Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Biocell
versão impressa ISSN 0327-9545
Biocell v.28 n.2 Mendoza abr./ago. 2004
Histomorphological and quantitative immunohistochemical changes in the rat pancreas during aging
F.L. Riccillo, M.I. Bracamonte, G.M. Cónsole and C.L.A. Gómez Dumm
Cátedra de Histología-Embriología "B", Facultad Ciencias Médicas, Universidad Nacional de La Plata, Argentina.
Address correspondence to: Lic. Fernando L. Riccillo. Cátedra de Histología-Embriología «B», Facultad de Ciencias Médicas, UNLP, CC 455, (1900) La Plata, ARGENTINA. Phone: (+54-221) 425 6735; Fax: (+54-221) 425 8989; E-mail: friccillo@museo.fcnym.unlp.edu.ar
Abstract: Although the endocrine pancreas is the purpose of several deep investigations, morphological data referred to the effect of aging on the gland are not homogeneous. The purpose of the current work was to analyze the changes occurring in the pancreas of aged rats, with especial reference to the islet cell populations. Six young (Y), old (O) and senescent (S) male Sprague-Dawley rats were used. The pancreas tails were processed for light microscopy and studied by means of routine stains as well as by immunohistochemical identification of insulin-, glucagon-, somatostatin-, and pancreatic polypeptide- secreting cells (Dako Envision System, DAB as chromogen). A progressive pancreatic histoarchitecture distortion was found among the aged animals. Even when the alterations were not uniformly observed, they appeared more evident and severe in the S group. The S rats showed significantly increased volume density and cell density of the B cell population, as well as larger number of islet profiles, when compared to O rats. A significant progressive increment of adipose tissue was also evident in aged animals. No abnormal changes were detected in the non- B cell populations of the different groups.
The quantitative changes found in aged animals suggest a possible compensatory reaction of the B cell population in an attempt to curb the influence of diabetogenic factors mounting with advanced age.
Key words: Aging. Pancreas. Islets. Immunohistochemistry. Morphometry.
Introduction
Aging has been defined as the sum of all changes produced with the passing of time, determining a decline in the morphological integrity and functional capacity of the endocrine, nervous and immune systems, together with a decrease in their ability to maintain homeostasis (Meites et al., 1987). When we consider the effects of aging on the endocrine pancreas, many evidence of changes in its normal function appeared, finding in the literature numerous data about this subject. In humans, increasing age is associated with decreasing glucose tolerance (De Fronzo, 1984) and represents a major risk factor for the development of non-insulindependent diabetes mellitus (NIDDM). Reaven and Reaven (1981) have shown in rats that insulin secretion decreases with age, even if the animals are calorie restricted and do not become obese.
One of the mechanisms probably implicated in these disorders could be related to functional deficiency of the insulin producing tissue (Draznin et al., 1985), associated with a structural damage at histo- or cytological level, reflecting changes in number and distribution of both B-cells and non B-cells (Orci, 1982). However, there is no agreement among several of the published data. These show different and contradictory results, especially those referred to the morphological aspect. In fact, the principal changes were described mainly in insulin-secreting cells, since most studies are focused on this population owing to its essential role in glucose regulation and general metabolism implications. Likewise, we also considered possible changes of the non B-cell populations, pointing out the paracrine relation ships that have been described within the islet (Orci, 1982; Stagner and Samols, 1992), related to the modulating action exerted over the endocrine function and also on the exocrine secretion.
Taking into account the above mentioned, the purpose of the present study was to analyze during aging, the general histomorphology of the whole pancreas, as well as the quantitative immunocytochemical changes in the islet cell-populations, in order to contribute to the knowledge of possible alterations throughout the life span, directed to a better understanding of the pancreatic metabolism.
Material and Methods
Animals and specimen collection
Young (Y, 4 months), old (O, 24 months) and senescent (S, 30 months) male Sprague-Dawley rats, weighing 311.22 ± 8.55 g (Y); 319.37 ± 16.23g (O) and 300.83 ± 9.69g (S) were used. Six animals for each group were housed in a temperature-controlled room (22 ± 2°C) on a 12:12-h light-dark cycle. Food and water were available ad libitum. Rats were sacrificed by rapid decapitation, and samples of the splenic portion (tail) of the whole pancreas were taken and dissected immediately.
Maintenance and treatment of animals were in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Immunohistochemistry and morphometry
Pancreas from 6 animals of each group were fixed in Bouins solution, dehydrated and embedded in paraffin. Serial sections (4 µm) of two different levels were processed for routine histological study (hematoxylineosin and Gomori trichrome) and for immunohistochemical identification (Sternberger et al., 1970) of insulin-, glucagon-, somatostatin-, and pancreaticpolypeptide-secreting cells: B, A, D and PP cells, respectively. It is important to point out that each of the tissue samples was especially oriented during inclusion, in such a way as to obtain the widest possible area of tissue to be evaluated. For immunodetection, sections were incubated at room temperature for 1h with readyto-use primary antibodies against either rat insulin, glucagon, somatostatin and pancreatic polypeptide (Biogenex Laboratories, San Ramón, CA, U.S.A.). After washing, the sections were immunostained by means of a Dako EnVision System. Diaminobenzidine (DAB) was used as chromogen. The specificity of the primary antiserum was monitored by replacing the first antiserum by normal rabbit serum or PBS. The sections were counterstained with hematoxylin.
Measurement of cell parameters was made by means of an image analysis system (Imaging Technology, software Optimas 5.2). Four serial sections were obtained from each level for each of the pancreatic hormones. All the sections were introduced in the PC using an analog Sony video camera (PAL system), after being converted to the RGB (red-green-blue) system necessary for digitizing and processing the sections. The totality of islets found was considered for each section, an image being generated for each islet detected (average = 15 images/section) depending on the size and nearness of the islets. For this purpose, a X25 objective was used. The islets under approximately 40 µm in diameter were not measured since they were considered possible error factors, as it could not be determined whether they were islets superficially cut or small groups of 4 or 5 cells. These measurements were recorded and processed automatically, and the following parameters were afterwards calculated: volume density (VD=Σ cell area /RA) and cell density (CD = number of cells / RA). Both of them were referred to different reference areas (RA): endocrine reference area (RAe) and total pancreas reference area (RAt). RAe represents the total endocrine area scanned, in which islet populations were scored. Then, with the sum (Σ) of the areas (A) of each endocrine cell type, referred to as RAe, we obtained the respective VD, which indicates cell mass, according to a usually accepted concept. We also calculated the VD of the endocrine pancreas and the VD of B-, A-, D- and PP-secreting cells, taking the total pancreas as a reference area (RAt). In this case, a panoramic image of each section was obtained using a x 2.5 objective lens in order to measure the RAt, which was outlined including the intraglandular adipose tissue, and excluding the connective interlobular septa, the larger vessels and nerves, as well as extraglandular adiposity. The number of cells was calculated dividing the immunostained area of each cell population by the mean individual cell area of each cell type. For this parameter, 100 cells of each type were recorded. The individual islet areas (µm2) and the number of islet profiles were also measured. The latter parameter was referred to as total pancreas (mm2). The islet profiles are representative of the number of islets, since the sections from between two levels were sufficiently separated as not to count twice the same islet. All of these data were calculated from the totality of islets finding in each slide of each experimental group. Finally, the VD of the pancreatic adipose tissue was also calculated, taking the total pancreas as a RA. For this parameter, the volume density logarithm was calculated.
Statistical analysis
Statistical analysis was performed using one-way analysis of variance, followed by Tukeys multiple-range test. Data were expressed as mean ± SEM.
Results
Microscopic observations previous to a quantitative analysis revealed a progressive distortion in the histoarchitecture of the pancreatic parenchyma and stroma of aged rats (O and S, Figs. 2, 3) compared to Y animals (Fig. 1). A marked increase in the adipose tissue was found at the expense of the decrement in the pancreatic exocrine mass. Consequently, the pancreatic exocrine/endocrine ratio appeared changed, as shown in table 2, when the VD of endocrine pancreas is analyzed referred to total pancreas. Insular polymorphism with many fragmented islets (Fig. 3) and areas of progressive interstitial fibrosis (Fig. 4) were observed. In addition, ductal structure was altered (Figs. 3 and 4). Thus, zones with adenomatous and cystic ductal hyperplasia were evident, showing ectasia and thinner ductal epithelium (Figs. 3 and 4 A , D). Basement membrane was strongly stained and augmented in thickness (Fig. 4 D, inset). Moreover, arteriosclerosis was observed as the main vascular alteration in aged animals. Finally, localized interstitial lymphocytic infiltrates were identified in some S rats (not shown).

FIGURE 1. Pancreatic islets (arrows) from a Y rat for immunohistochemical identification of B-cells. Section was counterstained with hematoxylin. (X 100).

FIGURE 2. Distorted pancreatic lobule from an O rat included in a normal area of the gland. Arrows: isolated small islets. Immunostained for B-cell identification. Section was counterstained with hematoxylin. (X 100).

FIGURE 3. Pancreas from an S rat for immunohistochemical identification of B cells. A number of specifically stained islets of different size and shape are observed over a distorted pancreatic parenchyma. In the upper left corner, an area of cystic ductal hyperplasia is seen. Section was counterstained with hematoxylin. (X 100).
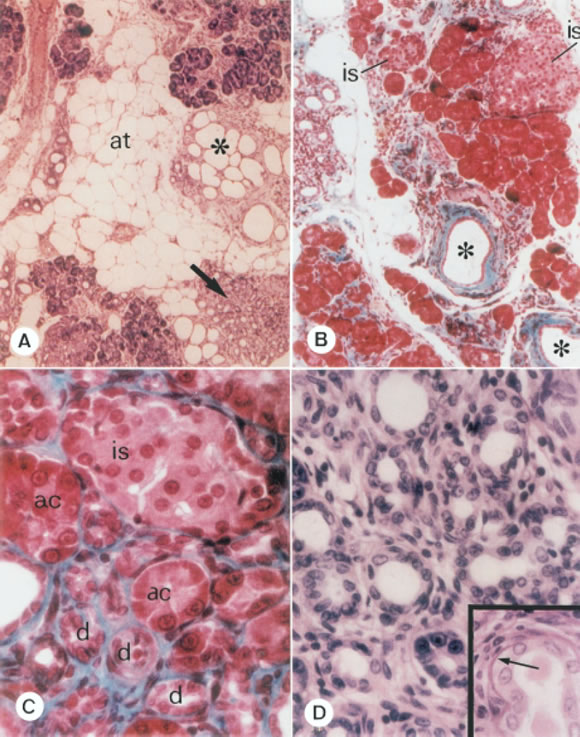
FIGURE 4. A) Pancreas from an S rat. Severe changes in the histoarchitecture are seen; at: adipose tissue; asterisk: cystic ductal hyperplasia; arrow: adenomatous ductal hyperplasia. On the right upper corner: acinar tissue coexisting with fibrosis. Hematoxylin-eosin (X 100). B) Pancreas from an S rat. Asterisks: isolated ductal ectasia with marked fibrosis. is: islets of different size. Gomori trichromic (X 100). C) Pancreas from an O rat, showing an area with different structures intermingled and separated by and evident fibrosis; is: islet; ac: acini; d: small ducts. Gomori trichromic (X 400). D) A high magnification view of ductal hyperplasia. Hematoxylin-eosin (X 400). Inset: ductal basement membrane showing increased thickness (arrow). Hematoxylin-eosin (X 400).
The morphometric analysis of the pancreas (Table 1) showed significant differences between the VD and CD of the islet B cells from O and S groups, while no significant changes were found in those parameters respecting A, D, and PP cells, among the different groups. Thus, VD and CD appeared significantly increased for B cell in S rats compared to O rats, referred to endocrine as well as total pancreas. When the total pancreas was taken as RA (Table 2), VD and CD from S animals increased respecting both O and Y rats. Instead, such parameters did not vary significantly in O rats when compared to Y group. On the other hand, the mean individual islet area (Fig. 5) was higher in O with respect to Y and S rats, the difference being significant only when compared to Y animals. In addition, O rats displayed a diminished number of islet profiles compared to both Y and S rats, such parameter being significantly higher in S animals when compared to O rats. The VD of the endocrine tissue was higher in S than in both O and Y rats but differences were not significant (Table 2). Finally, when the VD of pancreatic adipose tissue was compared between the aged groups of rats (Fig. 6), a significant increment was found in S rats with respect to both Y and O animals. This parameter also appeared increased in O animals when compared to Y group.
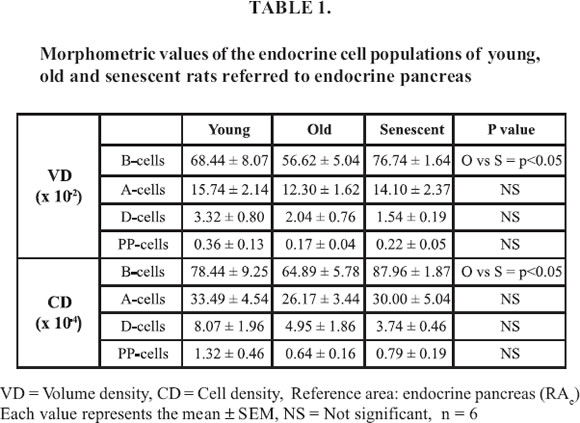


FIGURE 5. Mean individual islet areas and mean number of islet profiles from young (Y), old (O) and senescent (S) rats.

FIGURE 6. Adipose tissue in the pancreas of young (Y), old (O) and senescent (S) rats.
Discussion
Although many factors are involved in the progression of the aging process, it is difficult to separate those that are proper of aging itself, from those due to connected diseases that develop throughout the passage of time (Quay, 1995).
It has been demonstrated that aging produces a disruption in the neuroendocrine system regulation (Meites et al., 1987). Also, it was reported that aging in the rat is associated with diminished pancreatic exocrine functions (Hollander and Dadufalza, 1984). However, there is no total agreement about the changes undergone by the endocrine pancreas.
In this study we have focused on the morphological aspect of the pancreatic changes throughout the rat life span, from both a quantitative and qualitative point of view.
The progressive pancreatic tissue distortion was not uniformly detected among the aged animals, according to the expected wide range of expression in the aging process. This implies that not all the S and O rats presented similar degree of pancreatic pathology. However, it was clear that although most of the O rats developed structural alterations, these were more evident and severe in the S group.
The interstitial fibrosis progression could appear as a key factor in the distortion of both exocrine and endocrine pancreas histoarchitecture, reflected in the vascular (arteriosclerosis), ductal (ectasia as well as adenomatous and cystic hyperplasia) and insular (fragmentation and polymorphism) alterations. Furthermore, the progressive increment of the pancreatic (intraglandular) adipose tissue, highly noted in the S rats, appears as a substitution of the normal exocrine pancreas, frequently associated to pathologic processes, since aging can be considered a natural degenerative event. The implications of such abnormalities in the exocrine, endocrine and paracrine mechanisms, could also be considered.
Several reports exist in accordance with the observations concerning non-B cells (McEvoy, 1981) which show that these cells do not vary significantly through the life span. However, there is no agreement when the B-cell changes were considered. In accordance with our results, Reaven and Reaven (1981) showed an increment of both islet size and insular cell number. By contrast, DeClercq et al. (1988) published that in senescent Wistar rats the volume density as well as the numeric density of islets of Langerhans were decreased, although the proportion and localization of the different types of endocrine cells remained unchanged with aging. These contradictory observations might be due possibly to differences between the rat strains.
Considering the fact that decreased cellular proliferation is a general hallmark of aging (Hayflick, 1985), Ruhe et al. (1997) proposed that the maintenance of a normal organ function during senescence may be accomplished, in part, by an increase in the percentage of cells that are responsive to stimuli, and /or by an increase in the sensitivity of such responsive cells. However, the evidence that the B-cell mass exists in a dynamic state is becoming increasingly strong. This signify that the number of B cells is determined by the balance of cell renewal and cell loss, that is, turnover of B cells (Swenne, 1983; Finegood et al., 1995; Bernard and Ktorza, 1999; Bernard et al., 2001; Bonner-Weir, 2001).
The present morphometric analysis suggests a different behavior of the B-cell mass respecting the rest of the endocrine islet cell types, in which no significant changes were found when quantitative data were analyzed. Although no significant differences could be established between Y and O rats, our data showed that VD and CD of O animals decreased when compared to those of Y ones, when referred to endocrine tissue. However, when comparing O to Y rats, the mean individual islet areas significantly increased in the old animals, while the mean number of islet profiles significantly decreased.
We think that the increase of the VD of the B cells in S animals with respect to O ones reflects a possible increment of the B-cell mass induced by an aging associated insulin resistant state. The tendency of smaller and more numerous islets to appear in the S group, could be explained as an outcome of disruption of previous larger islets. Since we lack our own insulin plasma values in the present morphological study, we cannot extensively discuss the results with regard to the decreased insulin release in old rats reported by others. However, the probable compensatory increment in B-cell tissue cannot necessarily be related to a normal or increased insulin secretion.
Whatever the case may be, the relative increase in the number of islet profiles together with higher VD and CD of the B cells observed in the S rats, could re flect a compensatory increment of B-cell population in these animals. This B-cell behavior is generally associated with deficiency in the insulin secretion mechanism and / or insulin resistance states (Aizawa et al., 1994). Since B cells are responsible for the maintenance of the body glucose levels within a very narrow range, it is reasonable to think that this dynamic cell population undergoes compensatory changes to maintain euglycemia. However, it would be necessary to establish whether these small endocrine cell clusters could express some proliferating pattern representative of a replicative mechanism. Current investigations are being followed by our group in order to examine this aspect.
Our study allows us to think over the possibility that the appearance of the histomorphological changes in the pancreas of the older rats, mainly in the B-cell population, reflects the effect of aging upon the pancreatic structure, as an attempt to compensate a possible impairment in the insulin secretion pattern. This may be related to the decreased glucose tolerance (Jackson, 1990) and the insulin metabolism alterations (Coordt et al., 1995; Perfetti et al., 1996), that usually appear associated with advanced ages and their possible diabetic associated states.
Acknowledgements
The authors are grateful to Ms Gabriela Simonetto for the technical assistance. This work was supported by grants from Comisión de Investigaciones Científicas de la Provincia de Buenos Aires (CICBA), and Universidad Nacional de La Plata, (UNLP). Fernando Riccillo is recipient of a research fellowship from UNLP, GM Cónsole and CLA Gómez Dumm are Members of the Researchers Career, CICBA, Argentina.
References
Aizawa T, Komatsu M, Sato Y, Ishihara F, Suzuki N, Nishii N, Hashizume K, Yamada T (1994). Insulin secretion by the pancreatic beta cell of aged rats. Pancreas 9 (4): 454-459. [ Links ]
Bernard C, Berthault M-F, Saulnier C, Ktorza A (1999). Neogenesis vs. Apoptosis as main components of pancreatic B-cell mass changes in glucose-infused normal and mildly diabetic adult rats. FASEB J. 13: 1195-1205. [ Links ]
Bernard C, Ktorza A (2001). Endocrine pancreas plasticity under physiological and pathological conditions. Diabetes 50 (suppl.1): s30-s35. [ Links ]
Bonner-Weir (2001). B-cell Turnover. Its assessment and implications. Diabetes 50 (suppl.1): s20-s24. [ Links ]
Coordt MC, Ruhe RC, McDonald RB (1995). Aging and insulin secretion. Proc Soc Exp Biol Med 209(3): 213-222. [ Links ]
DeClercq L, Delaere P, Remacle C (1988). The aging of the endocrine pancreas of the rat. I. Parameters of cell proliferation. Mech Ageing Dev 43(1): 11-24. [ Links ]
De Fronzo RA (1984). Glucose intolerance of aging. Diabetes Care 4: 493-513. [ Links ]
Draznin B, Steinberg JP, Leitner JW, Sussman KE (1985). The nature of insulin secretory defect in aging rats. Diabetes 34: 1168-1173. [ Links ]
Finegood DT, Scaglia L, Bonner-Weir S (1995). Dynamics of B-cell mass in the growing rat pancreas: estimation with a simple mathematical model. Diabetes 44: 249-256. [ Links ]
Hayflick L (1985). Theories of biological aging. Exp Gerontol 20: 145-149. [ Links ]
Harman D (1981). The aging process. Proc Natl Acad Sci USA, 78: 7124-7128. [ Links ]
Hollander D, Dadufalza VD (1984). Aging-associated pancreatic exocrine insufficiency in the unanesthetized rat. Gerontology 30(4):218-22. [ Links ]
Jackson RA (1990). Mechanism of age-related glucose tolerance. Diabetes Care 13: 9-19. [ Links ]
McEvoy RC (1981). Changes in the volumes of the A-,B-, and D-cell populations in the pancreatic islets during the postnatal development of the rat. Diabetes 30: 813-817. [ Links ]
Meites J, Goya RG, Takahashi S. (1987). Why the neuroendocrine system is important in aging process. Exp Gerontol 22: 1-5. [ Links ]
Orci L (1982). Macro and micro domains in the endocrine pancreas. Diabetes 31: 538-565. [ Links ]
Perfetti R, Rafizadeh CM, Liotta AS, Egan JM (1996). Age-dependent reduction in insulin secretion and insulin mRNA in isolated islets from rats. Am J Physiol 269(6): E983-90. [ Links ]
Quay W (1995). Pancreatic regulation of nutrient metabolism. In: Hormones and Aging. P. Timiras, W. Quay, A. Vernadakys Eds. CRC Press, Boca Raton, chapter 10, pp. 169-199. [ Links ]
Reaven EP, Reaven GN (1981). Structure and function changes in the endocrine pancreas of aging rats with reference to the modulating effects of exercise and caloric restriction. J Clin Invest 68, 75-84. [ Links ]
Ruhe RC, Curry DL, McDonald RB (1997). Altered cellular heterogeneity as a possible mechanism for the maintenance of organ function in senescent animals. J Gerontol A Biol Sci Med Sci 52(1): B53-58. [ Links ]
Stagner JI, Samols E (1992). The vascular order of islet cellular perfusion in the human pancreas. Diabetes 41: 93-97. [ Links ]
Stemberger LA, Handy PH, Cuculis JJ, Meyer HC (1970). An unlabelled antibody method of immunocytochemistry. J Histochem Cytochem 18: 315-340. [ Links ]
Swenne I (1983). Effects of Aging on the regenerative capacity of the pancreatic B-cell of the rat. Diabetes 32: 14-19. [ Links ]
Received on January 16, 2003.
Accepted on March 29, 2004.














