Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Revista de la Sociedad Entomológica Argentina
versão impressa ISSN 0373-5680
Rev. Soc. Entomol. Argent. vol.70 no.3-4 Mendoza jul./dez. 2011
TRABAJOS CIENTÍFICOS
Revision and biogeography of the Neotropical dung beetle genus Scybalophagus (Coleoptera: Scarabaeidae)
Revisión y Biogeografía del género neotropical de escarabajos estercoleros Scybalophagus (Coleoptera: Scarabaeidae)
Ocampo, Federico Carlos* and Fredy Molano **
* Instituto de Investigaciones de las Zonas Áridas - Instituto de Ciencias Básicas, CCT-CONICET Mendoza. CC 507, 5500. Mendoza, Argentina; e-mail: focampo@mendoza-conicet.gob.ar .
** Laboratorio de Entomología, Museo de Historia Natural "Luis Gonzalo Andrade", Escuela de Ciencias Biológicas, Universidad Pedagógica y Tecnológica de Colombia, Boyacá, Colombia; e-mail: fredymol@gmail.com
Recibido: 21-V-2011
Aceptado: 9-VIII-2011
ABSTRACT. The South American genus Scybalophagus Martínez is comprehensively revised. The genus now includes five species, distributed in Argentina, Chile, Bolivia and Peru. All species are redescribed and diagnostic characters are provided along with illustrations of each species. Lectotypes are designated for Canthon lacordairei Laporte, 1840 (now Scybalophagus lacordairei) and Canthon rugosus Blanchard, 1845 (now Scybalophagus rugosus). Scybalophagus zumpti (Frey, 1963) (=Epirinus zumpti Frey) is now considered a junior synonym of S. rugosus (Blanchard). The biogeography of the genus and of each species is discussed and predictive distributions, based on environmental niche modeling, are provided for all species. Information on the biology and natural history of Scybalophagus species is discussed.
KEY WORDS. South American transition zone; Taxonomy; Dung beetles.
RESUMEN. El género suramericano Scybalophagus Martínez es revisado exhaustivamente. El género ahora incluye cinco especies distribuidas en Argentina, Chile, Bolivia y Perú. Se redescriben todas las especies y se proveen caracteres diagnósticos junto con ilustraciones para cada especie. Se designan lectotipos para Canthon lacordairei Laporte, 1840 (ahora Scybalophagus lacordairei) y Canthon rugosus Blanchard, 1845 (ahora S. rugosus). Scybalophagus zumpti (Frey 1963) (=Epirinus zumpti Frey) es ahora considerado un sinónimo junior de S. rugosus (Blanchard). La Biogeografía del género y cada especie es discutida y se presentan distribuciones potenciales, basadas en modelos de nicho ecológico, para todas las especies. Se discute la información sobre la biología e historia natural de las especies de Scybalophagus.
PALABRAS CLAVE. Área de transición sudamericana; Taxonomía; Escarabajos estercoleros.
INTRODUCTION
The genus Scybalophagus was described by Martínez (1953). The original description is a short communication in which Martínez also described one species, S. patagonicus Martínez, designated as the type species of Scybalophagus. Martínez (1953) included in the genus, S. fractipes (Harold), S. lacordairei (Laporte), S. pilosus (Felsche), and S. rugosus (Blanchard), all formally placed in Canthon Hoffmannsegg. Pereira (1953) synonymized S. fractipes with Canthon plicatipennis Blanchard. One year later, Martínez (1954) proposed a key to species and listed S. plicatipennis (Blanchard) in the genus Scybalophagus. Frey (1963) described Epirinus zumpti Frey from South Africa, species that one year later was transferred to the genus Pseudoepirinus by Ferreira (1964), this genus was named by Ferreira to accommodate the single species E. zumpti. Frey placed this species in the genus Epirinus based on the presence of the transversal carinae on the hind tibia, character shared by all species of Scybalophagus. After studying the type material of P. zumpti and comparing it with the New World canthonines, Scholtz & Howden (1987) synonymized Pseudoepirinus with Scybalophagus. The most comprehensive work on Scybalophagus was published by Halffter & Martínez (1968). In their contribution on American canthonines, Halftter & Martínez (1968) provided a description of Scybalophagus, a key to species, and comments on the species distribution and biology but did not produce a full taxonomic work neither they provided detailed information on species distribution. The biology of Scybalophagus species was discussed by Halffter & Matthews (1966).
The purpose of this contribution is to redescribe the genus Scybalophagus, provide a synopsis and diagnosis for each of its species, designate lectotypes for two species, propose a new synonym of Scybalophagus rugosus (Blanchard), provide distribution maps and discuss the ecological and historical biogeography for the genus and species distribution, and summarize the available information about their biology.
MATERIAL AND METHODS
Specimens were examined, dissected, and illustrated using a dissecting stereomicroscope (10 - 40 X). Mouthparts and male genitalia were dissected and cleaned in a dilute solution (~10%) of potassium hydroxide, and neutralized in a dilute solution (~ 10%) of acetic acid. The male genitalia was placed in a glycerin-filled vial pinned under the specimen. Body measurements, puncture density, puncture size, and density of setae are based on the following standards: Body length was measured from the apex of the pronotum (at the middle) to the apex of the elytra, plus head length from the apex of clypeal process to the base of the head (head was measured separately because its variable position renders it unpractical to measure total body length). Body width was measured across mid-pronotum. Puncture density was considered "dense" if punctures were nearly confluent to less than two puncture diameters apart, "moderately dense" if punctures were two to six diameters apart, and "sparse" if punctures were separated by more than six diameters apart. Puncture size was defined as "small" if punctures were 0.02 mm or smaller, "moderate" if 0.02-0.07 mm, and "large" if 0.07 mm or larger. Setae were defined as "sparse" if there were few setae, "moderately dense" if the surface was visible but with many setae, and "dense" if the surface was not visible through the setae. Elytral carinae were counted from the elytral suture. Specimen labels were copied literally using "/" between lines and ";" between labels. The names of the male reproductive organ structures were based on the work of Binaghi et al. (1969), Zunino (1972), Medina et al. (2003) and Medina & Molano (in prep).
Designation of lectotypes. Lectotypes are here designated to provide the nomenclatural stability of the taxon studied, according to Article 72 of the International Code of Zoological Nomenclature (ICZN, 1999).
Specimens for this research were collected or borrowed from and deposited in the following institutions and collections:
CMNC: Canadian Museum of Nature, Ottawa, Canada (R. S. Anderson, F. Génier).
IADIZA: Colección de Entomología del Instituto Argentino de Investigaciones de las Zonas Áridas, Mendoza, Argentina (F. C. Ocampo).
MACN: Museo Argentino de Ciencias Naturales, Buenos Aires, Argentina (A. Roig).
MNHN: Muséum National d'Histoire Naturelle, Paris, France (O. Montreuil).
MNNC: Museo Nacional de Historia Natural, Santiago, Chile (M. Elgueta)
UNSM: University of Nebraska State Museum, Lincoln, NE, USA (B. C. Ratcliffe).
USNM: United States National Museum, Washington D.C., USA (D. Furth).
Predictive models of species distribution
Species distribution models (SDMs) can be used to predict the potential distribution of a species which, in turn, are useful to test biogeographical, ecological, and evolutionary hypotheses (Graham et al., 2004). Predictive distributions are obtained by relating known collection localities of a species to a set of environmental variables that, presumably, reflect the ecological niche of the species (Guisan & Thuillier, 2005).
Georeferenced localities for each Scybalophagus species were obtained directly from specimens' labels or locality, data were georeferenced using the BioGeomancer workbench application (http://www.biogeomancer.org/index.html). These data were mapped to model species distribution using predictive methods based on 19 bioclimatic variables (Hijmans et al., 2005), plus soil type (categorical), and elevation. The resolution of the environmental layers was approximately 4.6 x 4.6 km. Data were analyzed using Maxent (Phillips et al., 2006). Maxent is considered one of the strongest methods for predicting species distribution, particularly for species sampled from a relatively low number of localities (Tognelli et al., 2009).
RESULTS
Scybalophagus Martínez (Figs. 1-45)
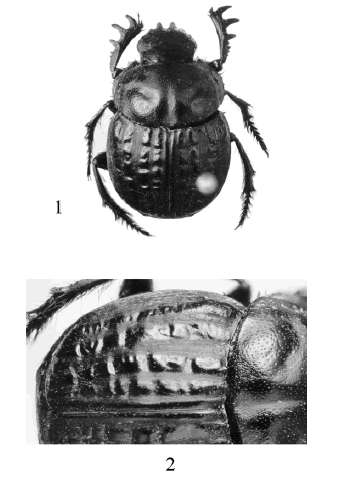
Figs. 1-2. Scybalophagus lacordairei. 1. Dorsal view. 2. Elytral sculpture detail.

Figs. 3-7. Male genitalia of Scybalophagus lacordairei. 3. Aedeagus. 4. Parameres dorsal view. 5. Basal sclerite. 6. Plate sclerite. 7. Elongated sclerite.

Figs. 8-9. Scybalophagus patagonicus. 8. Dorsal view. 9. Elytral sculpture detail.

Figs. 10-14. Male genitalia of Scybalophagus patagonicus. 10. Aedeagus. 11. Parameres dorsal view. 12. Basal sclerite. 13. Plate sclerite. 14. Elongated sclerite.

Figs. 15-16. Scybalophagus pilosus. 15. Dorsal view. 16. Elytral sculpture detail.

Figs. 17-21. Male genitalia of Scybalophagus pilosus. 17. Aedeagus. 18. Parameres dorsal view. 19. Basal sclerite. 20. Plate sclerite. 21. Elongated sclerite.

Figs. 22-23. Scybalophagus plicatipennis. 22. Dorsal view. 23. Elytral sculpture detail.
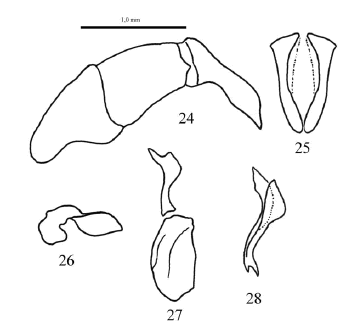
Figs. 24-28. Male genitalia of Scybalophagus plicatipennis. 24. Aedeagus. 25. Parameres dorsal view. 26. Basal sclerite. 27. Plate sclerite. 28. Elongated sclerite.

Figs. 29-34. Scybalophagus rugosus. 29. Dorsal view. 30. Elytral sculpture detail. 31. Dorsal view of head. 32. Metasternum. 33. Mesotibia. 34. Metatibia.

Figs. 35-39. Male genitalia of Scybalophagus rugosus. 35. Aedeagus. 36. Parameres dorsal view. 37. Basal sclerite. 38. Plate sclerite. 39. Elongated sclerite.

Fig. 40. Distribution map of Scybalophagus on Morrone's (2006) biogeographic scenario (Cha= Chaco, Mon= Monte, Pam= Pampa, Pat= Patagonia, Pun= Puna). Grey dots represent actual data points, grey areas represent the predictive distribution model where darker areas correspond to higer probabilities of presence based on the used variables.
Scybalophagus Martínez, 1953. Boletín de la Sociedad Entomológica Argentina 2: 3.
Scybalophagus Martínez, 1954. Natura 1: 66.
Pseudoepirinus Ferreira, 1964. Novos Taxa Entomológicos 37: 3. Syn.
Scybalophagus, Pereira & Martínez, 1956. Revista Brasilera de Entomologia 6: 94, 104, 183.
Scybalophagus, Halftter & Martínez, 1968. Revista de la Sociedad Mexicana de Historia Natural 29: 253.
Scybalophagus, Halffter & Martínez, 1977. Folia Entomologica Mexicana 38: 37
Scybalophagus, Scholtz & Howden, 1987. Journal of the Entomological Society of South Africa 50: 116.
Scybalophagus, Halffter & Matthews, 1966. Folia Entomologica Mexicana 12-14: 17, 261.
Type species: Scybalophagus patagonicus Martínez, 1953, original designation.
Diagnosis. The species of the genus Scybalophagus can be recognized from other New World canthonines by the following combination of characters: Medium to small size, color black, with or without copper reflections; clypeus bidentate (with two small medial teeth), teeth separated by U-shaped emargination, or cuadridentate (with two small medial teeth and smaller, lateral teeth); head lacking horns or tubercles; pronotum strongly transverse; elytra convex, lateral and apical margins rounded, lacking carinae; pygidium longer than wide, basal margin angulose; metasternum gibbose in middle; protibiae tridentate, with denticles on basal half; meso- and metatibiae with well developed transversal carinae on apical third (Figs. 33-34); meso- and metatarsi laterally flattened, triangular in shape.
Description. Body: oval, size medium (length 6.0-15.0 mm). Dorsal surface microgranulate, granulate, punctate or smooth. Head: as long as wide, without visible dorsal carinae, clypeogenal sutures evident, frontoclypeal suture not evident. Clypeus with two teeth separated by short U-shaped emargination, and with or lacking external lobe beside each tooth. Dorsal eye surface reduced, elongated. Head with occipital bead. Clypeal ventral process cariniform, slightly pointed. Mentum deeply emarginated at apex, emargination V-shaped. Labial palpus with first palpomere triangular; second palpomere cylindrical; third palpomere short, cylindrical, narrower than second. Prothorax: strongly transverse. Anterior angles nearly right-angled; posterior angles rounded. Lateral and posterior margins rounded. Proepisternum lacking carina. Elytra: Disc convex, with 9 striae; striae variable in size; interstriae variable in width. Humerus with or without humeral tubercle. Pseudoepipleuron well developed, medial margin carinated. Epipleuron, well developed, sinuous, tapered toward apex. Hind wings: developed (functional). Mesosternum: Short, narrowed medially. Mesoepimeron wider than long, setose. Meso-metasternal suture evident, rounded. Metasternum: anterior lobe gibbous, surface variably sculptured. Legs: Apico-anterior femoral pit absent. Protibia tridentate, teeth located on apical half, basal half with 3-6 denticles; dorsal surface, medial margin and ventral surface with longitudinal, setose carinae. Protibial spur dorso-ventrally flat, as long or longer than tarsal segments 1-4 combined, apex bifurcated or truncate. Protarsus short; segments 1-4 subequal, as long as wide; tarsomere 5 as long as tarsomeres 3-4 combined, laterally flattened, distally widened. Claws small, simple, falciform. Mesofemur elongated, wider in middle. Meso- and metatibia elongate, as long as meso- and metafemora (respectively), evenly widened toward apex; with well developed transversal carina on apical third, carinae setose; tibial apex wider than tibiae. Larger mesotibial spur sub conical; slender shorter spur, curved, flattened. Meso- and metatarsi laterally flattened, tarsomeres 1-4 sub equal in size, sub triangular; tarsomere 5 as long as 3-4 combined; claws simple, curved. Metatibial spur sub conical, longer than tarsomeres 1-2 combined. Pygidium as wide as long; lacking basal sulcus; disc flat or convex; basal margin strongly angulose. Male genitalia with symmetric parameres; internal sac of aedeagus with submedial lobe, and a set of raspules (brushes), basal lamella shaped as a "magnifying glass" and plate lamella consisting on several structures.
Distribution. Argentina, Bolivia, Chile, and Peru.
Phylogenetic relationships. The genus Scybalophagus is a well defined, monophyletic group of Canthonini. Halffter & Martínez (1968) indicated that Scybalophagus is possibly closely related to Canthon Hoffmannsegg, based on the morphology of these two genera. Although a detailed phylogenetic analysis of the New World Canthonini was never performed, analyses that included this genus indicate that Scybalophagus is an old lineage of South American Canthonini (Ocampo & Hawks, 2006; Monahan et al., 2007), hypothesis also suggested by Halffter & Martínez (1968).
Key to species of Scybalophagus (modified from Halffter and Martínez, 1968)
1. Elytral interstriae 2-6 uniform, strongly risen or not, shiny or not, with tubercles, large punctures or smooth; clypeus bidentate ........ ............................................... 2
1'. Elytral interstriae 2, 3, 5, and 6, strongly risen, shiny, with large transverse depressions; interstriae 4 flat, opaque (more evident on elytral basal half); clypeus quadridentate, (lateral teeth small) (Figs. 1-7) ......................... . . Scybalophagus lacordairei (Laporte)
2. Elytral disc with interstriae strongly "rugose", surface with large irregular, opaque punctures, punctures confluent or not, bearing a minute seta; pronotal surface densely punctate, punctures large, irregular (Figs. 29-39) ............................................. Scybalophagus rugosus (Blanchard)
2'. Elytral disc with interstriae mostly uniform (interstria 1 could have transverse depressions at middle), surface smooth, with small tubercles, or large setae; pronotal surface sparsely punctate, punctures small ............................................................... 3
3. Metasternum with large prominent gibba in middle; elytral surface with small tubercles or large setae ...................................... 4
3'. Metasternum uniformly convex, lacking prominent gibba (although gibba evident); elytral surface lacking tubercles or setae (Figs. 22-28) ..................................................... Scybalophagus plicatipennis (Blanchard)
4. Body surface shiny, color copper; elytral and margins of pronotum bearing large setae; metasternal gibba with surface smooth in middle, punctate on lateral and anterior margins. (Figs. 15-21) ............................................ Scybalophagus pilosus (Felsche)
4'. Body surface black, opaque; elytral surface with small tubercles, tubercles notorious on interstriae 2, 3, and 5; metasternal gibba with surface strongly, uniformly punctate (Figs. 8-14) ............................................................... Scybalophagus patagonicus Martínez
Scybalophagus lacordairei (Laporte) (Figs. 1-7, 41)

Fig. 41. Map of distribution of S. lacordairei (grey dots), and predictive distribution, darker areas represent higher probabilities of occurrence of the species based on 21 variables.
Scybalophagus lacordairei (Laporte, 1840) (Hyboma)
Hyboma lacordairei Laporte, 1840. Histoire Naturelle des Insects Coléoptères: 74.
Canthon gemmatum Blanchard, 1845. Voyage dans L'Amerique Méridionale pars Alcide D' Orbigny, Coléoptères: 160. syn.
Deltochilum lacordairei, Lucas, 1857. Voy Castelnau Col: 97.
Canthon lacordairei, Harold, 1868a. Berliner Entomologische Zeitschrift 12:11, 17.
Coprobius lacordairei, Burmeister, 1873. Stettiner Entomolgische Zeitung 34: 411.
Canthon lacordairei, Blackwelder, 1944. Bulletin of the United States Natural Museum 185: 199.
Scybalophagus lacordairei, Martínez, 1953. Boletin de la Sociedad Entomológica Argentina 2: 3.
Scybalophagus lacordairei, Martínez, 1954. Natura 1: 67.
Scybalophagus lacordairei, Halffter & Martínez, 1968: Revista de la Sociedad Mexicana de Historia Natural 29: 261.
Type material. Lectotype female (MNHN) labeled: "Museum Paris / PATAGONIE / (Patagones) / D'Orbigny 1834"; "Canthon / lacordairei / LECTOTYPE / Ocampo det.". "Scybalophagus / lacordairei / Ocampo det. 2010." Lectotype here designated. One paralectotype (MNHN) labeled as lectotype except: "Canthon / lacordairei / PARALECTOTYPE / Ocampo det." "Scybalophagus / lacordairei / Ocampo det. 2010." Paralectotype here designated.
Redescription. Color black. Length 7.43-10.44 mm, width 4.03-7.56 mm. Head (Fig.1): With two medial teeth separated by U-shaped emargination, and two lateral teeth, lateral teeth poorly developed, genoclypeal suture evident, with margin slightly indented; surface granulose; eyes dorsally elongated, ventrally globose. Pronotum (Fig. 1): Strongly transverse; surface irregular, granulose to punctate; anterior angle right-angled, posterior angle rounded. Elytra (Fig. 2): Surface irregular, interstriae 1, 2, 3, 5, and 7 with surface smooth, undulated form base to apex, interstriae 4 and 6, microrugose; striae evident; elytral lateral and apical margins broadly rounded. Pigydium: Surface flat, microgranulate sparsely setose, setae short, with minute tubercles, tubercles sparse. Venter: Proepisternum-, meso-, and metaepisternum setose, setae moderately long and moderately dense. Metasternum strongly gibbose in middle, surface sooth in middle, becoming punctate and sparsely setose toward apical and lateral margins. Male genitalia (Figs. 3-7): Aedeagus with phalobase longer than parameres; parameres symmetrical, narrowed at middle. Internal sac with two brushes on submedial lob; internal sac with sclerites as in Figs. 5-7.
Distribution (Fig. 41). ARGENTINA. Buenos Aires: Villarino (1); no more data (6); Puán (3); Bahía Blanca (4); Felipe Solá (1). La Pampa (Martínez, 1954; Halffter & Martínez, 1968). La Rioja: Anillaco (1). Mendoza: Ñacuñán (4). Río Negro (Martínez, 1954; Halffter & Martínez, 1968); "Patagonia" (3).
Remarks. Males can be recognized from females by the protibial spur, bifurcated in males and simple in females.
Biology. Adults of S. lacordairei are attracted to fresh dung, the species has been recorded in human and cow dung (Halffter & Martínez, 1968, FCO personal observation). Specimens where observed flying and rolling dung balls during the morning.
Scybalophagus patagonicus Martínez (Figs 8-14, 42)

Fig. 42. Map of distribution of S. patagonicus (grey dots), and predictive distribution, darker areas represent higher probabilities of occurrence of the species based on 21 variables.
Scybalophagus patagonicus Martínez, 1953. Boletín de la Sociedad Entomológica Argentina 2: 3.
Scybalophagus patagonicus, Martínez, 1954. Natura 1: 66, 68.
Scybalophagus patagonicus, Halffter & Martínez, 1968. Revista de la Sociedad Mexicana de Historia Natural 29: 264.
Type material. Holotype male (MACN) labeled: "ARGENTINA / Chubut / Teca / Soriano leg. / Coll. Martínez / Oct 946."; "HOLOTYPUS"; "Scybalophagus / patagonicus / A. Martínez. det 53." Allotype (MACN) labeled: "ARGENTINA / Neuquén / Zapala / J. Muñoz leg. / A. Martínez coll."; "ALLOTYPUS"; "Scybalophagus / patagonicus / A. Martínez. det. 53". Two paratypes (USNM) labeled: "ARGENTINA / Santa Cruz / Pto. Deseado / Dr. Donat leg. / Coll. C. Bruch." "PARATIPO"; "Scybalophagus / patagonicus / gen. sp. n. / A. Martínez det 53".
Redescription. Color dull black with slight copper reflection on pronotum. Length 8.78-12.58 mm, width 4.20-8.66 mm. Head (Fig. 8): With two medial teeth separated by wide U-shaped emargination, lacking lateral teeth, genoclypeal suture evident, with margin slightly indented; surface granulose on clypeus to sparsely punctate on frons; eyes dorsally elongated, ventrally globose. Pronotum (Fig. 8): Strongly transverse; surface punctate, punctures large setose, setae minute, slightly rugose in between punctures; anterior angle right-angled, posterior angle rounded. Elytra (Fig. 9): Surface irregular, with 2-3 tubercles at base, humeral tubercle developed, interstriae with surface microgranulate and with small tubercles, tubercles sparse; striae evident; declivital area with broad tubercle; elytral lateral and apical margins broadly rounded. Pigydium: Surface convex, microgranulate, sparsely setose, setae short. Venter: Pro-, meso-, and metaepisternum setose, setae moderately long and moderately dense. Metasternum strongly gibbose in middle, surface smooth in middle, with small tubercles and sparsely setose toward apical and lateral margins. Male genitalia (Figs.10-14): Aedeagus (Fig. 10), parameres symmetrical, longer than phalobase; internal sac with few brushes on submedial lob; internal sac with sclerites as in Figs 12-14.
Distribution (Fig. 42). ARGENTINA. Chubut: Cerro Negro (2); Teca (1), Leleque (1); Península Valdéz (1). Neuquén: RN 40 (17.5 km S Catan Lil) (1); RN 237 (Bajada del Collón Curá, 6.5 km NE Corral de Piedra) (2); San Martín de los Andes (1); Zapala (1). Río Negro: Maquinchao (Martínez, 1955). Santa Cruz: Puerto deseado (4).
Biology. With the exception that the species has been observed at daylight rolling a ball of human feces, nothing else is known about the biology of S. patagonicus, presumably it is attracted to ungulate's fresh dung but no records of this have been documented.
Scybalophagus pilosus (Felsche) (Figs. 15-21, 43)
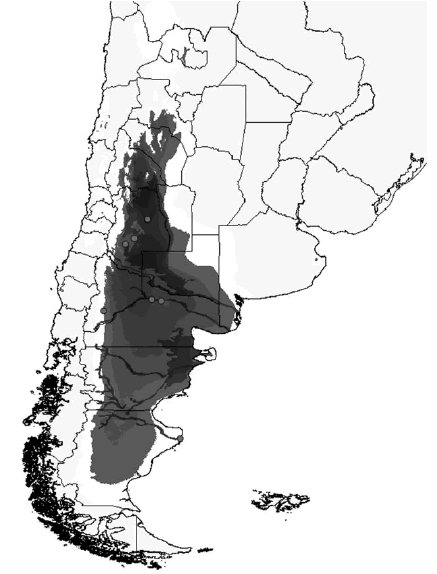
Fig. 43. Map of distribution of S. plicatipennis (grey dots), and predictive distribution, darker areas represent higher probabilities of occurrence of the species based on 21 variables.
Scybalophagus pilosus (Felsche, 1910): 339 (Canthon).
Canthon pilosus Felsche, 1910. Deutsche Entomologische Zeitschrift 4: 339.
Canthon pilosus, Schmidt, 1922. Arch fur Naturgesch 88: 63.
Canthon pilosum, Blackwelder, 1944. Bulletin of the United States Natural Museum 185: 200.
Scybalophagus pilosus, Martínez, 1953. Boletín de la Sociedad Entomológica Argentina 2: 3.
Scybalophagus pilosus, Martínez, 1954. Natura 1: 67.
Scybalophagus pilosus, Halffter & Martínez, 1968. Revista de la Sociedad Mexicana de Historia Natural 29: 263.
Type material: The holotype of this species was not studied. In the original description there is no reference to where the type material came from or where the specimen/s were deposited. The species can be reliable recognized based on the original description.
Redescription. Color black with light copper reflection. Length 6.59-8.45 mm, width 4.12-5.10 mm. Head (Fig. 15): With two medial teeth separated by wide U-shaped emargination, lacking lateral teeth, genoclypeal suture evident, with margin not indented; surface punctate on clypeus to sparsely punctate on frons; eyes dorsally elongated, ventrally globose. Pronotum (Fig. 15): Strongly transverse; surface punctate, punctures small setose, setae long (Fig. 15); anterior angle right-angled, posterior angle rounded. Elytra (Fig. 16): Surface irregular, with 2-3 tubercles at base, humeral tubercle developed, interstriae with surface smooth, setose, setae long; striae evident; elytral lateral and apical margins broadly rounded. Pigydium: Surface convex, punctate, sparsely setose, setae long. Venter: Pro-, meso-, and metaepisternum setose, setae moderately long and moderately dense. Metasternum gibbose in middle, surface smooth in middle, sparsely setose toward apical and lateral margins. Male genitalia (Figs. 17-21): Aedeagus (Fig. 17), parameres symmetrical, longer than phalobase; internal sac with brushes on submedial lob; internal sac with sclerites as in Figs. 19-21.
Distribution (Fig. 43). ARGENTINA. Mendoza: Ñacuñán (3); Reserva Laguna de Llancanelo (2); RP180 (2 km S el Nihuil) (3); Río Negro: Coronel Gómez (1); Villa Regina (1). Neuquén: Neuquén (1); Ojo de Agua (2). Santa Cruz: Puerto Deseado (Martínez, 1954).
Biology. Adults of S. pilosus are attracted to fresh dung, and they have been documented in human, cow (Halffter & Martinez, 1968, FCO personal observation) and in puma dung (FCO personal observation). Specimens were observed active at daylight.
Scybalophagus plicatipennis (Blanchard) (Figs. 22-28, 44)

Fig. 44. Map of distribution of S. pilosus (grey dots), and predictive distribution, darker areas represent higher probabilities of occurrence of the species based on 21 variables.
Scybalophagus plicatipennis (Blanchard, 1845), Voyage dans L'Amerique Méridionale pars Alcide D' Orbigny. Coléoptères: 164 (Canthon).
Canthon plicatipennis Blanchard, 1845. Voyage dans L'Amerique Méridionale pars Alcide D' Orbigny. Coléoptères: 164.
Canthon fractipes Harold, 1868a. Berliner Entomologische Zeitschrift 12: 101. (synonym).
Canthon plicatipennis, Harold, 1868a. Berliner Entomologische Zeitschrift 12: 140.
Canthon plicatipennis, Harold, 1868b. Coleopterologische Hefte 5: 57.
Coprobius fractipes, Burmeister, 1873. Stettiner Entomologische Zeitschrift 34: 416.
Canthon fractipes, Bruch, 1915. Revista del Museo de La Plata 17: 184.
Canthon plicatipennis, Schmidt, 1922. Archiv für Naturgesch 88: 67.
Canthon plicatipennis, Balthasar, 1939. Folia Zoologica Hydrobiol. 9: 202.
Canthon plicatipenne, Blackwelder, 1944. Bulletin of the United States Natural Museum 185: 201.
Scybalophagus plicatipennis, Martínez, 1953. Boletín de la Sociedad Entomológica Argentina 2: 3.
Canthon fractipes, Pereira, 1953. Dusenia 4: 394.
Scybalophagus plicatipennis, Martínez, 1954. Natura 1: 67.
Canthon plicatipennis, Pereira & d'Andretta, 1955. Revista Brasilera de Entomologia 4: 48.
Scybalophagus plicatipennis, Halffter & Martínez, 1968. Revista de la Sociedad Mexicana de Historia Natural 29: 261.
Type material. The type material of S. plicatipennis could not be found. The type should be deposited at the MNHN (Paris) but despite FCO's effort to find it in the museum's collection, the specimen could not be located. This species could be reliably identified based on the description and we consider that it is not necessary to designate a neotype at this time, specially considering that there is still hope to locate the material used by Blanchard to describe the species.
Redescription. Color black. Length 6.29-7.86, width 4.44-5.89 mm. Head (Fig. 22): With two medial teeth separated by wide U-shaped emargination, lacking lateral teeth, genoclypeal suture evident, with margin not indented; surface microrugose on clypeus to microgranulose on frons; eyes dorsally elongated, ventrally globose. Pronotum (Fig. 22): Strongly transverse; surface punctate, punctures small, sparse, glabrous; anterior angle right-angled, posterior angle rounded. Elytra (Fig. 23): Surface regular, interstriae with surface smooth; striae evident; elytral lateral and apical margins broadly rounded.
Pigydium: Surface convex, microgranulate, sparsely setose, setae short. Venter: Pro-, meso-, and metaepisternum setose, setae moderately long, sparse. Metasternum poorly gibbose in middle, surface sooth in middle, sparsely setose toward apical and lateral margins. Male genitalia (Figs. 24-28): Parameres symmetrical, longer than phalobase; internal sac with sclerites as in Figs. 26-28.
Distribution (Fig. 44). ARGENTINA. Catamarca: La Ciénaga (Martínez, 1955); Córdoba: Cruz del Eje (1); Mendoza: Ñacuñán (3); RN 40 (South of Pareditas) (6); R143 (Km 33) (1); Reserva La Payunia (Puesto los Relinchos) (1); La Rioja: Anillaco (2 km N) (2); Mascasín (11); San Luis: San Gerónimo (1). "Patagonia" (1). San Juan (Halffter & Martínez, 1968); La Pampa (Halffter & Martínez, 1968).
Biology. Adults of S. plicatipennis are attracted to human, cow, and rodent dung, and they have been observed at daylight rolling goat dung and small pieces of rodent (undetermined) dung (Halffter & Martínez, 1968, FCO personal observation).
Scybalophagus rugosus (Blanchard) (Figs. 29-39, 45)

Fig. 45. Map of distribution of S. rugosus (grey dots), and predictive distribution, darker areas represent higher probabilities of occurrence of the species based on 21 variables.
Scybalophagus rugosus (Blanchard, 1845) (Canthon).
Canthon rugosum Blanchard, 1845. Voyage dans L'Amerique Méridionale pars Alcide D' Orbigny. Coléoptères: 159.
Canthon tessellatus Erichson, 1847. in Weigmann Arch. für Naturgeschichte 13:105.
Canthon rugosus, Harold, 1868a Berliner Entomologische Zeitschrift 12: 19.
Canthon rugosus, Schmidt, 1922. Archiv für Naturgesch 88: 63.
Canthon rugosum, Guérin-Méneville, 1855. Verh. Zool. Bot. Ver. Wien 5:586.
Canthon rugosus, Balthasar, 1939. Folia Zoologica Hydrobiol. 9: 185.
Canthom rugosum, Blackwelder, 1944. Bulletin of the United States Natural Museum 185: 201.
Canthon rugosum, Gutiérrez, 1950. Anales de la Sociedad Científica Argentina 149: 54-55.
Scybalophagus rugosus, Martínez, 1953. Boletín de la Sociedad Entomológica Argentina 2: 3.
Scybalophagus rugosus, Martínez, 1954. Natura 1: 66, 68.
Scybalophagus zumpti (Frey) new synonym.
Epirinus zumpti Frey, 1963: 550.
Pseudoepirinus zumpti (Frey). Ferreira, 1964: 3.
Scybalophagus zumpti (Frey). Scholtz & Howden, 1987. Journal of the Entomological Society of South Africa 50: 116.
Type material: Lectotype male (MNHN) labeled: "Museum Paris / Chuquisaca / D'Orbigny 1834"; "Canthon / rugosum / Blanchard / LECTOTYPE"; "Scybalophagus / rugosus / Ocampo det. 2010."Lectotype here designated. Four paralectotypes (three at MNHN, one at IAZA) labeled as lectotype except: "Canthon / rugosum / Blanchard / PARALECTOTYPE"; Scybalophagus / rugosus / Ocampo det. 2010". Paralectotypes here designated.
Redescription. Color black. Length 8.06-14.35 mm, width 4.27-9.80 mm. Head (Figs. 29, 31): With two medial teeth separated by wide U-shaped emargination, lacking lateral teeth, genoclypeal suture evident, with margin slightly indented; surface rugose; eyes dorsally elongated, ventrally globose. Pronotum (Fig. 29): Strongly transverse; surface rugopunctate, punctures large setose, setae minute; anterior angle right-angled, posterior angle rounded. Elytra (Fig. 30): Humeral tubercles well developed; surface irregular, interstriae with surface smooth alternated with large dyscoidal punctures that can be as wide as interestria, punctures setose, setae minute; elytral lateral and apical margins broadly rounded. Pigydium: Surface slightly convex on apical half, flat on basal half, transversally rugose, sparsely setose, setae short. Venter (Fig. 32): Pro-, meso-, and metaepisternum setose, setae moderately long, sparse. Metasternum strongly gibbose in middle, surface smooth in middle, with small tubercles and sparsely setose toward apical and lateral margins. Male genitalia (Figs. 35-39): Aedeagus (Fig. 35), parameres symmetrical, parameres and phalobase subequal in length; internal sac with brushes on submedial lob; internal sac with sclerites as in Figs. 37-39.
Distribution (Fig. 45). ARGENTINA. Catamarca: RP 47, Cuesta de Capillitas (8); Cuesta minas Capillitas (10); La Hoyada (Sierra de Aconquija) (2). Jujuy: El Aguilar (2); La Quiaca (7). La Rioja: Anillaco (2 km N). Salta: Cachi, Piedra del Molino (4); Cachi, Izonza (3); Santa Rosa, Tastil (1). Tucumán: El Infiernillo (26); Tafí del Valle (2). BOLIVIA: La Paz: Alto La Paz (24); Chuquisaca (3); Sicasica (6). Cochabamba: Cochabamba (7); no more data (3); Guaqui (2); Oruro (4); Achacachi (2); Titicaca Basin (2); Lago Titicaca, Isla del Sol (2). CHILE: Parinacota: Volcán Tacora (1). Tarapacá: Caraguano (2). PERU: Puno (2); no more data (3).
Biology. Adults of S. rugosus are attracted to human, lama, guanaco, cow, sheep, and horse dung (Halffter & Martínez, 1968, FCO personal observation) and they have been observed rolling balls of this kind of dung. A varaible number of specimens (5-20) were observed in fresh horse dung pats during the morning.
Remarks. In a recent contribution on African Canthonini, Deschodt & Scholtz (2008) excluded S. zumpti from their work suggesting that the species is not present in South Africa and that the description of S. zumpti was based on mislabeled specimens from South America. Based on the examination of the type specimens of S. zumpti, on their comparison with S. rugosus, and considering that the area where S. zumpti was recorded has been widely surveyed and specimens never found, we consider that the latter was named based on specimens incorrectly labeled which, in fact, correspond to S. rugosus from South America (Scholtz, personal communication). Based on the above evidence we consider S. zumpti (Frey) as a junior synonym of S. rugosus (Blanchard).
BIOGEOGRAPHY
South America has been subject to many biogeographic studies due to the particular distribution patterns of its flora and fauna, specially the austral region, which has been considered to be different from the rest of South America (Jeannel, 1967; Monros, 1958; Kuschel, 1964). Several theories have been proposed to explain the origin and relationship of this region's biota with other temperate areas such as Australia, New Zealand, and South Africa. Nonetheless, these studies are centered on taxa shared with the other austral continents (referred as the austral component, Kuschel, 1964) but fewer studies have taken into consideration taxa from South America that are restricted to arid lands and have phylogenetic relationships with other taxa present in the Neotropics (Roig Juñent et al., 2008).
The genus Scybalophagus is distributed in western and southern Argentina, western Bolivia, Northern Chile, and Southern Peru, ranging from sea level in southern Argentina to above 4500 m in Bolivia and Peru. The species are distributed in the Chaco (South Western), Prepuna, Puna, Monte, Pampeana (South) and Patagonian biogeographic provinces (Fig. 40). According to Morrone (2006), Atacama, Prepuna, Puna, Monte, and the Coastal Peruvian Desert biogeographic provinces constitute the South American Transition Zone, although unpublished data and analysis including several animal and plant taxa indicate that the Patagonian province should be considered part of this transitional zone. Following the latter biogeographic scenario, Scybalophagus would be restricted almost exclusively to the South American Transition Zone. Considering the origin of its elements, this transition zone shows a mixture of Neotropical and Subantartic insect fauna (Willink, 1991; Morrone 1999, 2000, 2004, 2006; Roig-Juñent et al., 2008), being Scybalophagus included in the former group, with phylogenetic affinities with the Neotropical Canthonini (Ocampo and Hawks, 2006). In the South American Transition Zone, endemic, relictual taxa, coexist with other endemic taxa that would have speciated in the area but with sister groups in neighboring non-desert regions (e.g. Eucraniini (Scarabaeinae), Aclopinae, Allidiostomatinae [Scarabaeidae], Tauro-cerastes [Geotrupidae], Pycnochila [Carabidae], Vayella [Vespidae], Bufonacris [Tristiridae], to name a few).
The insect fauna characteristic of the South American Transition Zone is distinguished by strong adaptations to extreme environments and arid environments (precipitation is < than 400 mm year), with marked variations in day and night temperature, strong seasonality, and high solar radiation. According to Roig-Juñent et al. (2006), based on a biogeographic analysis using arthropod distribution data, the Puna, Prepuna, Monte, Chaco, and Patagonia biogeographic provinces are closely related, a hypothesis that is consistent with the Scybalophagus distribution.
Distribution of Scybalophagus species (Figs. 40-45)
As it was mentioned above, species of the genus Scybalophagus are mainly distributed in Argentina, with one species, S. rugosus, that is also known from Bolivia, Chile, and Peru.
Species in the genus show different levels of sympatry, being S. pilosus, S. lacordiarei, and S. plicatipennis, present in the same areas, and collected in the same locality: Ñacuñán, Mendoza, Argentina. Predictive models of species distribution for these three species indicate that they potentially share an extended area that covers central western Argentina, particularly Mendoza, San Luis, San Juan, and La Rioja.
Scybalophagus lacordairei has been previously known for southern Buenos Aires, center and eastern La Pampa, and center and northeastern Río Negro. Our records from Mendoza extend its known distribution to central western Argentina; the distribution of the species corresponds to the Monte biogeographic province (Fig. 41). The known distribution area of S. lacordairei ranges from near sea level up to 570 meters.
Scybalophagus pilosus is distributed in Chubut, Santa Cruz, Neuquén, Río Negro and Mendoza; the species predictive distribution model indicates that the species can be potentially distributed in La Pampa and San Juan; the distribution of the species is within the Monte and Patagonia biogeographic provinces (Fig. 43). The distribution area of S. pilosus ranges from near sea level up to 650 meters.
Scybalophagus patagonicus is distributed in Neuquén, Río Negro, Chubut and Santa Cruz; its distribution and species predictive distribution model correspond to the Patagonia biogeographic province (Fig. 42). The known distribution area of S. patagonicus ranges from near sea level up to 850 meters.
Scybalophagus plicatipennis is known to be distributed in Mendoza, San Luis, San Juan, Córdoba, and La Rioja, its distribution and species predictive distribution model correspond to the Monte biogeographic province (Fig. 44). The known distribution area of S. plicatipennis ranges from 450 to 1715 meters above sea level.
Scybalophagus rugosus is the species of the genus that reaches the farthermost northern distribution range, it occurs in the deserts of southern Peru, northeastern Chile, western Bolivia, and northwestern Argentina, down to La Rioja, always at altitudes above 930 meters and up to 4650 meters, being the species of dung beetles that reaches the highest altitude in the Americas and one of the dung beetles (and beetles in general!) that reaches the highest altitudes in the World (Fig. 45).
ACKNOWLEDGEMENTS
To all the curators and collection managers of the institutions listed in the material and methods section, we thank you very much for making specimens available for our study. This project was supported by NSF (The National Science Foundation, USA) through the following grant: Advances in Biological Informatics # 0743783, and by CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina) through the following grant: PIP #112-200801-01869. FCO thanks CONICET (Argentina) and the Instituto de Ciencias Básicas, Universidad Nacional de Cuyo for their permanent support to his research.
LITERATURE CITED
1. BALTHASAR, V. 1939. Eine Vorstudie zur Monographie der Gattung Canthon. Folia Zoologica et Hydrobiologica, Riga 9: 179-238. [ Links ]
2. BINAGHI G., G. DELLACASA & R. POGGI. 1969. Nuovi Caratteri diagnostici per la determinazione degli Onthophagus del gruppo ovatus (L.) e geonemia controllata delle specie Italiane del gruppo. Memorie della Società Entomologica Italiana. Genova 48(IB): 29-46. [ Links ]
3. BLACKWELDER, R. E. 1944. Checklist of the Coleopterous insects of Mexico, Central America, the West Indies and South America. Part. II. Bulletin of the United States Natural Museum 185: 189-341. [ Links ]
4. BLANCHARD, E. 1845. Coléoptères. In: Blanchard, E. & G.. A. Brullé: Insectes du Voyage dans l'Amérique méridionale de M. Alcide d'Orbigny (1826-1833). Berget-Levrault, Strasbourg. 6: 155-184. [ Links ]
5. BURMEISTER, H. 1873. Lamellicornia Argentina. Stettiner Entomolgische Zeitung 34: 403 -417. [ Links ]
6. BRUCH, C. 1915. Catálogo sistemático de los coleópteros de la República Argentina. Revista del Museo de La Plata 17: 184. [ Links ]
7. DESCHODT, C. & C. H. SCHOLTZ 2008. Systematics of South African forest-endemic dung beetles: new genera and species of small Canthonini (Scarabaeidae: Scarabaeinae). African Entomology 16: 91-106. [ Links ]
8. ERICHSON, W. F. 1847. in Weigmann, Conspectus Insectorum coleopterorumquae in Republica Peruana observata sunt. Archiv für Naturgeschichte 13:105. [ Links ]
9. FELSCHE, C. 1910. Über coprophage Scarabaeiden. Deutsche Entomologische Zeitschrift 4: 339-352. [ Links ]
10. FREY, G.. 1963. Neue Coprophagen und Melolonthiden mit Bestimmungstabellen (Col.). Entomologische Arbeiten aus dem Museum Georg Frey 14: 550-559. [ Links ]
11. FERREIRA, M. C. 1964. Contribuiçao para o estudo do género Epirinus Reiche (Col. Lamallicornia). Novos Taxa Entomológicos 37: 3-13. [ Links ]
12. GRAHAM, C. H., R. S. RON, J. C. SANTOS, C. J. SCHNEIDER & C. MORITZ. 2004. Integrating phylogenetics and environmental niche models to explore speciation mechanisms in dendrobatid frogs. Evolution 58: 1781-1793. [ Links ]
13. GUISAN, A. & W. THUILLIER. 2005. Predicting species distribution: offering more than simple habitat models. Ecology Letters 8: 993-1009. [ Links ]
14. GUTIÉRREZ, A. R. 1950. Scarabaeidae del norte de Chile. Anales de la Sociedad Científica Argentina 149: 54-55. [ Links ]
15. GUÉRIN-MÉNEVILLE, F. E. 1855. Catalogue des Insectes Coléoptères, recueillis par M. Gaetano Osculti, Pendant son exploration de la région éguatoriale, sur les bords du Napo et de l'Amazone. Verhandlungen der Zoologisch-Botanischen Gesellschaft in Wien 5: 586 [ Links ]
16. HALFFTER, G. & A. MARTÍNEZ. 1968. Revision monographica de los Canthonina americanos, 3ra parte. Revista de la Sociedad Mexicana de Historia Natural 29: 209-290. [ Links ]
17. HALFFTER, G. & A. MARTÍNEZ. 1977. Revision monographica de los Canthonina americanos, IV parte. Clave para géneros y subgéneros. Folia Entomológica Mexicana 38: 29-107. [ Links ]
18. HALFFTER, G. & E. G. MATTHEWS.1966. The natural history of dung beetles of the subfamily Scarabaeinae (Coleoptera: Scarabaeidae). Folia Entomologica Mexicana 12-14: 1-312. [ Links ]
19. HAROLD, E. VON. 1868a. Monographie der Gattum Canthon. Berliner Entomologische Zeitschrift 12: 1-144. [ Links ]
20. HAROLD, E. VON. 1868b. Diagnosen neuer Coprophagen. Coleopterologische Hefte 5: 57. [ Links ]
21. HIJMANS, R. J., S. E. CAMERON, J. L. PARRA, P. G. JONES & A. JARVIS. 2005. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965-1978. [ Links ]
22. INTERNATIONAL COMMISSION ON ZOOLOGICAL NOMENCLATURE (ICZN). 1999. International Code of Zoological Nomenclature, Fourth Edition. Adopted by the International Union of Biological Sciences. International trust for Zoological Nomenclature, London, xxix + 306 pp. [ Links ]
23. JEANNEL, R. 1967. Biogéographie de l'America australe. In: Deboutteville, D. E. & E.H. Rappaport (eds), Biologie de l'Amerique australe, CNRS and CONICET, Paris, pp. 401-460. [ Links ]
24. KUSCHEL, G. 1964. Problems concerning an austral region. In: Gressit, J. L. C. H. Lindroch, F. R. Forsberg, A. Fleming, and E. G. Turbott (eds), Pacific Basin Biogeography, bishop Museum Press, Honolulu, Hawaii, 443-449 [ Links ]
25. LAPORTE, F. L. N. C. (Comte de Castelnau) (1840) Historie naturelle des insectes, vol. 2. P. Duménil, Paris, 564 pp. [ Links ]
26. LUCAS, P. H.1857. Expédition dans les parties centrales de l'Amerique du Sud, de Rio de Janeiro a Lima, et de Lima au Para: exécutée par ordre du gouvernement français pendant les années 1843 a 1847, sous la direction de Francis de Castelnau. Animaux nouveaux ou rares recueillis pendant l'expédition dans les parties centrales de l' Amérique du Sud. Vol 3 Entomologie (de H. Lucas), Mollusques (de H. Hupé), Myriapodes et scorpions (de P. Gervais), pp. 1-104. [ Links ]
27. MARTÍNEZ, A.1953. Un nuevo género y especie de Canthonina (Coleop. Scarabaeid.). Boletín de la Sociedad Entomológica Argentina 2: 1-4. [ Links ]
28. MARTINEZ, A. 1954, Fauna de los Parques Nacionales I. Algunas Notas Entomológicas. Natura 1: 59-69 [ Links ]
29. MEDINA, C. A., C. H. SCHOLTZ & B. D. GILL. 2003. Morphological variation and systematics of Canthon and related genera of new world Canthonini dung beetles. Deutsche Entomologische Zeitschrift. Berlin 50(1):23-68 [ Links ]
30. MONAGHAN, M. T., D. J. G. INWARD, T. HUNT & A. P. VOGLER. 2007. A molecular phylogenetic analysis of the Scarabaeinae (dung beetles). Molecular Phylogenetics and Evolution. 45: 674-692. [ Links ]
31. MONROS, F. 1958. Consideraciones sobre la fauna del sur de Chile y revisión de la tribu Stenomelini (Coleoptera: Chrisomelidae). Acta Zoologica Lilloana 15: 143-153. [ Links ]
32. OCAMPO, F. C. & D. C. HAWKS. 2006. Molecular phylogenetics and evolution of the food relocation behaviour of the dung beetle tribe Eucraniini (Coleoptera: Scarabaeidae: Scarabaeinae). Invertebrate Systematics 20: 557-570. [ Links ]
33. PEREIRA, F. S. 1953. Notas sinonimicas. Dusenia4: 387-402. [ Links ]
34. PEREIRA, F. S. & M. A. V. D'ANDRETTA. 1955. The species of Deltochilum of the subgenus Calhyboma Kolbe. Revista Brasileira de Entomologia. 4: 7-50. [ Links ]
35. PHILLIPS, S. J., R. P. ANDERSON & R. E. SCHAPIRE. 2006. Maximum entropy modeling of species geographic distributions. Ecological Modeling 190: 231-259. [ Links ]
36. MARTINEZ, A. & F. S. PEREIRA. 1956. Dois géneros novos de Canthonini americanos (Col. Scarbaeoidea, Scarabaeidae). Papéis Avulsos de Departamento de Zoologia, Secretaria da Agricultura S. Paulo - Brasil. 12: 363-388. [ Links ]
37. MORRONE, J. J. 2000. Biogeographic delimitation of the Subantarctic Region and its provinces. Revista del Museo Argentino de Ciencias Naturales n.s. 2: 1-15. [ Links ]
38. MORRONE, J. J. 1999. Presentación preliminar de un Nuevo esquema Biogeográfi co de América del Sur. Biogeographica. 75: 1-16. [ Links ]
39. MORRONE, J. J. 2004. Panbiogeografía, componentes bióticos y zonas de transición. Revista Brasileira de Entomologia 48: 149-162. [ Links ]
40. MORRONE, J. J. 2006. Biogeographic areas and transition zones of Latin America and the Caribbean islands based on panabiogeographic and cladistic analyses of the entomofauna. Annual Review of the Entomology. 51: 467-94. [ Links ]
41. ROIG-JUÑENT, S., M. TOGNELLI & J. J. MORRONE. 2008. Aspectos Biogeográficos de los insectos de Argentina. In: Debandi, G., L. Claps & S. Roig-Juñent (eds.), Biodiversidad de artrópodos argentinos volumen 2. Pp. 1-20. Editorial Sociedad Entomológica Argentina, Mendoza. [ Links ]
42. ROIG-JUÑENT, S., M. C. DOMÍNGUEZ, G. E. FLORES & C. MATTONI. 2006. Biogeographic history of South American arid lands: A view from its arthropods using TASS analysis. Journal of Arid Environments 66: 404- 420 [ Links ]
43. SCHOLTZ, C. & H. HOWDEN. 1987. Revision of the African Canthonina. Journal of the Entomological Society of South Africa 50: 75-119. [ Links ]
44. SCHMIDT, A. 1922. Bestimmungstabelle der mir bekannten Cant-hon-Arten. 2. Verbretungsbiete der Canthon-Arten. 3. Neubes-chreibungen von Canthon, Saprosites, Mendidius, Euparia und Ataenius. Archiv für Naturgeschichte 88: 61-103. [ Links ]
45. TOGNELLI, M. E., S. A. ROIG-JUÑENT, A. E. MARVALDI, G. E. FLORES & J. M. LOBO. 2009. An evaluation of methods for modeling distribution of Patagonian insects. Revista Chilena de Historia Natural 82: 347-360. [ Links ]
46. WILLINK, A. 1991. Contribución a la zoogeografía de insectos argentinos. Boletín de la Academia Nacional de Ciencias de Córdoba 59: 125-147. [ Links ]
47. ZUNINO, M. 1972. Revisione delle specie paleartiche del genere Onthophagus. I.Il sotto genere Euonthophagus. Bollettino del Museo di Zoologia dell'Università di Torino 1: 1-28 [ Links ]














