Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Ameghiniana
versão On-line ISSN 1851-8044
Ameghiniana v.42 n.2 Buenos Aires mar./jun. 2005
Abundance and distribution of Lithophaga (Mytilidae) in extant and fossil oysters: taphonomic and paleobiological implications
Cecilia Mauna1 , Silvio Casadío1 , Ana Parras1 and Marcela Pascual2
1 Facultad de Ciencias Exactas y Naturales, Universidad Nacional de La Pampa, Uruguay 151, 6300 Santa Rosa, La Pampa, Argentina. ceciliamauna@universia.com.ar, scasadio@cpenet.com.ar , aparras@cpenet.com.ar
2 Instituto de Biología Marina y Pesquera Alte. Storni, 8520 San Antonio Oeste, Río Negro, Argentina. criadero@canaldig.com.ar
Abstract. In this study we analyze the abundance and distribution patterns of Lithophaga patagonica in valves of Ostrea puelchana and compare them to those of Lithophaga sp. observed on the fossil species Ostrea patagonica and Ostrea alvarezii from the late Miocene Puerto Madryn Formation. No specimen of the fossil oysters showed borings of Lithophaga sp. on the interior surface of the valves. This suggests that they were produced while the oysters were still living and, at the same time, that the oyster beds were buried rapidly after death. In Ostrea puelchana the boring abundance was significantly higher for the left valve and, within it, the areas more heavily bored were the umbones and the platform. The same results were obtained for valves of Ostrea alvarezii , suggesting that this oyster showed life habits similar to the living one. On the other hand, in Ostrea patagonica the abundance of Lithophaga borings was the same on both valves. This agrees well with its life habit, in which the shells mainly are oriented almost vertically. The left valve of Ostrea patagonica showed no preferential location for the borings. In the right valves of Ostrea patagonica the posterior and anterior margins show perforation values that are higher than expected. Results suggest that the life position of oysters is one of the factors influencing the abundance and distribution of Lithophaga borings. This information is useful to infer the life position of fossil oysters and to reconstruct their taphonomic history.
Resumen. A bundancia y distribución de las perforaciones de L ithophaga (m ytilidae ) en ostras fósiles y actuales : implicancias tafonómicas y paleobiológicas . En este trabajo se analizaron los patrones de distribución y la abundancia de Lithophaga patagonica sobre las valvas de Ostrea puelchana coleccionadas en el banco Las Grutas, Golfo San Matías y se compararon con los de Lithophaga sp. observadas en las especies fósiles Ostrea patagonica y Ostrea alvarezii provenientes de la Formación Puerto Madryn, Mioceno tardío. La ausencia de perforaciones en el interior de las valvas sugiere que fueron producidas mientras las ostras permanecían vivas y que las conchillas fueron enterradas rápidamente. En Ostrea puelchana la frecuencia de las perforaciones fue significativamente más alta para la valva izquierda y, en ésta, las áreas más perforadas fueron el umbo y el zócalo. Los mismos resultados fueron obtenidos para las valvas de Ostrea alvarezii . En Ostrea patagonica la frecuencia de las perforaciones de Lithophaga fueron estadísticamente iguales para ambas valvas. Esto concuerda con su hábito de vida, principalmente con las conchillas casi verticales. La valva izquierda de Ostrea patagonica no mostró una ubicación preferencial de las perforaciones, mientras que en la valva derecha los márgenes posterior y anterior mostraron una frecuencia de perforación más alta que la esperada. Los resultados sugieren que la posición de vida de las ostras es uno de los factores que influyen en la frecuencia y distribución de las perforaciones de Lithophaga . Esta información es útil para inferir la posición de vida de ostras fósiles y su historia tafonómica.
Key words. Lithophaga; Bioerosion; Oysters; Taphonomy; Miocene; Argentina.
Palabras clave. Lithophaga; Bioerosión; Ostras; Tafonomía; Mioceno; Argentina.
Introduction
Lithophaga or its borings in oyster shells along the Argentine Atlantic coast have been common since the late Miocene (Pascual et al ., 2001; Farinati and Zavala, 2002; Casadío et al ., in press). The extant Lithophaga patagonica (d'Orbigny, 1842) is known in South America from the coast of Santa Catarina (Brazil) to Bahía Camarones (Argentina), where it is a usual component of benthic communities associated with rocky shores (Pastorino, 1995).
Because of its size, Lithophaga patagonica is considered a macroborer and because it actively bores the substrate where it lives it is classed as a euendolith (Golubic et al ., 1975). Its body is sub-cylindrically elongated and its shell is thin (Blanco et al ., 1988). According to Blanco et al . (1988), the body shape -with a clear lengthwise growth component and a restricted increase in width and height- can be attributed to the anterior-posterior development of the boring mechanism and the resulting reduction of the cavity due to the carbonatic layer secreted by the organism and that covers the inner surface.
There are many studies on extant species of Lithophaga and their role in boring communities (Kleemann, 1980; Kelly and Bromley, 1984; Scott, 1988; Scott and Risk, 1988; Cantera and Contreras, 1988; Jones and Pemberton, 1988). Most of the studies concerning the role of Lithophaga in hard substrate communities are within a general context and treat these borers together with other bioerosion-producing organisms. In addition, they were all carried out in tropical or subtropical areas and mainly in coral reefs (Hein and Risk, 1975; Scott, 1988; Scott and Risk, 1988; Guzmán and Cortéz, 1989; Cortéz, 1991, 1992; Perry, 1998).
Other studies have focussed on determining the specificity in substrate selection by larvae of Lithophaga settling on corals (Highsmith, 1980; Mokady et al ., 1991, 1992, 1994; Brickner et al ., 1993; Krumm and Jones, 1993). This selection is very important because the postlarvae completely and irreversibly loose their mobility and are unable to shift substrates (Chia fide Mokady et al ., 1991). Physical and biological factors such as the texture and position of the substrate bacterial coverings and the presence of co-specifics may control the active selection of a substratum in larvae of marine invertebrate (Crisp, 1976; Barnett and Crisp, 1979; Weiner et al ., 1989). This may involve complex behavioral patterns, including active swimming and the use of sensory mechanisms such as photothaxis, geothaxis and chemothaxis (Burke, 1983).
According to Mokady et al . (1991, 1992), the selection of substrate and metamorphosis in living corals by Lithophaga larvae may have been controlled by chemical factors such as living tissue of the corals.
In the San Matías Gulf, Lithophaga patagonica is a common member of the community recorded in shells of the extant Ostrea puelchana d'Orbigny, 1842. Together with sponges they are the main boring organisms living within the shells of these oysters (Castellanos, 1957; Pascual et al ., 2001).
In this study we analyze the frequencies and distribution patterns of Lithophaga patagonica on valves of Ostrea puelchana and compare them to those of Lithophaga sp. observed on the fossil species O . patagonica d'Orbigny, 1842, and O . alvarezii d'Orbigny, 1842, from the late Miocene Puerto Madryn Formation.
In Ostrea puelchana the boring frequency was significantly higher for the left valve and, within it, the areas more heavily bored were the umbones and the platform. Similar results were obtained for valves of Ostrea alvarezii , suggesting that this oyster showed life habits similar to the living one. On the other hand, in Ostrea patagonica the frequency of Lithophaga borings was the same on either valve. This agrees well with its life habit, which was mainly with the shells placed almost vertically forming nests or concentrations of several specimens cemented to each other.
Results suggest that the life position of oysters is one of the factors influencing the abundance and distribution of Lithophaga borings. Therefore, this kind of information is useful for infering the life position of fossil oysters and also helpful in reconstructing their taphonomic history.
Ostrea puelchana d'Orbigny, 1842
Ostrea puelchana ranges from Río Grande do Sul (Brazil) to the San Matías Gulf (Argentina), where it forms large oyster grounds (Castellanos, 1957). It has a solid shell, with a flat right valve and a larger and convex left valve. The left valve can reach up to 11.4 cm high, 8.8 cm long, 2.5 cm thick and weigh as much as 0.104 kg. The right valve up to 9.2 cm high, 7.2 cm long, 2.0 cm thick, and may weigh up to 0.046 kg.
The demography of this species within the San Matías Gulf is very peculiar. It shows a bimodal population structure reflecting the existing size difference between sexes. Specimens smaller than 5.5 cm are predominantly epibiontic males, while larger specimens are mainly females (Pascual et al ., 1989; Pascual et al ., 2001).
Ostrea puelchana is larviparous and the dispersal period occurs during the veliger stage lasting up to 20 days (Pascual et al ., 1989; Pascual and Zampatti, 1995). After this period, if the pediveliger larva finds an adequate substrate, it attaches to it.
The attachment process is very fast and is produced on the mid-ventral margin of the left valve. After fixation, the larva undergoes metamorphosis and becomes a juvenile oyster (Pascual, 1993). Shortly after fixation the oyster may reach a size larger than the substrate to which it became attached. Therefore, from then on it lies free on the bottom and may be affected by currents.
According to Pascual et al . (2001) the life position that the oyster acquires is a characteristic that can change along its life and affect its stability in the water current.
Sixty one percent of oysters from the Las Grutas ground in the San Matías Gulf live with the right valves downwards. This position is more stable because it creates a suction effect that prevents the oyster from being dragged by the current (Pascual et al ., 2001). When analyzing the life position adopted by oysters according to their size class, a decreasing trend in the percentage of shells lying on the right valve can be noticed as size increases. The values recorded by Pascual et al . (2001) were 99% for oysters less than 3 cm wide (n=67), 78% for those between 3 and 7 cm wide (n=225) and 59% for sizes larger than 7 cm wide (n=1,487).
Ostrea puelchana has a unique characteristic among living oysters, i.e ., carriage. This consists in the development of a flat platform from the anterior margin of the left valve in females, used for settling of the larvae. Growth rate of the oysters in this platform is very slow because of an inhibitory effect of the female which maintains them in their protandric phase - i.e ., male-. This process assures female fertilization (Pascual et al ., 1989; Pascual, 1993, 1997).
Although there are no studies concerning space competition between the epibiontic males of Ostrea puelchana and specimens of Lithophaga patagonica , it seems likely that the development of perforations on the platform of the left valve of females may influence the reproductive success as it hampers the settling or permanence of the males.
Description of the Las Grutas oyster ground
The Las Grutas oyster ground lies in open areas of the San Matías Gulf (figure 1). During low tide it ranges from 2.5 to 6 m deep and the speed of the tidal currents range from 20 to 30 cm.s- 1 (Servicio de Hidrografía Naval, 1969). According to Fernández (1989), the average water temperature is lowest in August (8ºC) and highest in January (21ºC).

Figure 1. Location map. The asterisks show the position of the oyster ground at the Las Grutas and the Puerto Pirámide section / Localización del banco ostrero Las Grutas en el golfo San Matías y de Puerto Pirámide en la Península de Valdés, Chubut.
The area of the oyster ground that was included in the study by Pascual et al. (2001) was 2 km 2 . The bottom consists of silty to clayish rocks with limestone concretions. They are crossed by channels filled with sand where, besides the oysters, there are small areas colonized by algae. The density of oysters, maximum and average, was respectively 22 individuals/ m 2 and 8 individuals/m 2 (Pascual et al ., 2001).
Ostrea patagonica d'Orbigny, 1842
Ostrea patagonica is a large, heavy and thickshelled oyster, in which the left valve of the adult specimens may reach more than 30 cm high, 20 cm long, 5 cm thick and weigh as much as 3 kg. The right valve is nearly flat, up to 20 cm high, 15 cm long, 5 cm thick, and may weigh up to 2.5 kg. Valves show very strong commarginal growth lines, many of which rise to form ridges or crests that render a very rugose aspect to the external surface. In the Puerto Pirámide section, Ostrea patagonica shows two ecophenotypes, i.e., clustered and gryphaeate. Both types inhabited soft sandy and muddy bottoms in a shoreface to offshore environment. Bunched forms consist of nearly vertical assemblages several specimens thick. The specimens are cemented to each other. These clusters can be associated to form reefs of several hundred square meters and reach more than five meters thick. On the other hand, the gryphaeate forms grew isolated and the shells lay on the left convex valve with the ventral commissure elevated above the bottom surface.
Ostrea alvarezii d'Orbigny, 1842
Adult specimens of Ostrea alvarezii show, in general, shapes and sizes similar to those of O. puelchana . The shell is solid and the right valve is flat, while the left one is larger, convex and has strong radial ribs. The left valve can reach up to 10 cm high, 8 cm long, 1.5 cm thick and can weigh as much as 0.180 kg. Right valves can reach 8 cm high, 6 cm long, 0.5 cm thick, and may reach 0.080 kg. At the Puerto Pirámide section, Ostrea alvarezii grew isolated, free, mainly lying on the right valve and living in tidal channels with shelly-sandy bottoms.
Iribarne et al. (1990) mentioned the presence of small epibiontic oysters attached to the anterior margin of specimens of Ostrea alvarezii collected in the late Miocene at Gran Bajo del Gualicho (Río Negro). They suggested for this oyster a reproductive strategy similar to the one recorded for Ostrea puelchana .
Description of the beds with Ostrea patagonica and Ostrea alvarezii
Fossil Ostrea patagonica and O . alvarezii come from the Puerto Madryn Formation, which is very well exposed at Puerto Pirámide, in the province of Chubut (figure 1). The section exposed at Puerto Pirámide belongs, as suggested by del Río et al . (2001) and Casadío et al . (in press), to the upper part of a depositional sequence including a transgressive system tract (TST) and a highstand system tract (HST). The TST interval is represented by shelf sediments deposited below wave base, while those of the HST represent tidal channel and tidal flat deposits. Casadío et al. (in press) described 3 facies associations (figure 2).

Figure 2. Stratigraphic log at the Puerto Pirámide section / Perfil estratigráfico de la sección estudiada en Puerto Pirámide.
Facies association 1 comprises a group of lithofacies representing a fining-upwards cycle. It includes sandstone and massive bioturbated mudstones facies. The association includes trace fossils of the Cruziana ichnofacies. Invertebrates in life position are common. Ostrea patagonica is grouped in clusters of up to 30 specimens. Other taxa include isolated specimens of Ostrea alvarezii , Aequipecten paranensis , Amusium paris , Mytilus sp. and Pachymagas piramidesia in life position as well as several species of crabs. The facies grade upwards into a laminated mudstone facies with trace fossils belonging to the Zoophycos ichnofacies. The grain-size, trace fossil assemblages, and fossil preservation suggest a relatively low sedimentation rate in a middle shelf, low-energy environment below fair weather wave base. However, according to taphonomic information, sudden increases in sedimentation rate possibly related to storms can be noticed at the base of this association.
Facies association 2 predominates in the middle and upper part of the succession and includes facies of sandstone and mudstone intercalations. Flaser, wavy and lenticular bedding are common. The facies contains few invertebrate remains, but there are beds containing abundant Skolithos and Ophiomorpha . The grain size and sedimentary structures suggest relatively low energy, possibly deposited in a sandy to muddy-sandy tidal flat. This facies association is always related to facies association 3.
Facies association 3 comprises a group of lithofacies clustered to form fining-upwards sequences. The geometry of these beds is lenticular with an erosive base. At the base of these sets there are shell concentrations, within a sandy matrix. In these beds there are common specimens of Ostrea alvarezii . The beds may show medium to large scale trough cross-stratification. These deposits grade upwards into fine to medium sandstone and mudstone, showing heterolithic bedding.
The geometry of the beds, the grain-size and sedimentary structures suggest that these deposits are the infilling of tidal channels and tidal sand waves. Shelly-sandy dunes and waves migrated along the deepest part of these channels, while in other areas with weaker currents small ripples migrated on sandy tidal flats. The presence of mudstone intraclasts suggests that the channels were flanked by fine tidal flat sediments (facies association 2).
Materials and methods
One hundred and fifty seven specimens of Ostrea puelchana were analyzed. These were collected live in the Las Grutas oyster bank (40º 48' S; 65º 05' W). Fifty three valves of Ostrea patagonica and 186 valves of O . alvarezii were obtained from beds within the Puerto Madryn Formation, exposed at Puerto Pirámide (42º 35' 48'' S, 64º 15' 27'' W), province of Chubut.
Samples of the live specimens were taken randomly in the Las Grutas ground within the most densely packed zone of the oyster bank. The aim of such a choice was to facilitate collection and obtain the highest possible number of specimens. Sampling took place by divers during February 2003 at 3.5 meters depth.
For Ostrea puelchana , all borings were counted and, when possible, the specimens of Lithophaga patagonica were selected out and the following data were recorded: oyster specimen number, type of valve (left or right), sector of the valve containing the perforation, and specimen number (of L . patagonica ). The oyster valves were broken in pieces in order to ensure counting of all borings.
To record Lithophaga sp. borings in fossil oysters, the shells of these were superficially broken and the data recorded were: valve number, type of valve (left or right), and sector of the shell bored.
The location of borings on the shell was classified following a division of the shell surface into different sectors (figure 3). These divisions reflect distinct morphological features of the valves that may influence differential settling of Lithophaga sp. larvae on each one.
The left valves of Ostrea puelchana and O . Alvarezii were divided into six different sectors and the right valves into five (figure 3). For the left valve, these sectors are: apex (3%), platform (5%), anterior margin (16%), ventral margin (30%), posterior margin (21%) and center (25%). In the right valve there is no platform, so the areas into which it was divided are: apex (3%), anterior margin (21%), ventral margin (30%), posterior margin (21%) and center (25%).

Figure 3. Maps of external surface of the valves of Ostrea puelchana ( A ), Ostrea alvarezii ( B ) and Ostrea patagonica ( C ). A: apex, P: platform, AM: anterior margin, PM: posterior margin, VM: ventral margin, C: center / Áreas reconocidas en las valvas, vista externa de Ostrea puelchana ( A ), Ostrea alvarezii ( B ) y Ostrea patagonica ( C ). A: ápice, P: plataforma, AM: margen anterior, PM: margen posterior, VM: margen ventral, C: centro.
In Ostrea patagonica the areas and percentages for both valves were: apex (18%), anterior margin (11%), ventral margin (30%), posterior margin (11%), center (30%).
Statistic analysis and graphics were done using STATISTICA Version 99. Tests were performed for differences between proportions. Therefore, in order to determine the independence of valve area and frequency of boring, contingency tables were performed (Chi-square Independence Tests). The expected perforation value for each area was obtained multiplying the total borings for each valve by the area occupied by each sector.
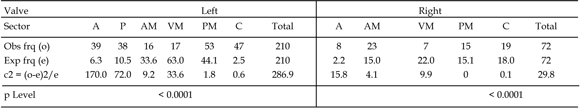
Table 1. Independence test between the valve sectors and boring frequencies for Ostrea puelchana / Test de independencia entre los sectores de las valvas de Ostrea puelchana y las frecuencias de perforación.

Table 2. Independence test between the valve sectors and boring frequencies for Ostrea patagonica / Test de independencia entre los sectores de las valvas de Ostrea patagonica y las frecuencias de perforación.

Table 3. Independence test between the valve sectors and boring frequencies for Ostrea alvarezii / Test de independencia entre los sectores de las valvas de Ostrea alvarezii y las frecuencias de perforación.
Results
The percentage of bored valves in Ostrea patagonica was 98% (n=53), which is significantly higher (p< 0.01) than for O. puelchana (35%, n= 314) and O . alvarezii (35%, n= 186).
In Ostrea patagonica 95% of right valves and 100% of left valves contained borings. On the other hand, in O. puelchana only 23% of right valves and 47% of left valves showed any borings. In Ostrea alvarezii the right valves with perforations were 18% while 51% of the left ones were bored. In Ostrea alvarezii and O. puelchana the percentage of borings in the left valve was significantly higher than in the right one (p<0.05). The percentages of right and left valves with borings in Ostrea puelchana and O . Alvarezii showed no significant differences (p>0.05).
The studied valves of Ostrea patagonica record 1,169 borings of Lithophaga sp., with an average of 21 borings on the right valve and 25 on the left one. In Ostrea puelchana there was a total of 282 borings, averaging 1.7 for the right valve and 3 for the left one. In Ostrea alvarezii there were 168 borings with an average of 1.6 in the right valve and 3 in the left one.
Most of the bored valves of Ostrea patagonica showed between 1 and 30 borings, with a maximum of 75 (figure 4). In Ostrea alvarezii and O. Puelchana most bored valves showed between 1 and 2 borings (maximum 12) and 1 and 3 borings (maximum 21) respectively (figure 4).

Figure 4. Number of borings in valves of Ostrea patagonica , Ostrea alvarezii and Ostrea puelchana / Número de perforaciones en las valvas de Ostrea patagonica , Ostrea alvarezii y Ostrea puelchana .
Analysis of the contingency tables (tables 1, 2, 3) shows that only the left valve of Ostrea patagonica showed no preferential location for the borings (p>0.05). The right valve of Ostrea patagonica and both valves of O . alvarezii and O . Puelchana showed preferential distribution of the borings (p<0.01). The most heavily bored sectors in comparison with the expected results on the right valve of Ostrea patagonica were the anterior and posterior margins (figure 5). In the left valve of Ostrea puelchana , the platform and the apex carried the highest number of borings while on the right valve it was the apex (figure 6). In both valves of Ostrea alvarezii the sectors containing more borings were the apex and anterior margin (figure 7). In all species, the ventral margin showed a boring frequency lower than expected.

Figure 5. Ventral view of the left valve ( A ) of Ostrea patagonica with borings in the anterior and posterior margins and center. The right valve ( B ) is bored in the ventral and anterior margins / Vista ventral de la valva izquierda ( A ) de Ostrea patagonica con perforaciones en el centro y en los márgenes posterior y anterior. La valva derecha ( B ) está perforada en los márgenes ventral y anterior. Scale bars / escalas = 1 cm .

Figure 6. Right ( A ) and left ( B ) valves of Ostrea puelchana . In the platform of the left valve there are an epibiont oyster male and Lithophaga patagonica . Apex of the right valve is bored by Lithophaga patagonica / Valvas derecha ( A ) e izquierda ( B ) de Ostrea puelchana . En la plataforma de la valva izquierda se observa un macho epibionte y un ejemplar de Lithophaga patagonica . El ápice de la valva derecha está perforado por Lithophaga patagonica . Scale bars / escalas = 1 cm.

Figure 7. Left valve of Ostrea alvarezii with apex and platform bored and right valve with apex and posterior margin bored / Valva izquierda de Ostrea alvarezii con el ápice y la plataforma perforadas y valva derecha con el ápice y el margen posterior perforados. Scale bars / escalas = 1 cm.
Conclusions
No specimen of the fossil oysters showed borings of Lithophaga sp. on the interior surface of the valves. This suggests that they were produced while the oysters were still living and, at the same time, that the oyster shells were rapidly buried after death.
In Ostrea patagonica the presence of Lithophaga sp. borings and the average number of them for both valves are higher than for the other two species considered. The reason for this could be either a preference of the larva for a particular substrate or else differential survival of the larvae.
The more rugose surface of the valves of Ostrea patagonica and of the left valves of O . puelchana and O . alvarezii could possibly trigger an active selection by the larvae (rugophilic behavior according to Bien et al ., 1999 and Bottjer, 1982). The larvae settling on rugose surfaces would be better protected from grazers, increasing their survival rate. Pascual (1997) recorded that the effect of chitonids on the shells of Ostrea puelchana was one of the main epibiont mortality causes.
Likewise, assuming similar life habits for Ostrea puelchana and O . alvarezii , i.e ., with the right valve preferably lying against the bottom, the larvae of Lithophaga sp. would have a higher survival chance on the left valve.
The beds with Ostrea patagonica reflect an environment of mainly low energy and low sedimentation rate. Therefore the oysters would have had a longer residence period on the bottom with the consequent exposure to larvae settling. Contrarily, Ostrea alvarezii is found in rocks representing tidal channels. Individuals here would have been covered by sediment for variable periods of time, in a manner similar to that observed in the extant population of Ostrea puelchana at Las Grutas.
Substrate hardness is another factor affecting larvae settling and subsequent boring rate (Kleemann, 1973, 1980). Ostrea puelchana and O . alvarezii have more compact and smaller shells than O . patagonica , in which larvae of Lithophaga sp. could have been thus more successful.
Left and right valves of Ostrea puelchana and O . alvarezii show preferential distribution of the borings they carry. In both Ostrea puelchana and O . Alvarezii the preferred sectors in the left valve are the platform and the apex and in the right valve are the apex and anterior margin. This means that borings occur more frequently in the thicker and more compact umbonal area, while along the thinner margins of the valves the number of borings is comparatively lower. Likewise, the higher number of borings on the apex of Ostrea alvarezii could be caused by the fact that –as in O . puelchana – this area remains exposed even when the rest of the shell has been covered by sediment after storm events. Tidal currents subsequently reexpose then (personal observations).
In the right valves of Ostrea patagonica the posterior and anterior margins show perforation values that are higher than expected. This could be attributed to the fact that the clustered ecophenotype oysters lived with their commissure perpendicular to the bottom, although most of them show a slight tilting towards the posterior or anterior margin. This tilt led to the exposure of the posterior and anterior margins, which thereby became more likely to be colonized by Lithophaga sp. larvae.
Acknowledgments
This study was partially supported by Universidad Nacional de La Pampa and Fundación Antorchas to S.C. We also thank to R. G. Bromley, A. D'Alessandro and M. Griffin for enlightening discussions and comments.
References
Barnett, B.E. and Crisp, D.J. 1979. Laboratory studies of gregarious settlement in Balanus balanoides and Elminius modestus in relation to competition between these species. Journal of the Marine Biological Associated of the United Kingdom 59: 581-590. [ Links ]
Bien, W.F., Wendt J.M. and Alexander, R.R. 1999. Site selection and behavior of sponge and bivalve borers in shells of the Cretaceous oysters Exogyra cancellata and Pycnodonte mutabilis from Delaware, U.S.A. Historical Biology 13: 299-315. [ Links ]
Blanco, G.A.C., Marasas, M.E. and Amor, A. 1988. Una comparación del crecimiento relativo en los Mitílidos Lithophaga patagonica y Brachydontes rodriguezi (Mollusca, Bivalvia). Anales Museo de Historia Natural Valparaíso 19: 65-74. [ Links ]
Bottjer, D.J. 1982. Paleoecology of epizoans and borings on some Upper Cretaceous chalk oyster from the Gulf Coast. Lethaia 15: 75-84. [ Links ]
Brickner, I., Kramarsky-Winter, E., Mokady, O. and Loya, Y. 1993. Speciation in the coral-boring bivalve Lithophaga purpurea : evidence from ecological, biochemical and SEM analysis. Marine Ecology Progress Series 101: 139-145. [ Links ]
Burke, R.D. 1983. The induction to metamorphosis of marine invertebrate larvae. Stimulus and response. Canadian Journal of Zoology 61: 17011-1719. [ Links ]
Cantera, J.R. and Contreras, R. 1988. Bivalvos perforadores de esqueletos de corales escleractinarios en la isla de Gorgona, Pacífico Colombiano. Revista Biología Tropical 36: 151-158. [ Links ]
Casadío, S., Feldmann, R., Parras, A. and Schweitzer, C. (In press). Miocene fossil decapoda (Crustacea: Brachyura) from Patagonia, Argentina and their paleoecological setting. Bulletin of the Carnegie Museum of Natural History . [ Links ]
Castellanos, Z.A. de. 1957. Contribución al conocimiento de las ostras del litoral Argentino ( Ostrea puelchana y O . spreta ). Ministerio de Agricultura y Ganadería de la Nación, Argentina 52 pp. [ Links ]
Cortéz, J. 1991. Los arrecifes coralinos de Golfo Dulce, Costa Rica: Aspectos geológicos. Revista Geológica de América Central 13: 15-24. [ Links ]
Cortéz, J. 1992. Los arrecifes coralinos de Golfo Dulce, Costa Rica: Aspectos ecológicos. Revista Biología Tropical 40: 19-26. [ Links ]
Crisp, D.J. 1976. Settlement responses in marine organisms. In: R.C. Newell (ed.), Adaptation to environment: essay on the physiology of marine animals , Butterworths, London, pp. 83-124. [ Links ]
del Río, C.J., Martínez, S.A. and Scasso, R.A. 2001. Nature and origin of spectacular marine Miocene shell beds of northeaster Patagonia (Argentina): paleoecological and bathymetric significance. Palaios 16: 3-25. [ Links ]
Farinati, E., and Zavala, C. 2002. Trace fossils on shelly substrate. An example from Miocene of Patagonia, Argentina. Acta Geológica Hispánica 37: 29-36. [ Links ]
Fernández, M.O. 1989. [ Parámetros hidrobiológicos del Golfo San Matías. Report Instituto de Biología Marina y Pesquera Almirante Storni, Río Negro, 10 pp. Unpublished.]. [ Links ]
Golubic, S., Friedmann, I. and Schneider, J. 1975. The lithobiontic ecological niche, with special reference to microorganisms. Journal of Sedimentological Petrology 51: 475-478. [ Links ]
Guzmán, H.M. and Cortéz, J. 1989. Coral reefs community structure at Caño Island, Pacific Costa Rica. Marine Ecology 10: 23-41. [ Links ]
Hein, F.J. and Risk, M.J. 1975. Bioerosion of coral heads: Inner patch reefs, Florida Reef Tract. Bulletin Marine Science 25: 133-138. [ Links ]
Highsmith, R.C. 1980. Burrowing by the bivalve mollusk Lithophaga curta in the living reef coral Montipora berryi and a hypothesis of reciprocal larval recruitment. Marine Biology 56: 155-162. [ Links ]
Iribarne, O.O., Pascual, M.S. and Zampatti, E.A. 1990. An uncommon oyster breeding system in Late Tertiary Patagonian species. Lethaia 23:153-156. [ Links ]
Jones, B. and Pemberton, S.G. 1988. Lithophaga borings and their influence on the diagenesis of corals in the Pleistocene Ironshore Formation of Grand Cayman Island, British Indies. Palaios 3: 3-21. [ Links ]
Kelly, S.R.A. and Bromley, R.G. 1984. Ichnological nomenclature of clavate borings. Palaeontology 27: 793-807. [ Links ]
Kleemann, K.H. 1973. Lithophaga lithophaga (Linné) (Bivalvia) in different limestones. Malacologia 14: 345-347. [ Links ]
Kleemann, K.H. 1980. Boring bivalves and their host corals from the Great Barrier Reef. Journal of Molluscan Studies 46: 13-54. [ Links ]
Krumm, D.K. and Jones, D.S. 1993. New coral-bivalve association ( Actinastrea-Lithophaga ) from the Eocene of Florida. Journal of Paleontology 67: 945-951. [ Links ]
Mokady, O., Bonar, D., Arazi, G. and Loya, Y. 1991. Coral host specificity in settlement and metamorphosis of the date mussel Lithophaga lessepsiana (Vaillant, 1865). Journal of Experimental Marine Biological Ecology 146: 205-216. [ Links ]
Mokady, O., Arazi G., Bonar, D. and Loya, Y. 1992. Settlement and metamorphosis specificity of Lithophaga simplex Iredale (Bivalvia: Mytilidae) on Red Sea corals. J ournal of Experimental Marine Biological Ecology 162: 243-251. [ Links ]
Mokady, O., Rozenblantt, S., Graur, D. and Loya, Y. 1994. Coralhost specificity of Red Sea Lithophaga bivalves: interspecific and intraespecific variation in 12S mitochondrial ribosomal RNA. Molecular Marine Biology and Biotechnology 3: 158-164. [ Links ]
Orbigny, A. d'. 1831-1842. Voyage dans l'Amerique Meridionale. Paris, vol. 5 part 3 Mollusques 758 pp. 9 Atlas 85 pls. Ed. Chez P. Bertrand. [ Links ]
Pascual, M.S. 1993. [ Contingencia y adaptación en la ecología reproductiva de Ostrea puelchana. Tesis Doctoral. Universidad Nacional de Mar del Plata, Argentina, 183 pp. Unpublished.]. [ Links ]
Pascual, M.S. 1997. Carriage of draw males by adult female puelche oysters: the role of chitons. Journal of Experimental Marine Biology and Ecology 212: 173-185. [ Links ]
Pascual, M.S. and Zampatti, E.A. 1995. Chemically mediated adult-larval interaction triggers settlement in Ostrea puelchana : applications in hatchery production. Aquaculture 133: 33-44. [ Links ]
Pascual, M.S., Iribarne, O.O., Zampatti, E.A. and Bocca, A.H. 1989. Female-male interaction in breeding system of puelche oyster ( Ostrea puelchana , d'Orbigny). Journal of Experimental Marine Biology and Ecology 132: 209-219. [ Links ]
Pascual, M.S., Zampatti, E.A. and Iribarne, O.O. 2001. Population structure and demography of the puelche oyster ( Ostrea puelchana d'Orbigny, 1841) grounds in northern Patagonia, Argentina. Journal of Shellfish Research 3: 1003-1010. [ Links ]
Pastorino, G. 1995. Moluscos costeros recientes de Puerto Pirámide, Chubut, Argentina. Academia Nacional de Ciencias 93: 1-30. [ Links ]
Perry, C.T. 1998. Macroborers within coral framework at Discovery Bay, north Jamaica: species distribution and abundance, and effects on coral preservation. Coral Reefs 17: 277-287. [ Links ]
Scott, P.J. 1988. Distribution, habitat and morphology of the Caribbean coral and rock boring Lithophaga bisulcata (d'Orbigny) (Mytilidae: Lithophaginae). Journal of Molluscan Studies 54: 83-95. [ Links ]
Scott, P.J. and Risk, M.J. 1988. The effects of Lithophaga (Bivalvia: Mytilidae) boreholes on the strength of the coral Porites lobata . Coral Reefs 7: 145-151. [ Links ]
Servicio de Hidrografía Naval, Cdo. J. Armada 1969. Carta H-262. Puerto San Antonio. (IV-1969). [ Links ]
Weiner, R.M., Walch, M., Labare M.P., Bonar, D.B. and Colwell, R.R. 1989. Effect of biofilms of the marine bacterium Alteromonas colwelliana (LST) on the settling of the oyster Cassostrea gigas (Thunberg, 1793) and C . virginica (Gmelin, 1971). Journal of Shellfish Research 8: 117-123. [ Links ]
Recibido: 19 de abril de 2004.
Aceptado: 30 de setiembre de 2004.














