Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Ameghiniana
versão On-line ISSN 1851-8044
Ameghiniana v.45 n.1 Buenos Aires jan./mar. 2008
Paleobiology of Pleistocene ground sloths (Xenarthra, Tardigrada): biomechanics, morphogeometry and ecomorphology applied to the masticatory apparatus
M. Susana Bargo1 and Sergio F. Vizcaíno1
1CIC-CONICET. División Paleontología Vertebrados, Museo de La Plata, Paseo del Bosque s/n, 1900 La Plata, Argentina. msbargo@fcnym.unlp.edu.ar , vizcaino@fcnym.unlp.edu.ar
Abstract. The fossil xenarthrans include giant forms, the ground sloths (Tardigrada), characteristic of the mammal fauna of the Pleistocene of South America. Although most authors agree in considering them as herbivorous, these forms have not been studied in terms of detailed morpho-functional analyses of their masticatory apparatuses. The aim of this work is the study the masticatory apparatus of the large Pleistocene ground sloths Glossotherium robustum, Lestodon armatus, Mylodon darwini and Scelidotherium leptocephalum (Mylodontidae) applying biomecanichal and morphogeometrical methods, and to compare with the information obtained for Megatherium americanum (Megatheriidae). The results are integrated with recent ecomorphological analyses that include three variables (hypsodonty index, dental occlusal surface area and relative width and shape of the muzzle) providing useful information for the inference of dietary habits and to propose a niche partitioning among these species. Glossotherium robustum and Lestodon armatus, the wide-muzzled sloths, were mostly bulk-feeders (i.e. ingest great amounts of food with each bite; probably grass and herbaceous plants). Mylodon darwini and Scelidotherium leptocephalum, the narrow-muzzled sloths, were mixed or selective-feeders (i.e. select plants or plant parts; grass and/or tree and shrubs foliage). The tooth design of mylodontids indicates that teeth were used mainly for crushing and grinding turgid and fibrous items respectively. Megatherium americanum was probably the most selective feeder among these sloths, and selectively fed on particular plants (shrubs) or plant parts (leaves, twigs, fruits). Its dentition was designed mostly for cutting soft but tough items which might include flesh, leaving open the possibility of an omnivorous diet.
Resumen. Paleobiología De Los Perezosos Terrestres (Xenarthra, Tardigrada) Pleistocenos: Biomecánica, Morfogeometría Y Ecomorfología Aplicadas Al Aparato Masticatorio. Los xenartros fósiles incluyen formas gigantes, los perezosos terrestres (Tardigrada), características de la fauna de mamíferos del Pleistoceno de América del Sur. Si bien la mayoría de los autores los han considerado herbívoros, estas formas no han sido objeto de un análisis morfofuncional detallado de sus aparatos masticatorios. El objetivo de este trabajo es estudiar el aparato masticatorio de los perezosos terrestres Glossotherium robustum, Lestodon armatus, Mylodon darwini y Scelidotherium leptocephalum (Mylodontidae) aplicando métodos biomecánicos y morfogeométricos, y compararlos con Megatherium americanum (Megatheriidae). Estos resultados son integrados con aquellos obtenidos de análisis ecomorfológicos que incluyen tres variables (índice de hipsodoncia, área de la superficie oclusal dentaria y ancho relativo y forma del hocico), proveyendo información para inferir probables hábitos alimenticios y proponer una partición de nichos. Glossotherium robustum y Lestodon armatus, formas de hocico ancho, no eran selectivos, se alimentaban al bulto (i.e. ingerían grandes cantidades de alimento con cada bocado; probablemente pastos y plantas herbáceas). Mylodon darwini y Scelidotherium leptocephalum, formas de hocico angosto, eran selectivos o intermedios (i.e. seleccionaban plantas o partes de plantas; pastos y/o hojas de árboles y arbustos). El diseño de los dientes indica que eran usados principalmente para triturar y moler alimentos semiduros o pulposos y fibrosos. Megatherium americanum era el más selectivo, y probablemente se alimentaba seleccionando ciertas plantas o partes de plantas (hojas, ramas, frutos). Sus dientes estaban diseñados principalmente para cortar alimentos blandos pero resistentes, que podrían incluir carne, lo que deja abierta la posibilidad de una dieta omnívora.
Key words. Mammalia; Xenarthra; Tardigrada; Paleobiology; Masticatory apparatus.
Palabras clave. Mammalia; Xenarthra; Tardigrada; Paleobiología; Aparato masticatorio.
Introduction
Sloths (Xenarthra: Tardigrada) are among the most conspicuous mammals in the Cenozoic faunas of South America and, as a group, they show a great diversity (with more than 80 genera, grouped in four families: Megatheriidae, Megalonychidae, Nothrotheriidae and Mylodontidae). During the early Miocene, the frequency and diversity of tardigrades increased considerably, including small to medium sized facultative arboreal forms (Scillato-Yané, 1986; White, 1997; McDonald and De Iuliis, 2008). But it is not until the Pleistocene, that a great number of gigantic sloths (Mylodontidae and Megatheriidae) are recorded. Webb (1985) proposed the term ground sloth for these forms belonging to different clades that reached the late Pleistocene-early Holocene. At present, tardigrades are represented only by two convergent genera: Choloepus (Illiger, 1811), the twotoed sloth, allied with members of Megalonychidae, and Bradypus (Linnaeus, 1758), the three-toed sloth, and the sister taxon of all the remaining sloths (Gaudin, 2004; figure 1). Carlini and Scillato-Yané (2004: 434) proposed a different view, considering that Bradypus differentiated through a process of heterochrony within Megalonychidae.

Figure 1. Phylogeny of Tardigrada (modified from Gaudin, 2004) / filogenia de los Tardigrada (modificado de Gaudin, 2004).
Although most authors agree in considering ground sloths as herbivorous, the South American forms have not been studied in terms of detailed morpho-functional analyses of their masticatory apparatuses. For more than a century general speculations on the dietary preferences of ground sloths have been proposed. Owen (1842, 1860) made remarkable descriptions of the skeletons of the ground sloths Glossotherium Owen, 1839 and Megatherium Cuvier, 1796, and gave extensive explanations about their possible diet and behavior. He based his conclusions on the morphology of the skull, combined with peculiarities of the rest of the skeleton, but always by analogy with living tree sloths primarily, and with other herbivorous extant mammals of similar size (i.e. elephant, giraffe). Owen (1842:159-160) wrote: …"The close correspondence between the Megatherium and the Mylodon [Glossotherium] in the modifications of the skeleton determining the peculiar forces acting from the hind upon the fore-parts, compels us to infer that they resembled each other in the mode of which they obtained their sustenance; and nevertheless, the difference in the form of the grinding surface of the teeth, as well as in their size and the depth of insertion, obviously indicates some difference in the substances comminuted… On the theory that the Megatherioids subsisted on foliage, it is most natural to suppose that the Mylodon and Megalonyx, with teeth most closely resembling those of the Sloths, would feed, like them, on the leaves and tender buds; while the Megatherium, whose essentially bradypodal teeth were more modified by their arrangement in a closer series, …so as concurrently to offer and obvious resemblance to the Elephant's dentition, would be thereby able to bruise the smaller branches, and to masticate these together with the buds and leaves"… "All the characteristics which co-exist in the skeleton of the Mylodon and the Megatherium conduce and concur to the production of the forces requisite of uprooting and prostrating trees;…" Latter in 1860, Owen stated about Megatherium …"Guided by the general rule that animals having the same kind of dentition have the same kind of food, I conclude that the Megatherium must have subsisted, like the Sloths, on the foliage of trees; but that the greater size and strength of the jaws and teeth, and the double-ridged grinding surface of the molars in the Megatherium, adapted it to bruise the smaller branches as well as the leaves, and thus to approximate its food to that of the Elephants and Mastodons".
Stock (1925) proposed that megatheres, together with megalonychids and nothrotheres, were probably browsers, while mylodontids were grazers. Cabrera (1926) discussed the diet of Megatherium, rejecting some theories on myrmecophagy or insectivory, and entirely concurred with Owen's statements on a folivorous diet. Winge (1941: 364) pointed that Megatherium "has progressed farthest in specialisation as a plant feeder" and that it "must undoubtedly have fed on unusually tough leaves which required much power of mastication". Scillato-Yané (1977) indicated that the Megatheriinae and Mylodontidae had a robust calcaneum, which might be used as a steady point to dig with the fore limbs to root out the grasses, which were the basis of their diet.
More recently, dietary habits were proposed based on some features of the masticatory apparatus. McDonald (1987) indicated that the Plio-Pleistocene South American Scelidotheriinae were selective-feeders because the long and narrow muzzle was appropriate to select plant parts. For the North American ground sloths, Naples (1987, 1989) proposed that Nothrotheriops shastense (Sinclair, 1905) (Nothrotheriidae) was a selective browser and Paramylodon harlani (Owen, 1843) (Mylodontidae) a browser/ grazer, instead of a strict grazer. McDonald (1995) considered that Megalonyx Harlan, 1825 (Megalo- nychidae), also from North America and Eremotherium Spillmann, 1948 (Megatheriidae), from North and South America, were browsers.
An alternative hypothesis to the ground sloths herbivory was proposed by Fari ña (1996) on the basis of a study that analyses the trophic relations of the Lujanian (late Pleistocene, early Holocene) megafauna. Fariña suggests that the ground sloths might have been opportunistic scavengers, especially Megatherium americanum (Cuvier, 1796). Fariña and Blanco (1996) support this hypothesis indicating that Megatherium was probably an active hunter based on the development of the olecranon process, which would allow it to stab effectively the prey.
In the last decade, several studies on the paleobiology of South American mammals, mostly xenarthrans, were conducted based on morpho-functional and biomechanical analysis of the feeding apparatus (Fari ña, 1985, 1988; Vizcaíno, 1994; Vizcaíno and Fariña, 1997; Vizcaíno and Bargo, 1998; Vizcaíno et al., 1998; De Iuliis et al., 2000; Pérez et al., 2000; Bargo, 2001a and b; Fariña and Vizcaíno, 2001; Vizcaíno and De Iuliis, 2003; Bargo, De Iuliis and Vizcaíno, 2006; Bargo, Toledo and Vizcaíno, 2006; Vizcaíno, Bargo and Cassini, 2006), and their paleoecological context, specially referred to the trophic relations (Fariña, 1996; Vizcaíno, 2000; Vizcaíno et al., 2006).
The great variation in skull and dental morphology, body size and proportion among ground sloths suggests that they had diversified to fill a variety of niches. Their marked differences in the skeletal and dental anatomy with other mammalian herbivores, and the lack of recent analogs, makes difficult to interpret the ecology of ground sloths, particularly their dietary habits. Even the extant tree sloths are too specialized to provide good models: with relative small body masses (less than 10 kilograms), they are strictly arboreal, folivorous (consuming mainly tree and liana leaves), and extremely silent during their rare and careful movements, spending most of their time well-hidden in the high canopy of Neotropical forests (Chiarello, 2008).
Several studies on the biomechanics of the masticatory apparatus have demonstrated correlations among the behavior, diet, and form of the skull, jaws, dentition, and musculature in extant mammals ( e.g., Maynard Smith and Savage, 1959; Turnbull, 1970; Moore, 1981; Naples, 1982, 1985; Smith, 1993; Spencer, 1995; Mendoza et al., 2002; Mendoza and Palmqvist, 2007, 2008). Particularly, Janis (1995) proposed that there are three variables that allow discriminating among ungulates of grazing, browsing and mixed feeder habits: hypsodonty index, lower premolar row relative length, and the relation between palatal width and muzzle width. However, it is worth mentioning that recently Mendoza and Palmqvist (2007, 2008) detected at some exceptions - e.g. the white rhino, Ceratotherium simum (Burchell, 1817)- to these generalizations considering each variable individually (see Discussion). In several recent papers we analyzed and adapted these variables (see Discussion) to the morphology of the tardigrades in order to facilitate their application to these mammals. (Bargo, De Iuliis and Vizcaíno, 2006; Bargo, Toledo and Vizcaíno, 2006; Vizcaíno, Bargo and Cassini, 2006).
This work summarizes the Doctoral Thesis of one the authors (Bargo, 2001a) as a review of the already published subjects, with the addition of the unpublished, updated, and improved information and results. The goal is the study of the morphology of the masticatory apparatus of the large Pleistocene mylodont ground sloths Glossotherium robustum (Owen, 1842), Lestodon armatus Gervais, 1855, Mylodon darwini Owen, 1839 and Scelidotherium leptocephalum Owen, 1840 (Mylodontidae) applying biomecanichal and morphogeometrical methods, and compare these taxa with Megatherium americanum (Megatheriidae; Bargo, 2001b) (figure 2). The results obtained are then integrated with recent ecomorphological analyses of these ground sloths, including the hypsodonty index (Bargo, De Iuliis and Vizcaíno, 2006), the dental occlusal surface area (Vizcaíno, Bargo and Cassini, 2006) and the relative width and shape of the muzzle (Bargo, Toledo and Vizcaíno, 2006), providing useful information for the inference of different dietary habits and, hence, allowing us to propose a paleoecological interpretation (niche partitioning) of the Pleistocene tardigrades.
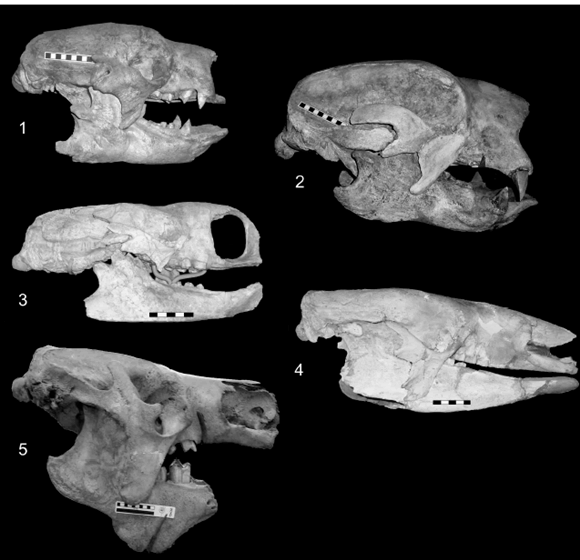
Figure 2. 1, Glossotherium robustum (MLP 3-140). 2.2, Lestodon armatus (MLP 3-30). Scale bar / escala: 10 cm. 3, Mylodon darwini (CN 43).4, Scelidotherium leptocephalum (MLP 3-402). Scale bar / escala: 5 cm. 5, Megatherium americanum (MNHN PAM 276). Scale bar / escala: 10 cm.
The use of terms browser and grazer
As becomes evident from the previous section, most authors refer to the dietary habits of fossil sloths as grazers or browsers. In some cases, they also use the feeding category of mixed-feeders.
However, as noted in studies on living herbivores (Hofmann and Stewart, 1972; Spencer, 1995), the terms browsing and grazing are of ambiguous nature. A review of the literature available on the subject reflects that the terms have been used to refer to the mode of food acquisition, as well as the type of food ingested, i.e. "browsing" may refer to selective feeding of any food type, as well as eating dicot material; "grazing" denotes grass eating, but is used to mean eating of forbs as well. Nature provides plenty of examples of these "ambiguities". For instance, the living cervid Ozotoceros bezoarticus (Linnaeus, 1758) which feeds mainly on grasses, but also plucks small morsels from nutritious plants of certain species, would be a browser from the point of view of the food acquisition and a grazer considering only the food ingested. Consequently, when applied to an extinct species -and to great extent also to living animals-, the reader (and probably the authors themselves) do not know if the terms refer to a mode of food acquisition selected by size and shape, and so is independent of the taxonomic nature of the item within certain ranges (i.e. herbivory), or the selection of specific taxa independent of the size and shape, or an uncertain degree of combination of both.
It seems that the basic problem while trying to classify mammals as either browsers or grazers is that both terms are based in different criteria, as it becomes apparent considering the meaning of the words. On one side, browse reflects capacity for searching and is used for feeding on leaves, young shoots, and other vegetation independently of its taxonomy (although it's definition also includes grazing among its meanings). On the other, graze implies some taxonomic, as well as structural constraints: to feed on growing grasses and herbage.
Hofmann and Stewart (1972) proposed a classification for ruminant ungulates as bulk and roughage eaters (grass eaters, and within them roughage grazers; fresh grass grazers; and dry regions grazers), selectors of concentrate juicy herbage (with tree and shrub foliage eaters, and fruit and dicot foliage selectors), and intermediate feeders (with some preferring grasses, and other preferring forbs, and shrub and tree foliage). This alternative classification was based on the stomach-structure and feeding habits of East African ruminants, but in some way it reflects physical properties of the consumed plants. It was proposed that bulk-feeders eat low quality plant material, and that this correlates with wide-muzzled animals to improve the quantity of food intake in each bite, while selective-feeders are narrow-muzzled facilitating selection of more nutritious small plant or plant parts (Janis and Ehrhardt, 1988; Solounias et al., 1988; Solounias and Moelleken, 1993). From this point of view, the previously mentioned cervid Ozotoceros bezoarticus would be a selective feeder, and its morphology clearly correlates with that behavior (Merino et al., 2005).
Following Solounias and Moelleken (1993), the terms grazers and browsers should be used specifically to express types of vegetation eaten, not to distinguish between selective and non-selective feeders. However, to assign a type of vegetation eaten is difficult if behavior is not observable and isotopic analyses are not available. In some cases, as the complex multivariate approach by Mendoza et al. (2002), observations on eaten food are highly statistically correlated to craniodental features for living ungulates. But, as the same authors pointed out, the discrimination of feeding habits in ungulates is a rather difficult task, due to functional, historical and biomechanical constraints, features that are highly emphasized in forms so distant phylogenetically, with almost no living relatives or evident analogs.
In an attempt to escape to this dead end, we analyze morphology based on a mechanical assessment (e.g. relationships between strength and speed, etc) and hence on how it deals with physical properties (hardness, wearing, size). Plotnick and Baumiller (2000) stated that this paleobiomechanic approach does not indicate if an organism has an optimal design, but determines whether structures were capable of doing a given function. Following this criterion, we will refer to dietary habits of ground sloths on the basis of the main physical properties of the food inferred from the morphological evidence.
Materials and methods
For the purpose of this study, nearly thirty skulls (most of them very complete) from different institutions from Argentina and abroad were examined. The acronyms and material studied are listed in Appendix 1.
The skull morphology of the mylodontids G. robustum, L. armatus, M. darwini and S. leptocephalum was described and then compared through morphogeometric methods. The masticatory muscles were reconstructed for the jaw mechanics analysis, and the occlusal patterns and mandibular movements determined through study of the craniomandibular joint, the form and arrangement of the dentition, including occlusal wear patterns, and the form and structure of the mandibular symphysis. These results were compared with those obtained by Bargo (2001b) for M. americanum.
Skull morphology and shape analysis. The morphology of the skull, mandible and dentition of the ground sloths were described comparatively, emphasizing only on those features that are relevant for the biomechanical analysis. The shapes of the skull and mandible were then compared using a morphogeometric method, which allows determining patterns of morphological variability and change. A superimposition technique (RFTRA, Resistant-Fit Theta- Rho-Analysis) was used. It analyzes changes in shape through the superimposition of one form onto another (base and target specimen, respectively) using the position of landmarks (homologous and geometrical points, or type I and II landmarks respectively sensu Bookstein, 1981) (see Benson et al., 1982; Chapman, 1990a and references therein). The distance coefficients obtained allow constructing distance matrices that, through a cluster analysis using UPGMA (Unweighted Pair-Group Method with Arithmetic Averages) generate dendrograms. RFTRA identifies and measures the homologous regions of change in shape by establishing congruence among those that have not changed. Although RFTRA has been applied especially to identify shape variability in a taxonomical context (Chapman, 1990b), recent studies on xenarthrans demonstrated this approach as useful in morpho-functional interpretations (Vizcaíno and Bargo, 1998; Vizcaíno et al., 1998; De Iuliis et al., 2000; Vizcaíno and De Iuliis, 2003).
Comparisons of pairs of specimens (skulls in lateral and palatal views, and mandibles in lateral views) of the four mylodontids were performed. In all cases, Glossotherium robustum was used as the base specimen. For this purpose, 19 landmarks (12 homologous, and 7 geometric were chosen for the lateral and palatal views of the skull, and 14 landmarks (5 homologous, and 9 geometric) for the lateral view of the mandible (Appendix 2, figure 3). Comparisons of G. robustum - M. americanum were performed and illustrated by Bargo (2001b: 186, figure 7).
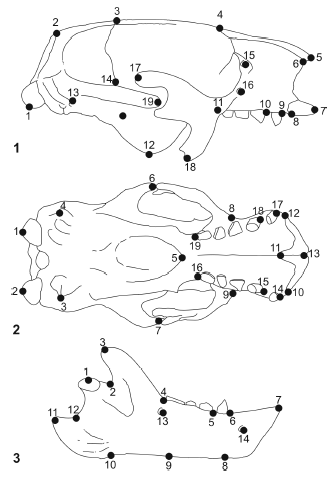
Figure 3. Skull in lateral (1) and palatal (2) views, and mandible in lateral view (3) of Glossotherium robustm showing the landmarks used for the morphogeometrical (RFTRA) analysis / cráneo en vista lateral (1) y palatal (2) y mandíbula en vista lateral (3) de Glossotherium robustum mostrando los landmarks utilizados para el análisis morfogeométrico (RFTRA).
Masticatory musculature. Muscle attachment sites are usually unambiguously indicated in mammals by features of the skull and jaws, such as roughened surfaces, scar lines, ridges, and crests. These features are usually more conspicuous in fossil than in living mammals, but the identification of them depends on the size of the fossil specimen, on their degree of preservation and the ontogenetic age of the individual. The areas of origin and insertion of the masticatory muscles were reconstructed based on these features, and the patterns of musculature in modern mammals (Maynard Smith and Savage, 1959; Turnbull, 1970), particularly those in tree sloths Bradypus and Choloepus (Macalister, 1869; Windle and Parson, 1899; Edgeworth, 1935; Sicher, 1944; Naples, 1982, 1985). Naples (1987, 1989) reconstructed in great detail the masticatory muscles, including the subdivisions of the m. temporalis, m. massetericus and m. pterygoideus of Nothrotheriops shastense and Paramylodon harlani. The material described by Naples, which one of the authors (MSB) was able to study, comes from the collection made from the Rancho La Brea Tar Pits housed at the Page Museum. The skulls and mandibles of these ground sloths are extremely complete, with an excellent degree of preservation, but the skulls and jaws recovered from the tar pits were disarticulated, and in most cases, it is not possible to establish individual correspondences between skulls and jaws from the same individual.
The nature and relationships of the skeletal features in the material examined for this work preclude as confident a reconstruction of the musculature detail that Naples was able to achieve. In any event, the scope of this paper does not require such detail reconstruction. Thus, only those features of the musculature that are relevant for analysis of the lines of action are described here. The musculature reconstructed for this analyses includes the m. temporalis, m. massetericus (including the m. zygomaticomandibularis), and m. pterygoideus.
Jaw mechanics. The application of biomechanics to the study of fossil vertebrates has proven to be a good approach to the testing of functional hypothesis (Plotnick and Baumiller, 2000). In this way, the jaws can be considered as a lever system, with the pivot at the craneomandibular joint, and the masticatory muscles providing the input force, whereas the output force is produced by the teeth on food. Then, the moment arms of the lines of action of the masticatory muscles can be estimated to analyze relationships between bite force and velocity. This procedure was applied to recent mammals (Maynard Smith and Savage, 1959; Turnbull, 1970), and then to fossils with a new geometric model proposed by Vizcaíno et al. (1998), which allows comparisons between fossil and extant mammals (figure 4). De Iuliis et al. (2000) and Vizcaíno and De Iuliis (2003) used this methodology with fossil giant armadillos, and Bargo (2001b) with the ground sloth Megatherium americanum. Total lengths of the mandibles were standardized at 11 cm to allow comparison among specimens of different sizes. Once the areas of origin and insertion of the masticatory muscles are reconstructed, the moment arms of the lines of action of the m. temporalis and m. massetericus can be estimated, based on the calculation of the averages of a given number of moment arms, generated from different points in the origin and insertion areas of each muscle. Due to the dorsoventrally expanded shape of the jugal in sloths, the different points of the origin of the m. massetericus were located in the upper, middle and lowest point (figure 4), which correspond to the posterior, middle and anterior points in armadillos. In this way, the values of the moment arms can be obtained independently of the localization of the line of action, which is difficult to determine in fossils. Interpretations on the relationships between bite force and velocity may be made by comparing the proportions of the combined moment arms of the m. massetericus and m. temporalis to those of different tooth positions (i.e., the anteriormost, middle, and posteriormost teeth). The analysis of teeth wear facets complements the mechanical analysis since it can be used to infer the direction of the mandibular movement during mastication (Greaves, 1973; Rensberger, 1973; Costa and Greaves, 1981). In extant mammals, the main jaw movement during the power stroke is upward and anteromedially directed (Hiiemae, 1978). Secondarily, an increase of distinct components occurs in different groups (e.g. anterior in rodents, vertical in carnivores, and lateral in herbivores). During these movements, teeth wear facets are produced, indicating the main direction of the jaw movement. Two types of wear facets can be described: those produced by tooth-tooth contact -which might have striations, indicating the orientation but not the direction of the movement- and those produced by tooth-food-tooth contact, characterized by the absence of striations. Also, the leading and trailing edges and leading and trailing interfaces can be identified. The interfaces enamel-dentine, continuous and discontinuous, indicates more clearly the direction of the upper and lower molar to each other during the masticatory movement.
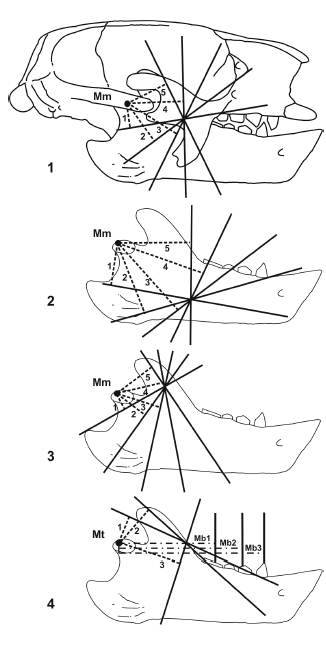
Figure 4. 1, Geometric model used for the estimation of moment arms of the masticatory muscles in Glossotherium robustum. Moment arms of the m. massetericus (Mm 1 to 5) from the middle point of the origin area of the muscle on the zygomatic arch / Modelo geométrico utilizado para estimar los brazos de momento de los músculos masticatorios en Glossotherium robustum; Brazos de momento del m. massetericus (Mm 1 a 5) a partir del punto medio del área de origen del m. massetericus sobre el arco cigomático; 2, from the anterior most point of the origin area / a partir del punto más anterior del área de origen; 3, and from the posterior most point of the origin area / y a partir del punto más posterior del área de origen; 4, Moment arms of the m. temporalis (Mt 1 to 3), and the bite (Mb 1 to 3, from the posterior most, middle and anterior most tooth respectively) / Brazos de momento del m. temporalis (Mt 1 a 3) y de la mordida (Mb 1 a 3 desde el diente más posterior, medio y más anterior, respectivamente. Lines of action of the muscles / Líneas de acción de los músculos Moment arms of the m. massetericus and m. temporalis / Brazos de momento del m. massetericus y m. temporalis. Moment arms of the bite / Brazos de momento de la mordida).
Ferigolo (1985) analyzed the internal structure of the xenarthrans teeth. Sloth's teeth lack enamel, and are composed of three tissues: an external layer of cementum, a thin layer of hard dentine, and a modified soft dentine, which has low resistance to abrasion, forming the core. The cementum and soft dentine are easily abraded, leaving the hard dentine, interposed between these two tissues. Wear facets with the leading and trailing edges, and especially the continuous and discontinuous interfaces, can be observed between both types of dentine. This methodology was applied by Naples (1982, 1989) in living tree sloths and Paramylodon harlani, respectively. In this study, wear facets and striations were analyzed in numerous lower teeth series of Glossotherium robustum, in order to contrast with the observations in Paramylodon by Naples (1989) and then compared with the other mylodontids and M. americanum.
Results
Cranial morphology
Extensive descriptions of the skulls of ground sloths were given by Owen (1842, 1856, 1857), Lydekker (1886, 1894), Ameghino (1889) and Kraglievich (1922, 1923, 1928 y 1934). McDonald (1987), De Iuliis (1996) and Esteban (1996) included detailed anatomical descriptions in their systematic revisions of the Scelidotheriinae, Megatheriinae and Mylodontinae, respectively. Naples (1989) studied the masticatory apparatus of Paramylodon harlani, describing in detail the skull and masticatory muscles in order to infer feeding behavior and diet.
The skulls of ground sloths show a number of osteological features that vary among different groups of tardigrades, but taken as a whole, distinguish them clearly from the rest of mammals: edentulous premaxillae, loosely fused to the maxillae (with the exception of Scelidotherium and Megatherium, were is generally strongly attached to the skull); elongated maxillae; incomplete postorbital bar, open zygomatic arch, with ascending and descending processes of the jugal well developed; pterygoids expanded as thin blades; mandible with a long predental space, with the length variable within the different groups. The dental morphology of sloths is also extremely different from other placental mammals, making difficult to establish tooth homologies. The most characteristic features are the lack of enamel, as well as the lack of deciduous dentition and the cuspation pattern observed in other mammals. Teeth are homodont (so called molariforms or caniniforms), hypsodont and ever growing (i.e., hypselodont), strongly reduced in number (dental formula is 5/4, except in Mylodon with 4/4 and the Pleistocene nothrotheres with 4/3) and separated by diastema variable in length.
This section presents comparative descriptions of the four species of mylodontid considered in this study, but only of those features of the cranium that are relevant for analysis of the mechanics of the masticatory apparatus. For a description of the skull of Megatherium americanum see Bargo (2001b).
Skull. The skull shape of Glossotherium robustum and Lestodon armatus is prismatic-rectangular and anteriorly widened, with L. armatus the larger of the two taxa (figures 2.1, 2). In both species the rostrum (muzzle) is formed mostly by the maxillae, quadrangular in lateral view, which bear anterior flanges, very convex and upwards and posteriorly directed, for the insertion of the caniniforms. In L. armatus the caniniforms are larger than in G. robustum, and anteriorly or mesially located from the first molariform, resulting in a longer diastema (figures 5.1, 3). The premaxillae of G. robustum and L. armatus are small, arrow-head shaped, with the posterior medial processes that attach loosely to the premaxillary processes of the maxillae. This feature contributes to the frequent loss of these bones in the specimens. The skull of Mylodon darwini is large, like L. armatus, rectangular shaped but much more elongated. The increase in length is reflected in the anterior portion (muzzle), due to an elongation of the premaxillae, maxillae and nasals, as demonstrated by the morphogeometric analysis (see below). M. darwini is clearly distinguishable from the other mylodontids because of the presence of a robust nasal arch in ontogenetically older individuals: the premaxillae, more robust than those of G. robustum and L. armatus, is firmly fused to the maxillae and extended dorsally to reach the nasals, forming an unusual complete arch, extensively described by Kraglievich (1934) (figures 2.3 and 6.1). The region of the muzzle is more elevated in comparison with the posterior part of the skull, a feature clearly observed in lateral view. Scelidotherium leptocephalum has a smaller skull, elongated and very narrow in comparison with the above mentioned mylodontinae (figures 2.4 and 6.3). The rostrum is longer than the posterior part of the skull, due to an elongation of the maxillae and specially the premaxillae, a feature clearly observed in the shape analysis performed. The premaxillae are also Vshaped, but unlike the mylodontines, the lateral rami are longer and deep. As in M. darwini, the premaxillae are strongly fused to the maxillae, so they are preserved with the skull in almost all specimens. An ascending process is observed in some specimens in the dorsal and medial part of the premaxillae, apparently supporting the nasal cartilage (Bargo et al., 2006). McDonald (1987) reported one specimen with the nasal cartilage ossified, forming a structure analogous to that of M. darwini.
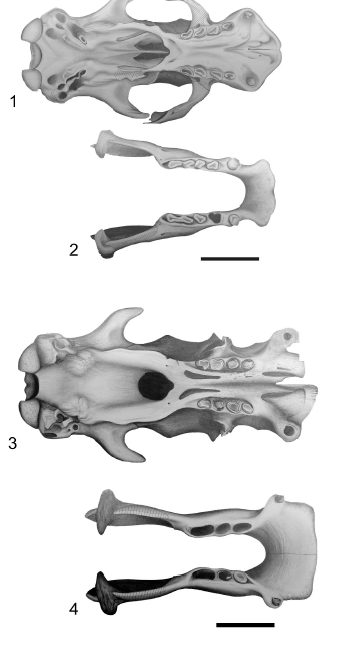
Figure 5. Glossotherium robustum skull (1) and mandible (2) in occlusal view. Lestodon armatus skull (3) and mandible (4) in occlusal view / Glossotherium robustum cráneo (1) y mandíbula (2) en vista oclusal. Lestodon armatus cráneo (3) y mandíbula (4) en vista oclusal. Scale bar / escala: 10 cm.
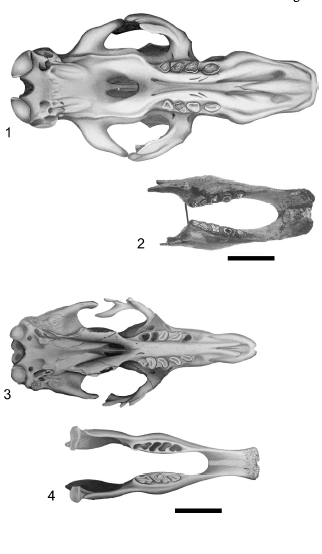
Figure 6. Mylodon darwini skull (1) and mandible (BM(NH) M- 16617 Holotype) (2) in occlusal view. Scelidotherium leptocephalum skull (3) and mandible (4) in occlusal view / Mylodon darwini cráneo (1) y mandíbula (BM(NH) M-16617 Holotipo) (2) en vista oclusal. Scelidotherium leptocephalum cráneo (3) y mandíbula (4) en vista oclusal. Scale bar / escala: 10 cm.
The zygomatic arch is very conspicuous in all ground sloths, and very similar in form in all of the mylodontids considered here (figure 2). Unfortunately, it is a frequently missing piece in fossils due to the fragility of the joint between the zygomatic process of the maxilla and the jugal. The jugal is expanded posteriorly in a vertical plate, with three or four processes. The ascending and descending processes are well developed, and their orientations vary slightly. The ascending process curves posteriorly, while the more expanded descending process is ventral and posteriorly oriented, showing in some species (e.g. S. leptocephalum) two or three small processes. The intermediate process is usually short, with a pointed apex directed slightly ventrally. The zygomatic process of the squamosal is a digitiform structure. The anterior and posterior parts of the arch are very close in all mylodontids, but never fuse so that the arch is incomplete, unlike Paramylodon or Megatherium, in which a secondary connection is formed.
The pterygoids do not differ markedly in the four considered species. They are inflated in their posterior part, and form expanded bones ventrally, like thin blades, with the ventral border rounded. The external surface is roughened, with many scars for the origin of the m. pterygoideus.
The palate is sub triangular in G. robustum and L. armatus (with the anterior the widest part ), less triangular in M. darwini, and parallel and very narrow in S. leptocephalum (figures 5 and 6). In all species it is flat in the transverse axis, but convex in the anteroposterior axis. It is located almost at the plane of the teeth occlusal surface, covering the lingual side of the teeth by a well marked flange, although the crowns of the teeth are visible on the labial surface of the maxillary border. The palate shows a marked roughened surface with many foramina (vascular perforations), and a V-shape notch in the anterior edge for the articulation of the premaxillae.
Craniomandibular joint (CMJ). The CMJ does not vary in its general morphology in the taxa here examined. It is located at the level of the occlusal plane, or just slightly over it. The glenoid fossa is poorly defined, with a shallow depression on the squamosal process which allows the mandibular condyle great freedom of motion (figures 5.1, 3 and 6. 1, 3). The mandibular condyle is also positioned at the level of the occlusal plane. It is wider mediolaterally than anteroposterioly, projecting farther medially than laterally relative to the coronoid process, and bears a short neck (figures 5.2, 4 and 6.4).
Mandible. The form of the mandibles of G. robustum and L. armatus do not differ markedly. The horizontal ramus is very deep at the level of the last molariform, decreasing gradually toward the caniniform, and increasing slightly again until the anterior border of the symphysis, which is situated at the occlusal surface level. The ventral border of the horizontal ramus is straight. The symphysis is strongly fused, as in all sloths, and elongated (the predental space is about half length of the dental series). It is elevated from the horizontal, in an angle of about 45º, but not surpassing the teeth occlusal plane. In occlusal view, the symphysis is very wide (particularly in L. armatus) with the anterior border slightly convex or some times straight (figure 5). The mandibles of M. darwini and S. leptocephalum are more elongated and slender than those of G. robustum and L. armatus. The elongation is produced in the predental space, as demonstrated by the shape analysis (see below), confirming a narrow and elongated predental spout (longer than the dental series) (figure 6), elevated over the teeth occlusal plane. The horizontal rami are also deep at the level of the last molariform, decreasing gradually to the anterior part.
The ascending rami are quite similar in the most relevant features of the four species. The angular area is prominent, expanded ventrally, surpassing slightly the ventral border of the horizontal ramus. The lateral surface is convex, showing well marked crests for the insertion of the m. massetericus, while the medial surface is concave. The angular process lies below the level of the occlusal plane. The coronoid process has a wide base, and it is not very high. It rises very inclined above the condyle, and then curves posteriorly.
Dentition. The dental formula is 5/4, except in Mylodon darwini, which has 4/4, due to the lost of the first upper molariform. Glossotherium robustum and Lestodon armatus posses a canine-like first tooth, termed the caniniform. The upper and lower dental series converge backwards in G. robustum, L. armatus and, in less degree, in M. darwini, while in S. leptocephalum they are parallel.
As mentioned above, sloth's teeth are composed of three types of tissues: cementum, and hard and soft dentine. In mylodontids, the outer layer of cementum is thin, and the occlusal surfaces of the molariforms are concave, due to the central soft dentine basin. The outer hard dentine forms sharp cutting edges (figure 7.1). In contrast, Megatherium americanum has an extremely thick layer of cementum which, together with the soft dentine, is easily abraded, leaving the hard dentine interposed between these two tissues, forming sharp, transverse crests separated by a deep valley (V-shaped) (figure 7.2).

Figure 7. Glossotherium robustum (1) upper left tooth series. Megatherium americanum (2) lower right tooth series / Glossotherium robustum (1) serie dentaria superior izquierda. Megatherium americanum (2) serie dentaria inferior derecha. Scale bar / escala: 5 cm.
Shape analysis
RFTRA analysis consists of comparisons of skulls, in lateral and palatal views, and mandibles in lateral views of pair of specimens, i.e. L. armatus, M. darwini and S. leptocephalum compared with G. robustum as the base specimen. Table 1 shows the distance coefficients for each pair of comparisons.
Table 1. Morphological distances obtained through shape analysis using RFTRA (Distance coefficients = D) / distancias morfológicas obtenidas a través del análisis de la forma usando RFTRA (coeficientes de distancia = D).

Glossotherium robustum - Lestodon armatus (figure 8). The overall shape of the skulls of G. robustum y L. armatus has the lowest morphological distance among the ground sloths included in this analysis, which indicates the highest similarity. The most relevant change is observed in the muzzle region. Lestodon armatus has the maxilla more lengthened in its mesial and ventral part, which becomes evident for the anterior displacement of the premaxillomaxillar suture. The caniniform is also anteriorly displaced, while the first molariform moves posteriorly, with the subsequent formation of a large diastema. The mandibles are also very similar. As in the skull, the most remarkable change is the anterior displacement of the caniniform and the longer diastema in L. armatus.
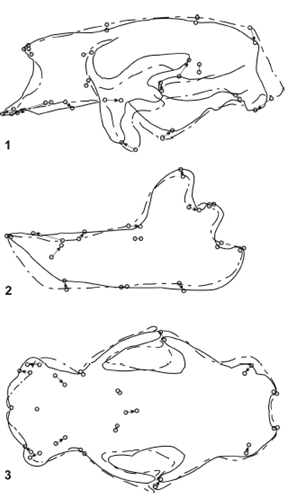
Figure 8. Results of RFTRA analysis on Glossotherium robustum - Lestodon armatus. Skulls (1) and mandibles (2) in lateral view, and skulls in palatal view (3) / Resultados del análisis mediante RFTRA en Glossotherium robustum -Lestodon armatus. Cráneos (1) y mandíbulas (2) en vista lateral, y cráneos en vista palatal (3).
Glossotherium robustum - Mylodon darwini (figure 9). The morphological distance between G. robustum - M. darwini is greater than that observed between G. robustum - L. armatus. The most remarkable change is observed in the anterior part of the skulls. Mylodon darwini has the muzzle much more elongated, which is clear in lateral and palatal view. This is due to an enlargement of the premaxillae, maxillae and nasals. Mylodon darwini has the palate more convex at the level of M1, and the molariform series is posteriorly displaced. A generalized narrowness of the skull of M. darwini is observed in palatal view, and the tooth series are displaced to the middle line, becoming almost parallel. The shapes of the mandibles are similar even though in M. darwini is more slender and elongated in its anterior part than in G. robustum.
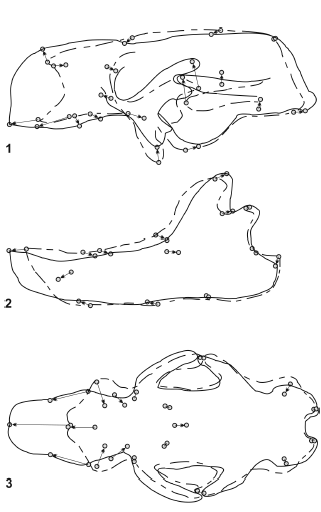
Figure 9. Results of RFTRA analysis on Glossotherium robustum - Mylodon darwini. Skulls (1) and mandibles (2) in lateral view, and skulls in palatal view (3) / resultados del análisis mediante RFTRA en Glossotherium robustum -Mylodon darwini. Cráneos (1) y mandíbulas (2) en vista lateral, y cráneos en vista palatal (3).
Glossotherium robustum - Scelidotherium leptocephalum (figure 10). The skulls of these species show remarkable differences reflected in the morphologi- cal distance, which is even greater than those of the previous comparisons. The overall shape of the skull of S. leptocephalum is more slender than G. robustum. It is particularly more shallow, elongated and narrow, which is evident in lateral and palatal view. The enlargement of the skull is restricted to the muzzle region; the premaxillae and the nasals are notably lengthened. The tooth series are almost parallel and the molariforms are displaced posteriorly, which shortens the tooth series and lengthens the muzzle. The distance coefficients of the mandibles are lower than those of the skulls. The most notable changes in S. leptocephalum are the predental space more lengthened and narrow, the anterior part of the symphysis lower, the posterior displacement of the teeth and the condyle more elevated.
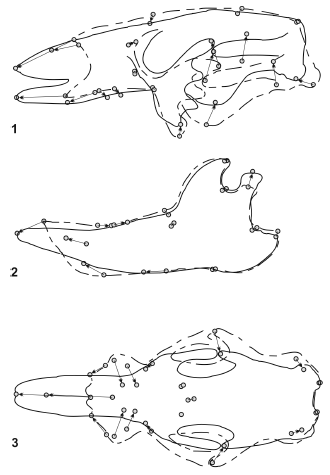
Figure 10. Results of RFTRA analysis on Glossotherium robustum- Scelidotherium leptcephalum. Skulls (1) and mandibles (2) in lateral view, and skulls in palatal view (3) / Resultados del análisis mediante RFTRA en Glossotherium robustum-Scelidotherium leptocephalum. Cráneos (1) y mandíbulas (2) en vista lateral, y cráneos en vista palatal (3).
Glossotherium robustum-Megatherium americanum. These specimens show the highest distance coefficients (skull and mandible in lateral view) in relation with comparisons among mylodontids. Bargo (2001b: figure 7) described the most important changes: the snout of M. americanum is extended anteriorly and slightly depressed dorsoventrally; the basicranium is elevated well above the alveolar plane, and the braincase is shorter. The zygomatic process of the squamosal and the ascending process of the jugal lie further dorsally, while the tip of the descending process of the jugal lies at nearly the same level as in G. robustum. The molariform series is displaced posteriorly, leaving a long predental space. The angular, condylar and coronoid processes of the mandible are markedly displaced dorsally in M. americanum respect to G. robustum. The horizontal ramus, except at the well-developed ventral bulge, is shallower. The predental space is longer, due to the more distal tooth row.
Masticatory musculature
The origin and insertion areas of the masticatory musculature in G. robustum, L. armatus, M. darwini and S. leptocephalum do not differ markedly. They follow the same pattern, showing minor variations in the shape or roughness of the attachment areas.
M. temporalis. This muscle is usually divided into superficial and deep portions in most living mammals (Turnbull, 1970), including tree sloths. Naples (1989) indicated that the m. temporalis was probably undivided in Paramylodon harlani. In the mylodontid ground sloths analyzed here, features in the skull that might indicate divisions of the muscle are ambiguous, thus the m. temporalis is recognized as a unit. It aroses from the temporal fossa, which is elongated, well defined, with a scarred surface, and covered most of the dorsal and lateral parts of the frontal and parietal bones. Mylodontids lack a sagittal crest, but the dorsal origin of the muscle is marked by the temporal line, which extends anteriorly to the prominent postorbital processes, and posteriorly nearly to the margins of the nuchal crests. The m. temporalis inserted, probably tendinously as in other mammals (Turnbull, 1970), on the roughened lateral, anterior, and medial surfaces of the coronoid process.
M. massetericus. The masseteric musculature is complex in almost all mammals. It is usually subdivided into superficial and deep components and the m. zygomaticomandibularis. Although the m. massetericus superficialis and m. massetericus profundus may be recognized, the subdivisions of the m. m. superficialis cannot be reliably reconstructed in mylodontids. The m. m. superficialis arose laterally from the zygomatic arch, as is indicated by the scarred central and lower part of the descending process of the jugal, and inserted mainly on the lateral surface of the angular process. The m. m. profundus presumably arose on a smooth depression of the antero-medial surface of the descending process of the jugal, and inserted on the base of the coronoid process, anterodorsally to the m. m. superficialis. The m. zygomaticomandibularis was probably large in mylodontids. This is reflected by the well developed and elongated ascending process of the jugal. This muscle arose from the scarred anteromedial surface of this process, and inserted on the smooth depression of the masseteric fossa at the base of the coronoid process, and above the m. m. profundus.
M. pterygoideus. The pterygoid musculature in sloths is large relative to that of other mammals (Edgeworth, 1935; Naples, 1985, 1989; Turnbull, 1970), and is subdivided in the m. pterygoideus lateralis and medialis, as typically occurs in other mammals. The m. pterygoideus medialis arose from a depression in the lateroventral surface of the elongated pterygoid flange, and inserted on the concave and prominently scarred medial surface of the large angular process. The m. pterygoideus lateralis originated on the lateral surface of the pterygoid, probably above the m. pterygoideus medialis and inserted in a roughened depression on the anteromedial edge of the mandibular condyle.
Jaw mechanics
Bite force and velocity: estimation of moment arms. The estimation of the moment arm of the masticatory muscle's line of action allows the comparison of the relative forces of the muscles and bite, and, more significant, the analysis of the relation between bite force and velocity, comparing the proportions of the combined moment arms of the m. massetericus and m. temporalis with those of the bite.
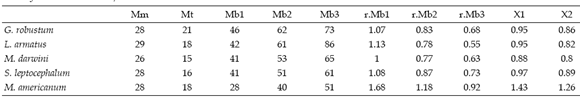
The moment arms of the m. massetericus (Mm), m. temporalis (Mt), and bite (Mb) of Glossotherium robustum, Lestodon armatus, Mylodon darwini, and Scelidotherium leptocephalum were estimated, and compared with those obtained previously by Bargo (2001b) for Megatherium americanum (table 2a, b and c). The values of the moment arms show little variation among the different species. However, the figures of the ratio of muscle moment/bite moment (r.Mb), which provide a measure of the relative bite force generated at different points along the tooth row and the bite velocity, are remarkable. Megatherium americanum has the highest values all along the tooth row, while mylodontids have similar values between them (table 2c). High ratios indicate strong, rather than fast mandibular movements. Hence, the masticatory apparatus of M. americanum is designed to generate larger bite forces than those of mylodontids. The means for the posterior teeth (X1) and for the whole series (X2) provide a comparative measure of the bite force generated at the posterior part of the mandible and its total bite force, respectively. Accordingly, mean values indicate that M. americanum has the strongest bite all along the molariform series (X2 = 1.26), and especially at the posterior ones (X1= 1.43), while mylodontids have a less powerful bite with little variation among them.
Table 2.a, Moment arms of m. massetericus, calculated from the uppermost, middle and lowermost point of the origin area of the muscle. Mm1 - Mm5: momemt arms of m. massetericus generated from five lines of action. X: mean. Values are in mm /a, Brazos de momento del m. massetericus, calculados a partir de los puntos más superior, medio y más inferior del área de origen del músculo. Mm1 - Mm5: brazos de momento del m. massetericus generados a partir de cinco líneas de acción. X: promedio. Los valores son en mm.
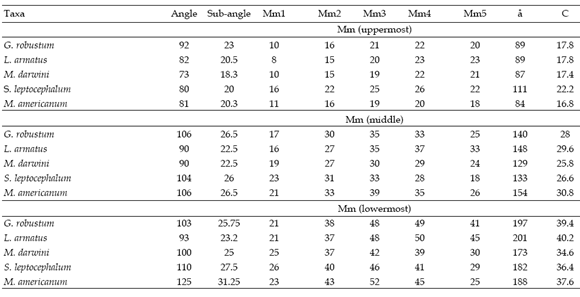
Table 2.b, Moment arms of m. temporalis, calculated from the posteriormost (Mt1), middle (Mt2) and anteriormost (Mt3) points of the origin area of the muscle. X: mean, Values are in mm / brazos de momento del m. temporalis, calculados desde los puntos mas posterior (Mt1), medio (Mt2) y más anterior (Mt3) del área de origen del músculo. X: promedio. Los valores son en mm.
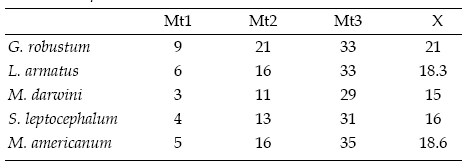
Table 2.c, Summary of mean values of moment arms and ratios muscle/bite / resumen de los valores promedio obtenidos de los brazos de momento y las razones músculo/mordida.
Mandibular movements inferred from tooth wear facets and striations. The analyses of tooth wear facets and particularly the leading and trailing interfaces between hard and soft dentines in Glossotherium robustum suggest that the main mandibular movement was produced in anteromedial direction. Although striations are not abundant, some have been observed in the hard dentine, and they support this direction. This pattern coincides with that described by Naples (1989) for the North American ground sloth Paramylodon harlani. The wear facets observed in Lestodon armatus and Mylodon darwini are not as evident as those of G. robustum, but the few striations observed would indicate that the main mandibular movement was in anteromedial direction, as in generalized recent mammals (Hiiemae, 1978). The teeth of S. leptocephalum do not show tooth-tooth contact wear facets in the hard dentine, nor does it show striations. Nevertheless, leading and trailing interfaces were observed in some teeth, having the same orientation as in G. robustum, which would probably indicate the same direction during the main mandibular movement.
The occlusal surfaces of the teeth of M. americanum are completely different from those of mylodontids (figure 7). The molariforms are bilophodont, that is, they bear two prominent, sharp, and transversely oriented lophs separated by a deep V-shaped valley. This feature produced an interlocking occlusion: in the upper teeth, mesial lophs occlude between two successive lower teeth, while the distal lophs occlude in the deep valleys enclosed by the anterior and posterior lophs of the lower teeth. This type of occlusion avoids the wear pattern described by Greaves (1973), but generates tooth-tooth contact facets, predominantly vertical and bearing clear striations, and tooth-food-tooth contact facets (compression).
Discussion
Biomechanical and morphogeometrical evidence
It is generally accepted that a high mandibular condyle improves the mechanical advantage of the m. massetericus by increasing the moment arm of the lines of action, as occurs in living ungulates (Maynard Smith and Savage, 1959; Turnbull, 1970; Greaves, 1980). Moreover, a high glenoid cavity distributes the bite force uniformly along the tooth series, which results advantageous in those herbivores that process great amounts of food (Greaves, 1980; Spencer, 1995).
In G. robustum, L. armatus, M. darwini and S. leptocephalum, the cranio-mandibular joint (CMJ) lies at the level of the tooth series, or little above it. So, a low moment arm of the m. massetericus (Mm) should be expected. However, the Mm is high, similar to the giant armadillos (pampatheres) (Vizcaíno et al., 1998; De Iuliis et al., 2000) which have the condyle elevated well above the tooth row and share many features with some living ungulates, in which the main masticatory effort is generated by the m. massetericus. The latter is also true for the ground sloths, where the m. massetericus is more developed than the m. temporalis. Therefore, it becomes evident that there is another morphological arrangement in ground sloths that allows keeping the Mm elevated. The mechanical advantage would have been given by the great development of the zygomatic arch, particularly the descending process of the jugal, which elongates significantly the lever of the m. massetericus.
On the contrary, M. americanum has the mandibular condyle well elevated above the tooth series, but the Mm is low and similar to mylodontids. As noted by De Iuliis (1996), the angular process in M. americanum has a more dorsal position, accompanying the condyle, averting in this way a dramatic rearrangement of the muscular attachment sites and force vectors. Bargo (2001b: figure 7) corroborated through shape analysis that both the condyle and the angular process are elevated nearly to the same degree, compared with the mandible of G. robustum, and the same is true for the masseteric fossa. Consequently, the estimated Mm is the same in both forms.
The moment arm of the m. temporalis (Mt) of mylodontids is, as expected, lower than Mm, and similar among the different species, and the same is true for M. americanum (Bargo, 2001a).
The combined moment arm -i.e. ratio of muscle moment (Mm +Mt) to bite moment (Mb)- provides a relative measure of the effective bite force generated by the musculature. In this way, large ratios indicate forceful biting rather than rapid jaw movements. Hence, the masticatory apparatuses of G. robustum, L. armatus, M. darwini and S. leptocephalum, which have lower ratios than M. americanum (see Table 2c), are designed to generate lesser bite forces all along the tooth row compared to the megatheriid. Moreover, the tooth rows are displaced distally in M. americanum compared with mylodontids, as demonstrated by RFTRA analysis (Bargo 2001b), which result in shorter moment arms for bite positions, throughout the tooth row, which in turn increase the bite force. Lestodon armatus shows the lowest value among my- lodontids in the first tooth (caniniform), given that it is markedly displaced anteriorly, suggesting less force but high velocity.
Both, megatheriids and mylodontids, have a wide articular condyle (more extended laterally than anteroposteriorly) and slightly convex, and the glenoid fossa shallowly concave. This arrangement would have permitted considerable freedom of motion of the mandible, both mediolateral and anteroposterior. There is more evidence that complements the latter, but allows two different interpretations for both groups. The analysis of wear facets, the continuous and discontinuous interfaces, and striations in mylodontids indicate that the main masticatory movement was in anteromedial direction. Moreover, the anterior and posterior parts of the zygomatic arch are not fused, nor in contact, which lesses its ability to withstand the great forces generated by the antagonistic actions of m. massetericus and m. pterigoideus during lateral movements. This feature would indicate that, even though existing, the lateral movements were not very strong and continuous during mastication. In the case of megatheriids, the particular tooth morphology (i.e. interlocking occlusion, bilophodonty) would be maintained by performing essentially orthal movements, and this is confirmed by the analysis of wear facets. Similarly, the zygomatic arch is large and robust, and the anterior and posterior parts may be in contact (even fused in aged individuals, De Iuliis, 1996), unlike the mylodonts in this study which have an incomplete arch. The stoutly built zygomatic arch, particularly the great development of descending process, leaves a narrow space between it, the horizontal ramus and, in part, the ascending ramus, suggesting a physical restriction to lateral movements (Bargo, 2001b).
Hiiemae and Crompton (1985) summarized the mechanical principles of tooth design in relation to the nature of the food. They recognized three basic patterns: A) a mortar and pestle system suitable to crush hard and brittle (e.g., nuts) or turgid (e.g., fruit pulp) food; B) blades to cut soft but tough food (e.g., muscle and skin); and C) a serial array of low profile blades acting as a milling machine for tough and fibrous food (e.g., grass). The first two patterns coincide respectively with "routes" 1 and 2, and the third with "routes" 3 and 4 of Janis and Fortelius (1988: 224-225). The teeth of ground sloths do not fit strictly within any of these patterns, but can be assigned to an intermediate situation. In mylodontids, the teeth are oval or semi-oval and show different degrees of lobation. The hard and soft dentine layers results in differential wear, generating a concave occlusal surface, comparable to pattern A of Hiiemae and Crompton (1985). The outer hard dentine forms sharp cusps that, during the masticatory movement would act as cutting edges. The combination of this morphology with the evidence that the direction of the mandibular movement is mostly anteromedial, indicates that mylodontid teeth would represent an intermediate situation between Hiiemae and Crompton's patterns A and C, i.e. for crushing and grinding. According to Janis and Fortelius (1988), moderately tough and abrasive food such as leaves require reciprocal blades for comminution of food items, a function which is accomplished by orthal chewing with bilophodont teeth ("route" 2). The dentition of Megatherium americanum represents a battery of high lophs, with sharp, cutting edges, similar to the dentition of tapirs and kangaroos. This morphology would represent an intermediate condition between Hiiemae and Crompton's patterns A and B, i.e. mainly for cutting, and crushing. This morphology does not rule out processing food with similar physical properties (soft but tough, fleshy) of animal source, like muscle or skin (Bargo, 2001b). The morphogeometrical analysis demonstrated that the most important localized changes occur in the mandibles and the muzzles (the palatal region), while the shape of the neurocranium was more conservative, probably due to strong phylogenetic constraints. The palatal region and mandibles can be better interpreted in a functional context and, consequently, would be more influenced by dietary adaptations as it was proposed for armadillos (Vizcaíno and Bargo, 1998).
Ecomorphological evidence
Mendoza et al., (2002) demonstrated that, at least in ungulates, the adaptation to a given trophic niche involves complex patterns of covariation among many morphological characters of the skull and mandible. The lack of living analogs for ground sloths precludes performing extensive ecomorphological analyses to establish unequivocal correlations between feeding behavior and morphological variables. However, some craniodental variables were applied and demonstrated to be useful in explaining differences in feeding behavior, as will be discussed below.
As became evident from the previous section, the two groups analyzed -Mylodontidae and Megatheriidae- are morphofunctionally distinct from each other in their masticatory apparatuses, but taxa within each group are markedly similar to each other. Recent ecomorphological analyses of these ground sloths include three craniodental variables: hypsodonty index, relative width and shape of the muzzle, and dental occlusal surface area (OSA). Hypsodonty index was standardized as depth of the mandible, measured at the level of the third molariform tooth, divided by length of the molariform tooth row. The index of relative muzzle width was calculated as the ratio between the palatal width, measured as a mean of the anterior and posterior width of the palate, and the maximum muzzle width (MMW). Because the premaxillae are reduced in sloths, the MMW is generally on the maxilla. Finally, the OSA was estimated digitizing the outlines of the teeth in occlusal view, in order to collect the surface contour of each tooth; the area enclosed by these points was calculated by a numerical integer approximation. (see Bargo, De Iuliis and Vizcaíno, 2006; Bargo, Toledo and Vizcaíno, 2006; Vizcaíno, Bargo and Cassini, 2006 for further explanations). The results of these studies offer relevant information that, coupled with the morphological and biomechanical evidence presented in this contribution, allows a paleobiological interpretation on the dietary habits of the ground sloths.
The masticatory apparatus of mylodontids was not particularly suited for producing strong bite forces during mastication, and the main masticatory movement was anteromedial. This, in turn, suggests that mylodonts were not well suited for extensive oral food processing, and the main action was crushing and, in less degree, grinding. In contrast, the feeding apparatus of Megatherium americanum was well designed for generating very strong, predominantly orthal movements that were used mainly for cutting rather than crushing and grinding.
The analysis of dental occlusal surface area (OSA) in xenarthrans by Vizcaíno, Bargo and Cassini (2006) supports these proposals. These authors found that mylodontids have extremely low OSA values in comparison with living herbivorous mammals of equivalent body size, which also suggests that mylodonts had poor food oral processing. This fact was probably compensated with by high fermentation in the digestive tract, or lower metabolic requirements, or a combination of both. Surprisingly, the OSA value of M. americanum is the one expected, or even higher, for a mammal of its size, and much larger than those of mylodontids. It is clear then that M. americanum was better suited for oral food processing in the oral cavity, and most likely had a lower fermentation capacity and/or higher metabolic requirements.
Bargo, Toledo and Vizcaíno (2006) analyzed the relationship between dietary habits and shape and width of the muzzle of the five species of ground sloths considered here, and examined models of food intake by reconstructing musculature and cartilages of the muzzle. According to these authors, ground sloths can be divided in two groups with different feeding behaviours: wide-muzzled sloths (Glossotherium robustum and Lestodon armatus) that were mostly bulk-feeders (i.e. ingest great amounts of food with each bite; probably roughage and grass eaters), and narrow-muzzled sloths (Mylodon darwini, Scelidotherium leptocephalum and Megatherium americanum) that were mixed or selective feeders (i.e. select plants or plant parts; grass and/or tree and shrubs foliage eaters). The muscle reconstruction indicates that the upper lip, formed by the m. incisivus superior, was probably square-shaped and not prehensile in widemuzzled sloths, as in the white rhinoceros, C. simum.
This fact, coupled with the absence of incisors, indicates that G. robustum and L. armatus simply used the upper lip coupled with the tongue to pull out grass and herbaceous plants. Similarly, narrow-muzzled sloths (M. darwini, S. leptocephalum and M. americanum) had a thick, cone-shaped and prehensile upper lip, useful for food intake as in the black rhinoceros, Diceros bicornis Linnaeus, 1758, used to select particular plants or plant parts (e.g. leaves and twigs). It is worth noting that, following Mendoza and Palmqvist (2008), the white rhino is a typical grazer but its muzzle is relatively narrower than in many mixed feeders. One plausible hypothesis is that in some cases it is the lip morphology what determines functional muzzle width, supporting the need to consider muscular anatomy to complete these discussions (see Bargo, Toledo and Vizcaíno, 2006 and references therein).
Finally, the comparative study of hypsodonty in Pleistocene ground sloths by Bargo, De Iuliis and Vizcaíno (2006) suggests that differences in crown height may be explained by a combination of variables (rather than any single), including dietary preferences (nature of food items), habitat (close or open, temperate or tropical) and behavior (feeding at ground level or higher, digging, etc.). Recently, Mendoza and Palmqvist (2008) demonstrated that high-crowned teeth represent an adaptation of ungulates against tooth wear resulting from the airborne grit and dust accumulated on the herbaceous plants of open environments. But the absence of enamel, which would make the teeth less durable and subject to faster wear, must be considered as responsible for much of the hypsodonty observed in sloths, as well as in all xenarthrans, obscuring the interpretation of the individual contribution of each these variables (see Bargo, De Iuliis and Vizcaíno, 2006 for a discussion on this matter). Within mylodontids, S. leptocephalum has the highest hypsodonty index (HI), followed by M. darwini, while L. armatus and G. robustum have the lowest indices. On the other hand, M. americanum has the highest HI, even when compared with other megatheriines (i.e. Eremotherium Spillman, 1948 from northern South America and North America, and other Megatherium species from north central and north western South America). We cannot determine the degree to which higher hypsodonty values in megatheriids and mylodontids correspond to feeding on abrasive grasses rather than browsing on foliage, as has been done for living ungulates (Janis, 1988; Solounias and Dawson-Saunders, 1988), simply because we cannot know the proportion of grass in their diet. However, one obvious factor in explaining differences in hypsodonty in ground sloths is the increased presence of grit caused by environmental differences resulting from geographic distribution, or environmental change over time, or particular habits. For example, the differences in hypsodonty between the megatheriines E. laurillardi (Lund, 1842) and M. americanum might be explained as adaptations to different environments, as reflected by their geographical distributions (see De Iuliis et al., 2000; Bargo, De Iuliis and Vizcaíno, 2006). Differences in environment over time, such as from closed to open, were apparently important in North American Paramylodon (McDonald, 1995). Digging behavior in S. leptocephalum and G. robustum, including but not limited to searching for food, was demonstrated by morphologic and biomechanical analyses of the limbs (Bargo et al. 2000; Vizcaíno et al., 2001). Also, the narrow-muzzled sloths S. leptocephalum and M. darwini would have used their stoutly built muzzles to root up for searching for food. These particular habits must have played a considerable role in shaping the dental characteristics of these sloths. In each of those cases, the important agent was the relative abundance of abrasive soil particles.
Several discoveries of mummified remains and dung of different ground sloths taxa, from South and North America, have provided additional evidence for the inference of their diets (McDonald and De Iuliis, 2008). For instance, the dominant vegetation identified from dung of Mylodon darwini, found in a cave at Ultima Esperanza, in Southernmost Patagonia, was grasses and sedges (Moore, 1978); which in some way supports the morphological information given here (low OSA values = low metabolic requirements or low quality food; narrow-muzzled = selective and mixed feeder) for that taxon. The novel application of stable isotopes analyses in xenarthrans (e.g. Coltrain et al., 2004; Kalthoff and Tütken, 2007), or DNA analysis, will provide a better understanding of the sloth's diet.
Conclusions
The results provided by this morphological and biomechanical study, coupled with the ecomorphological data, allow inferring different dietary habits for the most common species of Pleistocene ground sloths. Next, it is possible to infer a probable niche differentiation among these species, given that they inhabited within the same habitat.
Within mylodontids, Glossotherium robustum and Lestodon armatus, the wide-muzzled sloths, were most likely bulk-feeders. Their lips coupled with the tongue were used to pull out grass and herbaceous plants, which probably was the main dietary item. Mylodon darwini and Scelidotherium leptocephalum, the narrow-muzzled sloths, were mixed or selective- feeders with a prehensile lip that was used to select particular plants or plant parts. These species could have also used their muzzles (as hogs) to root up food items, such as roots and tubers. Mylodontids have also clear adaptations to digging in their forelimbs, using their claws to help searching for food. The tooth design of mylodontids, in relation to the nature of food, indicates that teeth were mainly for crushing and grinding turgid and fibrous items respectively.
Megatherium americanum was probably the most selective feeder, with a prehensile lip very thick and strong, and more developed than in the narrow-muzzled mylodontids. This condition probably enabled M. americanum to selectively feed on particular plants (shrubs) or plant parts (leaves, twigs, fruits). The dentition was designed mostly for cutting soft but tough items which might include flesh, leaving open the possibility of an omnivorous diet The use of alternative methods (biomechanics, morphogeometry and ecomorphology) to complement the basic morphologic analysis of the masticatory apparatus of forms that have no clear analogs, demonstrates to be very insightful for the inferences of dietary habits. However, as mentioned above, is clear that more evidence (e.g., coprological, biogeochemical, palynological) is required in order to reconstruct a more accurate understanding of the feeding behavior of these giant ground sloths.
Appendix 1. Acronyms and list of material / acrónimos y lista de material.
Acronyms
BM(NH): Natural History Museum, Londres, Inglaterra.
CN: Zoological Museum, Copenhagen, Denmark.
MACN: Museo Argentino de Ciencias Naturales "Bernardino Rivadavia", Buenos Aires, Argentina.
MLP: Museo de La Plata, La Plata, Argentina.
MMCIPAS: Museo Municipal y Centro de Investigaciones Paleontológicas de Salto, provincia de Buenos Aires, Argentina.
MMP: Museo Municipal de Ciencias Naturales "L. Scaglia", Mar del Plata, Argentina.
MNHN: Museo Nacional de Historia Natural de Montevideo, Uruguay.
MNHN-BOL: Museo Nacional de Historia Natural, La Paz, Bolivia.
MNHNP: Muséum National d'Histoire Naturelle, Paris, France.
MRSC: Museo Paleontológico Real de San Carlos "Armando Calcaterra", Colonia, Uruguay.List of materials
MYLODONTIDAE
Glossotherium robustum
MACN 1114. Complete skull with dentition. "Upper Pampean", unknown locality.
MACN 11074. Complete skull and right dentary. Lujanian, Arrecifes river, Buenos Aires Province, Argentina.
MACN 11769. Skull missing zygomatic arches and dentition. Pleistocene, Sauce Chico stream, Tornquist, Buenos Aires Province, Argentina.
MACN 12715. Skull missing dentition. "Upper pampean", Gorchs, F.C.S., Salado river, Buenos Aires Province, Argentina.
MLP 3-136. Complete skull, with jugals and premaxillae reconstructed. Figured by Lydekker (1894), Pl. XLVIII, figs. 1 y 1a y
Pl. XLIX, fig. 2. "Upper Pampean", unknown locality.
MLP 3-137. Skull, mandible (missing m2), and part of the skeleton. "Pampean", Olivera, Buenos Aires Province, Argentina.
MLP 3-138. Skull, mandible, and part of the skeleton missing left foot and lumbar vertebrae. Figured by Lydekker (1894), Pl. LI. "Upper pampean", San Antonio de Areco, Buenos Aires Province, Argentina.
MLP 3-139. Skull, right dentary and incomplete skeleton of a juvenile specimen. "Upper pampean", Olivera, Buenos Aires Province, Argentina.
MLP 3-140. Skull, mandible, and part of the skeleton. Figured by Lydekker (1894), Pl. XLIX, fig.1, Pl.L y Pl. LII, fig. 1. "Upper pampean", Río Luján, Olivera, Buenos Aires Province, Argentina.
MMCIPAS 1042/1043. Skull missing premaxillae and mandible. Pleistocene, Salto, Buenos Aires Province, Argentina.
MMP 1489-M. Skull missing jugals, caniniforms and right M1. Statigraphic provenance and locality unknown.
MMP 1490-M. Skull missing jugals and caniniforms; molariforms not well preserved. Statigraphy and locality unknown.
MNHN 1390. Skull missing premaxillae, jugals and dentition, except right M3. Pleistocene (Libertad Fm.), Arroyo Las Limetas, Conchillas, Colonia Department, Uruguay.
MRSC 920. Skull missing jugals. Pleistocene, Arroyo San Juan, Colonia Department, Uruguay.Lestodon armatus
MACN 10830. Skull and mandible. "Lower pampean", North of
Mar del Plata sea cliffs, Buenos Aires Province, Argentina.
MACN 11687. Skull of a juvenile specimen. "Pampean", Carcarañá river, Santa Fe Province, Argentina.
MLP 3-3. Skull with caniniforms and mandible with molariforms but missing caniniforms; incomplete skeleton but restored. Skull figured by Lydekker (1894), Pl. LIII, Figs. 1 y 1a. "Pampean", San Antonio de Areco, Buenos Aires Province, Argentina.
MLP 3-29. Skull and mandible partially restored. Jugals, left upper caniniform, right premaxilla, right m4 and left m2-4 are missing. Skull figured by Lydekker (1894), Pl. LIII, Fig. 2. "Pampean", unknown locality.
MLP 3-30. Skull and mandible partially restored. Right M2 and M4, and left m2 are missing. Caniniforms restored. "Pampean", unknown locality.
MRSC 807. Complete skull. Stratigraphy unknown, Colonia, Uruguay.
MRSC 1020. Skull and mandible. Stratigraphy unknown, Colonia, Uruguay.Mylodon darwini
BM(NH) M-16617 (ex RCS 472, ex RCS 3940). Holotype. Mandible with dentition; tip of the simphysis, coronoid, angular and condilar processes missing. Late Pleistocene, Punta Alta cliffs, Bahía Blanca, Buenos Aires Province, Argentina.
CN 43. Skull and nearly complete skeleton. Statigraphic provenance unknown, Buenos Aires Province, Argentina.
MACN 991. Right dentary with the molars partially restored. "Pampean", Río Salado, Buenos Aires Province, Argentina.
MACN 5980. Complete isolated premaxillae with part of the nasal arch. "Lower pampean", Miramar, Buenos Aires Province, Argentina.
MACN 11502. Incomplete left dentary (missing angular and coronoid processes, and part of the symphisis) with m2 and m4.
"Lower pampean"?, Río Carcarañá cliffs, Santa Fe Province, Argentina.
MACN 15348. Incomplete skull, restored in the dorsal part of the nasals, the nasal arch, the pterigoid blades and the jugals. "Lower pampean"?, Buenos Aires Province, Argentina.
MLP 3-122. Incomplete skull, with the nasal arch but missing dentition. Figured by Lydekker (1894), Pl. LIV. "Middle pampean", Buenos Aires Province, Argentina.
MLP 3-762a. Incomplete skull, with the nasal arch, jugals and dentition missing. "Upper pampean", Olavarría, Buenos Aires Province, Argentina.
MLP 3-763. Incomplete skull, with the nasal arch, jugals and dentition missing. "Upper pampean", Olavarría, Buenos Aires Province, Argentina.
MLP 3-764. Complete skull with dentition. Dorsal tip of the nasal arch mising. "Pampean", Olavarría, provincia de Buenos Aires, Argentina.
MLP 36-VIII-12-1. Incomplete skull without dentition. "Lower pampean", Estación Bunge, Buenos Aires Province, Argentina.
MMCIPAS 2458. Incomplete skull; zygomatich arches, premaxillae, anterior part of the nasals and dentition mising. Pleistocene, Salto, Buenos Aires Province, Argentina.
MNHN-BOL-V 006470. Skull very well preserved, but without dentition and right jugal. Pleistocene, Mojotorillo, Potosi Department, Bolivia.Scelidotherium leptocephalum
MLP 3-671. Skull and mandible and several limb bones. "Pampean", Olavarría, Buenos Aires Province, Argentina.
MLP 3-401. Skull and mandible and almost complete skeleton. "Pampean", Buenos Aires Province, Argentina.
MLP 3-420. Skull and mandible. "Upper Pampean", Buenos Aires Province, Argentina.
MMP 9-S. Skull of a juvenile specimen missing the zygomatic arches. Ensenadan, northeastern sea cliffs of Mar del Plata, Playa Santa Elena, Buenos Aires Province, Argentina.
MMP 31-S. Skull missing the zygomatic arches and the right molariforms. Sea cliffs of Camet, Mar del Plata, Buenos Aires Province, Argentina.
MMP 127-S. Skull missing zygomatic arches, mandible, atlas, ulna, radio and other limb bones. Lujanian (Cobo Fm.) 100 m North of Arroyo Santa Clara, Buenos Aires Province, Argentina.
MMP 157-S. Skull and mandible missing dentition. Playa Estrada, Mar del Plata, Buenos Aires Province, Argentina.
MMP 458-S. Skull and mandible. Skull mssing right M1, M4 and M5; mandible missing left m1- m4. Femur, patella, tibia, fragment of fibula and radio. Parque Camet, Mar del Plata, Buenos Aires Province, Argentina.
MMP 549-S. Skull, missing the zygomatic arches; mandible, with the coronoid processes and condyles incomplete. Part of the apendicular skeleton. Lujanian (Cobo Fm.), sea cliffs of Santa Clara del Mar, Buenos Aires Province, Argentina.
MMP 614-M. Skull missing the zygomatic arches and part of the skeleton. Rivera, Buenos Aires Province, Argentina.
MMP 1155-M. Skull missing the jugals, left M1-M5and right M3- M4; mandible missing dentition and right coronoid process incomplete. Ensenadan, Mar del Plata, Buenos Aires Province, Argentina.MEGATHERIIDAE
Megatherium americanum
MLP 2-64 Skull and mandible, with part of the hyoid apparatus. "Pampean", Argentina. Figured in Lydekker (1894: pl. 45, fig.1).
MLP 2-56. Complete mandible. "Pampean", Argentina. Figured in Lydekker (1894: pl. 45, fig. 1a).
MACN 1000, nearly complete mounted skeleton. Río Salado, Buenos Aires Province, Argentina.
MACN 2832. Skull and mandible, some hyoid pieces, vertebrae, fragment of scapula. "Pampean", Carcarañá river, Santa Fe, Argentina.
MACN 5002. Skull and mandible, femur, humerus and ulna. Palermo, Buenos Aires, Argentina. (Type of Megatherium gallardoi).
MNHNP 276. Skull and mandible with the symphysis broken. Stratigraphic provenance and locality unknown.
Appendix 2. Landmarks used for the morphogeometric analysis (H: homologous landmark; G: geometric landmark) / landmarksutilizados para el análisis morfogeométrico (H: landmarks homólogos; G: landmarks geométricos).
Skull: lateral view
1. Ventral margin of the occipital condyle (G).
2. Dorsal margin of the sagittal crest (H).
3. Parietofrontal suture on the sagittal plane (H).
4. Nasofrontal suture on the sagittal plane (H).
5. Anterior end of the nasal (G).
6. Nasointermaxillare (anterior end of the nasopremaxillar suture) (H).
7. Anterior end of the premaxilla (G).
8. Premaxillomaxillar suture on the ventral margin (H).
9. Mesial margin of first molariform tooth (H).
10. Mesial margin of second molariform tooth (H).
11. Distal margin of the last molariform tooth (H).
12. Ventral-most margin of the pterygoid (G).
13. Auditory foramen (H).
14. Squamoso-parieto-frontal suture (H)
15. Lacrimal foramen (H)
16. Infraorbital foramen (H)
17. Dorsal end of the ascending process of the jugal (G)
18. Ventral end of the descending process of the jugal (G)
19. Anterior end of the squamosal (G)Skull: palatal view
1. Posterior end of the right occipital condyle (G).
2. Posterior end of the left occipital condyle (G).
3. Left estilohyal fossa (H).
4. Rigth estilohyal fossa (H).
5. Palation (posterior point of the palate in the middle line) (H).
6. Anterior end of the right squamosal (G).
7. Anterior end of the left squamosal (G).
8. Rigth Infraorbital foramen (H).
9. Left Infraorbital foramen (H).
10. Premaxillomaxillar suture on the left margin (H).
11. Premaxillomaxillar suture on the middle line of the palate (H).
12. Premaxillomaxillar suture on the rigth margin (H).
13. Prosthion (anterior point of the premaxilla in the middle line) (H).
14. Mesial margin of first left molariform tooth (H).
15. Mesial margin of second left molariform tooth (H).
16. Mesial margin of the last left molariform tooth (H).
17. Mesial margin of first right molariform tooth (H).
18. Mesial margin of second right molariform tooth (H).
19. Mesial margin of the last right molariform tooth (H).Mandible: lateral view
1. Dorsal end of the condyle (G).
2. Junction between the condylar and coronoid processes (G).
3. Dorsal tip of the coronoid process (G).
4. Distal margin of the last molariform tooth (H).
5. Mesial margin of the second molariform tooth (H).
6. Mesial margin of the first molariform tooth (H).
7. Anterior symphyseal margin (G).
8. Intersection of the ventral margin of the dentary with the line extending down perpendicularly from the line drawn between landmarks 7 and 11 and at ¼ the distance between 7 and 11 (G).
9. Intersection of the ventral margin of the dentary with the line extending down perpendicularly from the line drawn between landmarks 7 and 11 and at ½ the distance between 7 and 11 (G).
10. Intersection of the ventral margin of the dentary with the line extending down perpendicularly from the line drawn between landmarks 7 and 11 and at ¾ the distance between 7 and 11 (G).
11. Posterior margin of angular process (G).
12. Junction between the angular process and the condyle (G).
13. External foramen of the dentary channel (H).
14. Anterior mental foramen (H).
Acknowledgments
MSB express her gratitude to her colleagues (S. Vizcaíno, G. De Iullis, R. Fariña and G. Cassini) and student N. Toledo for their valuable and enthusiastic contribution on this research. The authors acknowledge the following persons for the access to study the collections: R. Pascual and M. Reguero (Museo de La Plata), J. Bonaparte and A. Kramarz (Museo Argentino de Ciencias Naturales "Bernardino Rivadavia", Buenos Aires), A. Dondas (Museo Municipal de Ciencias Naturales "L. Scaglia", Mar del Plata), J. Ramírez (Museo Municipal de Salto, Buenos Aires Province), F. Anaya (Museo Nacional de Historia Natural, La Paz, Bolivia), A. Mones (Museo Nacional de Historia Natural de Montevideo, Uruguay), Mrs. Calcaterra (Museo Paleontológico Real de San Carlos "A. Calcaterra", Colonia, Uruguay). P. Christiansen for facilitating photographs of Mylodon darwini from the Museum of Copenhagen. Finally, we thank the reviewers H.G. McDonald and P. Palmqvist for his valuable comments and suggestions. This is a contribution to the projects PIP-CONICET 5240, PICT 26219 and UNLP N 474.
References
1. Ameghino F. 1889. Contribución al conocimiento de los mamíferos fósiles de la República Argentina. Actas de la Academia Nacional de Ciencias (Córdoba) 6: 1-1027. [ Links ]
2. Bargo, M.S. 2001a. [El aparato masticatorio de los perezosos terrestres (Xenarthra, Tardigrada) del Pleistoceno de la Argentina. Morfometría y biomecánica. Tesis Doctoral Universidad Nacional de La Plata, La Plata, Argentina. 400 pp. Unpublished.]. [ Links ]
3. Bargo, M.S. 2001b. The ground sloth Megatherium americanum: skull shape, bite forces, and diet. Acta Paleontologica Polonica, Special Issue 46: 41-60. [ Links ]
4. Bargo, M.S. De Iuliis, G. andVizcaíno, S.F. 2006. Hypsodonty in Pleistocene ground sloths. Acta Paleontologica Polonica 51: 53- 61. [ Links ]
5. Bargo, M.S., Toledo, N. and Vizcaíno, S.F. 2006. Muzzle of South American ground sloths (Xenarthra, Tardigrada). Journal of Morphology 267: 248-263. [ Links ]
6. Bargo, M.S., Vizcaíno, S.F., Archuby, F.M. and Blanco R.E. 2000. Limb bone proportions, strength and digging in some Lujanian (Late Pleistocene-Early Holocene) mylodontid ground sloths (Mammalia, Xenarthra). Journal of Vertebrate Paleontology 20: 601-610. [ Links ]
7. Benson, R.H., Chapman, R.E. and Siegel, A.F. 1982. On the measurement of morphology and its change. Paleobiology 8: 328- 339. [ Links ]
8. Bookstein, F.L. 1981. Coordinate systems and morphogenesis. En: T.G. Connelly, L.L. Brinkley, and B.M. Carlson (eds.), Morphogenesis and Pattern Formation. Raven Press, New York, pp. 265-287. [ Links ]
9. Burchell, W.J. 1817. Note sur une nouvelle espèce de Rhinoceros (Rh. simus). Bulletin des Sciences, par la Société Philomatique: 96- 97. [ Links ]
10. Cabrera, A. 1926. Sobre la alimentación del megaterio. Boletín de la Real Sociedad Española de Historia Natural 26: 388-391. [ Links ]
11. Carlini, A.A. and Scillato-Yané, G.J. 2004. The oldest Megalonychidae (Xenarthra: Tardigrada); phylogenetic relationships and an emended diagnosis of the family. Neues Jarburch Für Geologie und Paläontologie Abhandlungen 233: 423- 443. [ Links ]
12. Chapman, R.E. 1990a. Conventional Procrustes approaches. En: F.J. Rohlf y F.L. Bookstein (eds.), Proceedings of the Michigan Morphometrics Workshop. Special Publication Nr. 2, University of Michigan, Ann Arbor, pp. 251-267. [ Links ]
13. Chapman, R.E. 1990b. Shape analysis in the study of dinosaur morphology. En: K. Carpenter y P.J. Currie (eds.), Dinosaur Systematics: Perspectives y Approaches. Cambridge University Press, pp. 21-42. [ Links ]
14. Chiarello, A.G. 2008. Sloth ecology: an overview of field studies. En: W.J. Loughry y S.F. Vizcaíno (eds.), The Biology of the Xenarthra, University Press of Florida, pp. 269-280. [ Links ]
15. Coltrain, J.B., Harris, J.M., Cerling, T.E., Ehleringer, J.R., Dearing, M.-D., Ward, J. and Allen, J. 2004. Rancho La Brea stable isotope biogeochemistry and its implications for the palaeoecology of late Pleistocene, coastal California. Palaeogeography, Palaeoclimatology, Palaeoecology 205: 199-219. [ Links ]
16. Costa, R.L. and Greaves, W.S. 1981. Experimentally produced tooth wear facets and the direction of jaw motion. Journal of Paleontology 55: 635-638. [ Links ]
17. Cuvier, G. 1796. Notice sur le squelette d'une très-grande espèce de quadrupède inconnue jusqu'à présent, trouvé au Paraquay, et déposé au cabinet d'histoire naturelle de Madrid. Magasin Encyclopèdique: ou Journal des Sciences, des Lettres et des Arts 1796, 1: 303-310; 1796, 2: 227-228. [ Links ]
18. De Iuliis, G. 1996. [A systematic review of the Megatheriinae (Mammalia: Xenarthra: Megatheriidae). PhD Dissertation University of Toronto, Canada. 719 pp. Unpublisehd.]. [ Links ]
19. De Iuliis, G., Bargo, M.S. and Vizcaíno, S.F. 2000. Variation in skull morphology and mastication in the fossil giant armadillos Pampatherium spp. y allied genera (Mammalia: Xenarthra: Pampatheriidae), with comments on their systematics and distribution. Journal of Vertebrate Paleontology 20: 743-754. [ Links ]
20. Edgeworth, F.H. 1935. The cranial muscles of Vertebrates. Cambridge: Cambridge University Press, 493 pp. [ Links ]
21. Esteban, G. 1996. [Revisión de los Mylodontinae cuaternarios (Edentata, Tardigrada) de Argentina, Bolivia y Uruguay. Sistemática, Filogenia, Paleobiología, Paleozoogeografía y Paleoecología. Tesis Doctoral Facultad de Ciencias Naturales e Instituto Miguel Lillo, Universidad Nacional de Tucumán. 235 pp. Unpublisehd.]. [ Links ]
22. Fariña, R.A. 1985. Some functional aspects of mastication in Glyptodontidae (Mammalia). Fortschritte der Zoologie 30: 277-280. [ Links ]
23. Fariña, R.A. 1988. Observaciones adicionales sobre la biomecánica masticatoria en Glyptodontidae (Mammalia; Edentata). Boletín de la Sociedad Zoológica de Uruguay (2a. época) 4: 5-9. [ Links ]
24. Fariña, R.A. 1996. Trophic relationships among Lujanian mammals. Evolutionary Theory 11: 125-134. [ Links ]
25. Fariña, R.A. and Blanco, R.E. 1996. Megatherium, the stabber. Proceedings of the Royal Society of London 263: 1725-1729. [ Links ]
26. Fariña, R.A. and Vizcaíno, S.F. 2001. Carved teeth and strange jaws: How glyptodonts masticated. Acta Paleontologica Polonica, Special Issue 46: 87-102. [ Links ]
27. Ferigolo, J. 1985. Evolutionary trends of the histological pattern in the teeth of Edentata (Xenarthra). Archives Oral Biology 30: 71- 82. [ Links ]
28. Gaudin, T.J. 2004. Phylogenetic relationships among sloths (Mammalia, Xenarthra, Tardigrada): the craniodental evidence. Zoological Journal of the Linnean Society 140: 255-305. [ Links ]
29. Gervais, P. 1855. Recherches sur les mammifères fossiles de l'' Amérique méridionale. Comptes Rendus de l' Académie des Sciences 40: 1112-1114. [ Links ]
30. Greaves, W. S. 1973. The inference of jaw motion from tooth wear facets. Journal of Paleontology 47: 1000-1001. [ Links ]
31. Greaves, W.S. 1978. The jaw lever system in ungulates: a new model. Journal of Zoology 184: 271-285. [ Links ]
32. Greaves, W.S. 1980. The mammalian jaw mechanism: the high glenoid cavity. The American Naturalist 116: 432-440. [ Links ]
33. Harlan, R. 1825. Fauna Americana: being a description of the mammiferous animals inhabiting North America. Philadelphia: Anthony Finley; J. Harding, pp. 199-203. [ Links ]
34. Hiiemae, K.M. 1978. Mammalian mastication: a review of the activity of the jaw muscles and the movements they produce in chewing. En: P.M. Butler y K.A. Joysey (eds.), Development, function and evolution of teeth, Academic Press, London, pp. 359-398. [ Links ]
35. Hiiemae, K.M. and Crompton, A.W. 1985. Mastication, food transport, and swallowing. En: M. Hildebrand, D.M. Bramble, K.F. Liem y D.B. Wake (eds.), Functional Vertebrate Morphology, Harvard University Press, Cambridge and London, pp. 262- 290. [ Links ]
36. Hofmann, R.R. and Stewart, D.R.M. 1972. Grazer or browser: a classification based on the stomach structure and feeding habits of East African ruminants. Mammalia 36: 226-240. [ Links ]
37. Illiger, C. 1811. Prodromus systematis mammalium et avium additis terminis zoographicis utriusque classis. C. Salfeld, Berolini, 301 pp. [ Links ]
38. Janis, C.M.. 1995. Correlations between craniodental morphology and feeding behavior in ungulates: reciprocal illumination between living and fossil taxa. En: J. Thomason (ed.), Functional Morphology in Vertebrate Palaeontology, Cambridge University Press, pp. 76-98. [ Links ]
39. Janis, C.M. 1988. An estimation of tooth volume and hypsodonty indices in Ungulate mammals, and the correlation of these factors with dietary preference. En: D.E. Russell, J.P. Santoro y D. Sigogneau-Russell (eds.), Teeth revisited: Proceedings of the VII International Symposium on Dental Morphology, Memoirs de Musée national d'Histoire naturelle (serie C) 53: 367-387. [ Links ]
40. Janis, C.M. and Ehrhardt, D. 1988. Correlation of the muzzle width and relative incisor width with dietary preference in ungulates. Zoological Journal of the Linnean Society 92: 267-284. [ Links ]
41. Janis, C.M. and Fortelius, M. 1988. On the means whereby mammals achieve increased functional durability of their dentitions, with special reference to limiting factors. Biological Review 63: 197-230. [ Links ]
42. Kalthoff, D. and Tütken, 2007. Stable isotope composition of extant xenarthran teeth and their potencial fr the reconstruction of the diet of fosil xenarthrans (Mammalia). 8º International Congress of Vertebrate Morphology (Paris), Abstracts: 62. [ Links ]
43. Kraglievich, L. 1922. Estudio sobre los Mylodontinae. Análisis comparado de los valores craneométricos de los milodontinos de Norte y Sud América. Anales del Museo Nacional de Historia Natural de Buenos Aires 31: 457-464. [ Links ]
44. Kraglievich, L. 1923. Descripción comparada de los cráneos de Scelidodon rothi Ameghino y Scelidotherium parodii n. sp. Anales del Museo Nacional de Historia Natural de Buenos Aires 33: 57- 103. [ Links ]
45. Kraglievich, L. 1928. "Mylodon darwini" Owen es la especie genotipo de "Mylodon" Owen. Rectificación de la nomenclatura genérica de los milodontes. Physis 9: 169-185. [ Links ]
46. Kraglievich, L. 1934. Contribución al conocimiento de Mylodon darwini Owen y especies afines. Revista del Museo de La Plata 34: 255-292. [ Links ]
47. Lydekker, R. 1886. Description of three species of Scelidotherium. Proceedings of the Zoological Society of London: 496-497. [ Links ]
48. Lydekker, R. 1894. Contributions to a knowledge of the fossil vertebrates of Argentina, Part II: The extinct edentates of Argentina. Anales del Museo de La Plata (Paleontología Argentina) 3: 1-118. [ Links ]
49. Linnaeus, C. 1758. Systema Naturae per regna tria naturae, secundum classis, ordines, genera, species cum characteribus, differentiis, synonymis, locis. Laurentii Salvii, Stockholm, 824 pp. [ Links ]
50. Lund, P.W. 1842. Blik paa Brasiliens Dyreverden for Sidste Jordomvaeltning. Fjerde Afhandling: Fortsaettelse af Pattedyrene. Detkongelige Danske Videnskabernes Selskabs Skrifter. Naturvidenskabelige og Mathematisk Afhandlinger 9: 137-208. [ Links ]
51. Macalister, A. 1869. On the myology of Bradypus tridactylus; with remarks on the general anatomy of the Edentata. Annual Magazine of Natural History 4: 51-67. [ Links ]
52. Maynard Smith, J. and Savage, R.J.G. 1959. The mechanics of mammalian jaws. School Sciences Review 141: 289-301. [ Links ]
53. McDonald, H.G. 1987. [A systematic review of the Plio-Pleistocene scelidothere ground sloths (Mammalia: Xenarthra, Mylodontidae). PhD Dissertation, University of Toronto, Toronto. 478 pp. Unpublished.]. [ Links ]
54. McDonald, H.G. 1995. Gravigrade Xenarthrans from the early Pleistocene Leisey Shell Pit 1A, Hillsborough County, Florida. Bulletin of Florida Museum of Natural History 37: 345-373. [ Links ]
55. McDonald, H.G. and De Iuliis, G. 2008. Fossil history of sloths. En: W.J. Loughry y S.F. Vizcaíno (eds.), The Biology of the Xenarthra, University Press of Florida, pp. 39-55. [ Links ]
56. Mendoza, M. and Palmqvist, P. 2008. Hypsodonty in ungulates: an adaptation for grass consumption or for foraging in open habitat? Journal of Zoology 274: 134-142. [ Links ]
57. Mendoza, M., Janis, C. and Palmqvist, P. 2002. Characterizing complex craniodental patterns related to feeding behavior in ungulates: a multivariate approach. Journal of Zoology London 258: 223-246. [ Links ]
58. Merino, M.L., Milne, N. and Vizcaíno, S.F. 2005. A morphometric study of deer (Mammalia, Cervidae) crania from Argentina using three dimensional landmarks. Acta Theriologica 50: 91- 108. [ Links ]
59. Moore, D.M. 1978. Post-glacial vegetation in the south Patagonian territory of the giant ground sloth, Mylodon. Botanical Journal of the Linnaean Society 77: 177-202. [ Links ]
60. Moore, W.J. 1981. The Mammalian Skull. Cambridge University Press, 369 pp. [ Links ]
61. Naples, V.L. 1982. Cranial osteology and function in the tree sloths, Bradypus and Choloepus. American Museum Novitates 2739: 1-41. [ Links ]
62. Naples, V.L. 1985. Form and function of the masticatory musculature in the tree sloths, Bradypus and Choloepus. Journal of Morphology 183: 25-50. [ Links ]
63. Naples, V.L. 1987. Reconstruction of cranial morphology and analysis of function in the Pleistocene ground sloth Nothrotheriops shastense (Mammalia, Megatheriidae). Contributions in Science, Los Angeles County Museum of Natural History 389: 1-21. [ Links ]
64. Naples, V.L. 1989. The feeding mechanism in the Pleistocene ground sloth, Glossotherium. Contributions in Science, Los Angeles County Museum of Natural History 415: 1-23. [ Links ]
65. Owen, R. 1839. Fossil Mammalia (2). En: C.R. Darwin (ed.), The Zoology of the Voyage of the M.S.H. Beagle, London, 1(7): 41-64. [ Links ]
66. Owen, R. 1840. Fossil Mammalia (4). En: C.R. Darwin (ed.), The Zoology of the Voyage of the M.S.H. Beagle, London, 1(13): 81- 111. [ Links ]
67. Owen, R. 1842. Description of the skeleton of an extinct gigantic sloth, Mylodon robustus, Owen, with observations on the osteology, natural affinities, and probable habits of the megatherioid quadruped in general. R. and J. E. Taylor, London, 176 pp. [ Links ]
68. Owen, R. 1843. Letter from Richard Owen, Esq., F.R.S., F.G.S. etc., etc., on Dr. Harlan's notice of new fossil Mammalia. American Journal of Sciences and Arts 44: 341-345. [ Links ]
69. Owen R. 1856. On the Megatherium (Megatherium americanum Cuvier and Blumenbach). III. The skull. Philosophical Transactions of the Royal Society of London 146: 571-589. [ Links ]
70. Owen R. 1857. On the Scelidothere (Scelidotherium leptocephalum) Owen. Philosophical Transactions of the Royal Society of London 147: 101-110. [ Links ]
71. Owen, R. 1860. Memoir on the Megatherium or Giant Ground-Sloth of America (Megatherium americanum, Cuvier). Taylor y Francis, London, 84 pp. [ Links ]
72. Plotnick, R. and Baumiller, T.K. 2000. Invention by evolution: functional analysis in paleobiology. En: D.H. Erwin y S.L. Wing (eds.), Deep Time. Paleobiology's perspective. Supplement to Paleobiology 26: 305-321. [ Links ]
73. Pérez, L.M., Scillato-Yané, G.J. and Vizcaíno, S.F. 2000. Estudio morfofuncional del aparato hioideo de Glyptodon sp. (Cingulata, Glyptodontidae). Ameghiniana 37: 293-299. [ Links ]
74. Rensberger, J.M. 1973. An occlusion model for mastication and dental wear in herbivorous mammals. Journal of Paleontology 47: 515-528. [ Links ]
75. Scillato-Yané, G.J. 1977. Octomylodontinae: nueva subfamilia de Mylodontidae (Edentata,Tardigrada). Descripción del cráneo y mandíbula de Octomylodon robertoscagliai n.sp., procedentes de la formación Arroyo Chasicó (Edad Chasiquense, Plioceno temprano) del sur de la Provincia de Buenos Aires (Argentina). Algunas consideraciones filogenéticas y sistemáticas sobre los Mylodontoidea. Publicaciones del Museo Municipal de Ciencias Naturales de Mar del Plata L. Scaglia 2: 123-140. [ Links ]
76. Scillato-Yané, G.J. 1986. Los Xenarthra fósiles de Argentina (Mammalia, Edentata). 4º Congreso Argentino de Paleontología y Bioestratigrafía (Mendoza), Actas 2: 151-165. [ Links ]
77. Sicher, H. 1944. Masticatory apparatus of the sloths. Fieldiana: Zoology 29:161-168. [ Links ]
78. Sinclair, W.J. 1905. New Mammalia from the Quaternary caves of California. University of California Publications, Bulletin of the Department of Geology 4: 145-161. [ Links ]
79. Smith, K.K. 1993.The form of the feeding apparatus in terrestrial vertebrates: studies of adaptation and constraint. En: J. Hanken y B.K. Hall (eds.), The skull, volume 3: Functional y Evolutionary mechanisms. The University of Chicago Press, Chicago y London, pp. 150-196. [ Links ]
80. Solounias, N. and Dawson-Saunders, B. 1988. Dietary adaptations and palaeoecology of the late Miocene ruminants from Pikermi and Samos in Greece. Palaeogeography, Palaeoclimatology, Palaeoecology 65: 149-172. [ Links ]
81. Solounias, N. and Moelleken, S.M. 1993. Dietary adaptation of some extinct ruminants determined by premaxillary shape. Journal of Mammalogy 74: 1059-1071. [ Links ]
82. Solounias, N., Teaford, M. and Walker, A. 1988. Interpreting the diet of extinct ruminants: the case of a non-browsing giraffid. Paleobiology 14: 287-300. [ Links ]
83. Spencer, L.M. 1995. Morphological correlates of dietary resource partitioning in the African Bovidae. Journal of Mammalogy 76: 448-471. [ Links ]
84. Spillman, F. 1948. Beiträge zur Kenntnis eines neuen gravigraden Riesensteppentieres (Eremotherium carolinense gen. et spec. nov.), seines Lebensraumes und seiner Lebensweise. Paleobiologica 8: 231-279. [ Links ]
85. Stock, C. 1925. Cenozoic gravigrade edentates of Western North America with special reference to the Pleistocene Megalonychinae and Mylodontidae of Rancho La Brea. Carnegie Institution of Washington Publications 331: 1-206. [ Links ]
86. Turnbull, W.D. 1970. Mammalian masticatory apparatus. Fieldiana Geology 18: 149-356. [ Links ]
87. Turnbull, W.D. 1976. Restoration of masticatory musculature of Thylacosmylus. En: C.S. Churcher (ed.), Essays on Palaeontology in Honour of Loris Shano Russel, Athlon, Royal Ontario Museum Life Sciences Miscellaneous Publication, pp. 169-185. [ Links ]
88. Vizcaíno, S.F. 1994. Mecánica masticatoria de Stegotherium tessellatum Ameghino (Mammalia, Xenarthra) del Mioceno temprano de Santa Cruz (Argentina). Algunos aspectos paleoecológicos relacionados. Ameghiniana 31: 283-290. [ Links ]
89. Vizcaíno, S.F. 2000.Vegetation partitioning among Lujanian (late Pleistocene-early Holocene) armored herbivores in the pampean region. Current Research in the Pleistocene 17: 135-137. [ Links ]
90. Vizcaíno, S.F. and Bargo, M.S. 1998. The masticatory apparatus of Eutatus (Mammalia, Cingulata) and some allied genera. Evolution y paleobiology. Paleobiology 24: 371-383. [ Links ]
91. Vizcaíno, S.F. and De Iuliis, G. 2003. Evidence for advanced carnivory in fossil armadillos (Mammalia: Xenarthra: Dasypodidae). Paleobiology 29: 123-138. [ Links ]
92. Vizcaíno, S.F. and Fariña, R.A. 1997. Diet and locomotion of the armadillo Peltephilus: a new view. Lethaia 30: 79-86. [ Links ]
93. Vizcaíno, S.F., De Iuliis, G. and Bargo, M.S. 1998. Skull shape, masticatory apparatus, and diet of Vassallia y Holmesina (Mammalia: Xenarthra: Pampatheriidae). When anatomy constrains destiny. Journal of Mammalian Evolution 5: 291-322. [ Links ]
94. Vizcaíno, S.F., Bargo, M.S. and Cassini, G.H. 2006. Dental occlusal surface area in relation to body mass, food habits and other biologic features in fossil Xenarthrans. Ameghiniana 43: 11-26. [ Links ]
95. Vizcaíno, S.F., Zárate, M., Bargo, M.S. and Dondas, A. 2001. Pleistocene large burrows in the Mar del Plata area (Buenos Aires Province, Argentina) and their probable builders. Acta Paleontologica Polonica, Special Issue 46: 157-169 [ Links ]
96. Vizcaíno, S.F., M.S. Bargo, R.F. Kay and N. Milne. 2006. The armadillos (Mammalia, Xenarthra) of the Santa Cruz Formation (early-middle Miocene). An approach to their paleobioloy. Palaeogeography, Palaeoclimatology, Palaeoecology 237: 255-269. [ Links ]
97. Webb, S.D. 1985. The interrelationships of tree sloths y ground sloths. En: G.G. Montgomery (ed.), The Evolution y Ecology of Armadillos, Sloths, and Vermilinguas. Smithsonian Institution Press, Washington, pp. 105-112. [ Links ]
98. White, J.L. 1997. Locomotor adaptations in Miocene Xenarthrans. En: R.F. Kay, R.H. Madden, R.L. Cifelli, y J.J. Flynn (eds.), Vertebrate Paleontology in the Neotropics. The Miocene Fauna of La Venta, Colombia, Smithsonian Institution Press, 16: 246-264. [ Links ]
99. Windle, B.C.A. and Parsons, F.G. 1899. On the myology of Edentata. Proceedings of the Zoological Society of London 1: 314- 339. [ Links ]
100. Winge, H. 1941. Edentates (Edentata). En: S. Jensen, R. Spärck y H. Volsoe (eds.), The Interrelationships of the Mammalia genera, Reitzels Forlag, Copenhagen, pp. 319-341. [ Links ]
Recibido: 22 de agosto de 2006.
Aceptado: 4 de diciembre de 2007.














