medium. The need to perform an adequate follow-up of the recovery obtained after each assay makes it indispensable to use a rapid and reliable technique for the quantitative determination of the metals present in columbo-tantalites and in the residues of extractive metallurgy. The purpose of this work was to develop suitable methods for the quantitative determination of niobium, tantalum, iron and manganese in the reactants, products and residues of columbo-tantalites pressure leaching, using hydrofluoric and carboxylic acids. After optimizing the sample preparation procedure, the technique of X-ray fluorescence was selected as the method giving the most reproducible and accurate results. The results were compared to those obtained by the gravimetric method of classical analysis for Nb and Ta as well as to those obtained by means of ICP-OES.
II. EXPERIMENTAL
II.1. Reactants
The columbo-tantalite samples came from mineral ores in the province of San Luis, Argentina. Characterization by XRD showed that besides columbo-tantalite, the ore also contained quartz, feldspar and mica (Fig. 1a). The results of the ore characterization by XRF, shown in Fig. 1b, indicate that the ore stoichiometry is (Mn0.53 Fe0.47) (Nb0.66 Ta0.34). The standards were prepared from Ta2O5, Nb2O5, MnO2 (Fluka A G, Switzerland), Fe2O3 prepared from FeCl3.6H2O (Cicarelli) (Kodama, 1963), boric acid (Analar, U.K.) and high purity natural quartz. All the remaining reagents (carboxylic acids, HF, KHSO4, etc.) were of "pro analysi" quality.

Fig. 1. Characterization of the ore a: XRD; b: XRF.
II.2. Equipment
The ore was ground in a N.V. TEMA vibratory disc mill with chromium-nickel steel tray and separated into fractions of different particle size (between -10 +20 y -325 mesh ASTM) in a Fritsch Analysette 03-502 lab sieve. The solid samples were pelleted at 15,000 kPa with a hydraulic press. The characterization of starting materials and residues by XRD was performed on a Rigaku D-MAX-III C diffractometer (30 kV and 20 mA, with Ni filter and Cu Kα radiation). The measurements using the XRF technique were performed on a Philips PW 1400 spectrometer, equipped with a chromium tube. The measurements by ICP-OES were conducted on a Baird ICP 2070 equipment, with a nebulizer suitable to work in HF medium. The Nb and Ta extraction assays were carried out on a Parr reactor of 450 ml capacity, built in Monel, using HF and mixtures of HF and carboxylic acids as leaching agents.
II.3. Work methodologies
Measurements by ICP-OES were carried out using a calibration curve obtained from standards of known concentration, in 10% (v/v) HF medium. The XRF measurements on solid and liquid samples were performed by two different methodologies: the calibration curve method and the standard addition method. The liquid samples presented to the equipment had an HF concentration of approximately 10 % (v/v). A known mass of the solid samples, previously conditioned, was pelleted on a previously prepared boric acid support (Perino and Gasquez, 2000).
II.4. Preparation of standards and samples
A. ICP-OES technique
The stock solutions for the preparation of standards were separately prepared for each of the elements to be investigated. In the case of Nb and Ta, the technique described by Ruiz et al. (1996) was used. The Fe and Mn solutions were obtained as follows: approximately 100 mg of the metal were placed into a Teflon flask and 2 ml of distilled water and 3 ml of concentrated HNO3 were added. This mixture was then heated in sand bath until almost dry and 10 ml of concentrated HF were added. Finally, the solution was taken to a volume of 100 ml with distilled water. Adequate aliquots of these solution were used to prepare the different standards, using 10% (v/v) HF as diluent. The concentration range of the standards was varied according to the probable content of the element to be measured in the sample. The samples presented to the ICP-OES equipment were the solutions obtained from the fusion of the ore with KHSO4 and subsequent dissolution with tartaric acid (Ruiz et al. 1996), as well as those obtained from the filtrate after ore leaching in the Parr reactor. These solutions were conveniently diluted with 10% (v/v) HF so that their concentration was within the linear range of the calibration curve.
B. XRF technique
The measurements using X-ray fluorescence were performed on liquid and solid samples. The liquid samples were prepared following a procedure similar to the one describe in Section A. The analyzed solid samples came from the untreated ore in some cases and from the residues of the leaching process in others.
B.1. Liquids
B.1.1) Standard addition method
The stock solutions for each element were obtained as described in Section A. Increasing amounts of the element to be determined were separately added to equal aliquots of the sample so that the concentration of the sample was within the concentration ranges of the additions. Appropriate volumes of these solutions were placed in the measurement cells (Chemplex, covered with 6.3 μ - thick Mylar), and radiation was measured at the peak and at both of it sides. The net intensity was obtained by subtracting the average value of the background intensity to the peak intensity.
B.1.2) Use of the calibration curve
The standards and samples were prepared in a similar fashion to that described in Section A. The XRF measurements were performed as indicated in Section B.1.1).
B.2. Solids
B.2.1) Standard addition method
The standard addition method was investigated using two diluents: boric acid and quartz. At this stage, and with the purpose of determining the method reliability, only samples coming from unleached ore were used. The standards used were pure Nb, Ta, Fe and Mn oxides.
B.2.1.a) Adequate constant masses of the ore were mixed with increasing amounts of the corresponding oxide and variable amounts of boric acid, separately for each element, until a total mass of 100 mg was obtained. The used granulometry was -325 mesh. The solids were mixed in a manual agate mortar up to homogeneity and the sample was subsequently pelleted. Measurements were carried out by reading the radiation of the peak and both sides of the peak. The net intensity was calculated as described above.
B.2.1.b) In a first stage, a procedure similar to that described in the above section was used, but using quartz as diluent. Since the results obtained up to this point had not been totally satisfactory, a study was performed in order to optimize the sample method preparation. The investigated variables and the procedure used are described in the following section.
Ore-diluent ratio
Pellets were prepared by mixing, first manually and then in agate mechanical mortar, 100 mg of the ore with different quartz amounts to cover a concentration range (w/w) of the ore in the pellet between 0.5 (1:1 ratio) and 0.050 (1:20 ratio). The mixing time was three hours, and particle size was -270 +325 mesh. The measurements in the XRF equipment were performed as described in previous sections.
Mixing time
Pellets were prepared following a similar procedure to that described above. The ore-quartz ratio was 1:15, particle size was -270 +325 mesh and the mixing time varied between 1 and 12 hours.
Particle size
The study of the effect of granulometry was performed using three fractions of different particle size: -140 +200; -270 +325 and -325 mesh. The ore-quartz ratio was 1:15 and the mixing time was 1 hour. After selecting the working variables for each of the elements to be determined, 200 mg aliquots of the ore were mixed with 3000 mg of quartz and with increasing amounts of the respective oxides. Once the different mixtures were obtained, 100 mg pellets were prepared with each one of them. The measurements of fluorescence radiation were performed following a procedure similar to that described above.
B.2.2) Use of the calibration curve
The standards for obtaining the calibration curves of each elements were prepared by mixing different masses of the oxides, as shown in Table 1.
Table 1. Standards of Nb, Ta, Fe and Mn for the measurements on pelleted samples.

Note: The composition in FeO and in MnO was calculated from the Fe2O3 y MnO2 oxides used for preparation of the standards.
The mixing methods and conditions used were those that were considered optimum regarding to the mixing time and particle size; 100 mg pellets were used.
III. RESULTS AND DISCUSSION
The study was performed using the above described ore samples, whose composition was determined using six replicates, by gravimetry and UV-visible absorptiometry, for Nb and Ta (Ruiz et al., 1993). Fe and Mn were determined by redox volumetry (Kodama, 1963).
This validation method was considered the most adequate, since standard materials with similar composition to the samples subjected to leaching assays were not available. The percent composition (w/w), n=6 and α = 0.05, was: Nb2O5: 39.94 (s = 0.1855); Ta2O5: 34.07 (s = 0.1115); FeO: 7.71 (s = 0.1198) y MnO: 8.40 (s = 0.12848). Results obtained by a prestigious international laboratory using different methods matched ours by ± 3%.
A. ICP-OES technique
ICP-OES had the advantage of offering a rapid procedure for the analysis of the ore sample and the solutions coming from the ore leaching. However, the results obtained by this technique were not accurate enough to be considered satisfactory. Errors were usually above 10%, and were probably caused by difficulties in the nebulization process of the samples. For this reason, ICP-OES was only used in those leaching process situations which required the quantification of very low concentrations.
B. XRF technique
The measurements of X-ray fluorescence were carried out under the following working conditions:
- Gas flow detector and scintillation detector in tandem
- First order spectral lines
- Tube anode: Cr, operated at 60 kV and 40 mA
- Analyzing crystal: LiF 200
- Nb line: Lα (2θ = 21.360); Ta line: Lα (2θ = 44.420); Fe line: Kα (2θ = 57.52); Mn line: Kα (2θ = 62.97)
- Exposition conditions: in air
- 40 s counting time for all the analyzed elements
B.1. Liquids
The results obtained with liquid samples in HF medium were not totally satisfactory, either for those coming from ore dissolution or from leaching in the reactor. The important dispersion observed in the results was probably caused to the formation of a solid film on the Mylar, favored by the action of the X radiation.
B.2. Solids
Comparison of the results obtained by standard addition using boric acid and quartz as diluents demonstrated that dilution with quartz yields more accurate results. For this reason, the study of the variables for the preparation of the samples and standards (ore-diluent ratio; mixing time and particle size) was done using quartz as diluent and an univariate methodology. The recommended values, i.e., those giving the maximum fluorescent intensity for each variable were the following: ore-quartz ratio, 1:15; mixing time, 6 h (30 min manually and 5.30 h in mechanical mortar); particle size, -325 mesh. Figure 2 shows that after a mixing time of 6 hours the X-ray intensity did not increase significantly.

Fig. 2. Effect of mixing time of the solids on fluorescent intensity.
Figure 3 shows the results obtained in the application of standard addition for the Nb, Ta, Fe and Mn oxides
The degree of linearity is acceptable for all the analyzed elements in the concentration range used. Also, the ore composition obtained by this method differs by 3 % from that obtained using the reference method.

Fig. 3. Application of standard addition method for Nb, Ta, Fe and Mn oxides.
Although the standard addition method is relevant for the comparison and validation of experimental results, this technique is not suitable for determining the composition of residues coming from leaching process, since relatively large amounts of sample are needed. Therefore, we investigated the possibility of performing calibration curves using standards prepared with pure oxides (Section B.2.2). Figure 4 shows the calibration curves obtained for Nb, Ta, Fe and Mn.

Fig. 4. Calibration curves using standards prepared with mixture of pure oxides.
Tables 2 and 3 inform the results obtained by application of standard addition and calibration with pure oxides for an ore sample and a leaching residue sample, compared to those obtained by the reference method.
Table 2. Determination of Nb, Ta, Fe and Mn in ores.
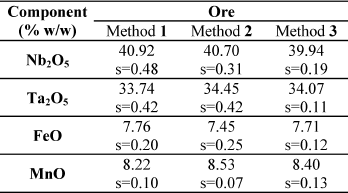
Note: 1: Standard addition method; 2: Calibration with standards prepared with pure oxides; 3: Reference method.
Table 3. Determination of Nb, Ta, Fe and Mn in residues.

From Tables 2 and 3, it can be seen that the results agreement betwen ± 4%, for all the elements studied, both in the ore sample and in the residue of acid leaching.
IV. CONCLUSIONS
The analytical methodologies developed for niobium, tantalum, iron and manganese are satisfactory for a wide range of concentrations and are applicable to ore and leaching residue samples. The calibration by means of standards prepared with pure oxides has proved to be useful due to its simplicity, versatility and analytical performance.
V. ACKNOWLEDGEMENTS
The authors wish to thank the valuable suggestions by Dr. J. A. Riveros de la Vega and the assistance of Dr. D. L. Martínez in the ICP-OES measurement. This work was supported by Universidad Nacional de San Luis and Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina.
REFERENCES
1. Angelelli, V., Yacimientos metalíferos de la República Argentina I, Comisión de Investigaciones Científicas de la Provincia de Buenos Aires, Argentina, pp. 203-205, 314-322 (1984). [ Links ]
2. Arcidiácono, E. C., "Contribución al conocimiento de columbo-tantalitas de las provincias de Córdoba y San Luis", Anales de la Asociación Química Argentina, 28 (2), 171-184 (1974). [ Links ]
3. Atkinson, R. H., Steigman, J. and Hiskey, C. F., "Analytical chemistry of Niobium and Tantalum. Critical analysis of existing methods", Analytical Chemistry, 24, 477-480 (1952). [ Links ]
4. Balaes, A. M. E., Dixon, K. and Wall, G. J., "Application of X-ray-fluorescence spectrometry to the analysis of Ta-Nb-Sn slags and associated by-products", Society for Applied Spectroscopy, 41 (3), 509-512 (1987). [ Links ]
5. Charlot, G., Les Méthodes de la Chimie Analytique, Masson et Cie, Paris, pp. 814-815 (1961). [ Links ]
6. Galliski, M. A., "Distrito minero El Quemado, departamentos La Poma y Cachi, provincia de Salta, II: Geología de sus pegmatitas", Revista de la Asociación Geológica Argentina, 38 (3-4), 340-380 (1983). [ Links ]
7. Gibalo, I. M., Analytical Chemistry of Niobium and Tantalum, Vol. 2, Ann Arbor/Humphrey Science Publishers, London, pp. 45-62, 334-335 (1970). [ Links ]
8. Gupta, C. K. and Suri, A. K., Extractive Metallurgy of Niobium, CRC Press, USA, pp. 21, 94-21 (1994). [ Links ]
9. Habashi, F., Handbook of Extractive Metalurgy, Vol. 3, Wiley-VCH, Germany, pp. 1404-1406, 1418-1421 (1997). [ Links ]
10. Kodama, K., Methods of Quantitative Inorganic Analysis, John Wiley & Sons, London, pp. 254, 285 (1963). [ Links ]
11. Kubová, J., Polakovicová, J., Stresko, V. and Medved, J., "Determination of Niobium and Tantalum in geological materials by atomic emission spectroscopy with inductively coupled plasma", Chemical Papers, 47 (4), 225-229 (1993). [ Links ]
12. Parker, R.J. and Brocchi, E., "Analysis of Nb and Ta in slags by X-ray-fluorescence spectrometry", Transactions Institute of Mining and Metallurgy, Section C, 90, C111-112 (1981). [ Links ]
13. Perino, E. and Gázquez, J., "Control e influencia del tamaño de partículas y presiones de compactación en preparaciones de pequeñas cantidades de muestras en polvo pelletizadas, en análisis por fluorescencia de rayos X", Avances por Técnicas de Rayos X, X, 141-145 (2000). [ Links ]
14. Roychowdhury P., Roy, N. K. and Das, A. K., "Determination of Tin, Tungsten, Niobium and Tantalum in rocks and minerals by inductively coupled plasma-atomic emission spectrometry", Atomic Spectroscopy, 104-105 (1995). [ Links ]
15. Ruiz, M. del C., González, J. and Olsina, R., "Analysis of Niobium, Tantalum and Titanium extracted from tantalite and columbite chlorination", Journal of Chemical Technology and Biotechnology, 57, 375-378 (1993). [ Links ]
16. Ruiz, M. del C., González, J., Olsina, R. and Perino, E., "X-ray fluorescence of Niobium, Tantalum and Titanium from Argentinian ores", Latin American Applied Research, 26, 209-213 (1996). [ Links ]
17. Tolley, R. J., Tantalum-Niobium International Study Center, 69, p. 3 (1992). [ Links ]
Received: March 12, 2002.
Accepted for publication: August 16, 2002.
Recommended by Subject Editor Ana Lea Cukierman.














