Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Darwiniana, nueva serie
versión impresa ISSN 0011-6793versión On-line ISSN 1850-1699
Darwiniana v.44 n.1 San Isidro ene./jul. 2006
Contribution to the study of alkalophilic and alkali-tolerant Ascomycota from Argentina
L. A. Elíades1, M. N. Cabello1 & C. E. Voget2
1 Instituto Spegazzini (UNLP), calle 53 N 477, 1900 La Plata, Buenos Aires, Argentina; mcabello@netverk.com.ar (author for correspondence).
2 CINDEFI, Facultad de Ciencias Exactas, UNLP-CONICET, Calle 47 y 115, 1900 La Plata, Buenos Aires, Argentina.
Abstract. Elíades, L. A., M. N. Cabello & C. E. Voget. 2006. Contribution to the study of alkalophilic and alkali-tolerant Ascomycota from Argentina. Darwiniana 44(1): 64-73.
Several species of alkalophilic and alkali-tolerant soil Ascomycota were isolated and reported here, namely: Emericellopsis minima; Melanospora zamiae; Neosartorya stramenia; Neurospora tetrasperma; Talaromyces flavus var. flavus; Talaromyces trachyspermus; Talaromyces stipitatus and Westerdykella dispersa. Neosartorya stramenia, Neurospora tetrasperma and Westerdykella dispersa were recorded for the first time for Argentina.
Keywords. Soil fungi; Ascomycota; Alkalophilic; Alkali-tolerant; Argentina.
Resumen. Elíades, L. A., M. N. Cabello & C. E. Voget. 2006. Contribución al estudio de Ascomycota alcalofílicos y alcalino-tolerantes de Argentina. Darwiniana 44(1): 64-73.
Se informa sobre 8 especies de Ascomycota alcalofílicos y alcalino-tolerantes de suelo: Emericellopsis minima; Melanospora zamiae; Neosartorya stramenia; Neurospora tetrasperma; Talaromyces flavus var. flavus; Talaromyces trachyspermus; Talaromyces stipitatus y Westerdykella dispersa. Neosartorya stramenia, Neurospora tetrasperma y Westerdykella dispersa son nuevos registros para Argentina.
Palabras clave. Hongos del suelo; Ascomycota; Alcalofílico; Alcalino-tolerante; Argentina.
Original recibido el 21 de septiembre de 2005;
aceptado el 10 de mayo de 2006
INTRODUCTION
Fungi are often dominant members of soil microbiota, especially in acidic environments, and may operate over a wider pH range than that of many heterotrophic bacteria (Gadd 2004). While there are numerous reports on fungi isolated from acidic to neutral soils, there are only a few reports about fungi from alkaline soils. Alkaline soils were examined for their fungal biota by Rai et al., (1971) and Nagai et al., (1995,1998). Soils with large amounts of calcium carbonate were studied by Vardavakis (1990) in Greece, -whereas in Argentina, Cabello & Arambarri (2002) and Elíades et al. (2004) have reported fungi from alkaline soils. In all these contributions only a few Ascomycota have been cited.
The native dry forest dominated by Celtis tala -and Scutia buxifolia is the main woodland community of the eastern plain called Pampa in Buenos Aires Province, Argentina. Woody ranges are located almost parallel to the coast of the La Plata River. These trees grow in soils with calcareous structure of biological origin (mollusk shells) classified as Rendoll (Sánchez et al., 1976). Between these woody ranges, salt meadow covers the inter-ranges; the alluvial soils with contrasting structures and high sodium content are characterized as Natracualf, whereas the moist meadow with defective drain and hydromorphic, non-alkaline soils is described as Argialbol.
The aim of the present work was to characterize and illustrate morphological features of alkalophilic and alkali-tolerant soil Ascomycota at two different depths in each soil: Rendoll, Natracualf and Argialbol by using soil washing methods for isolation. The ability of these fungi to grow at different pH was investigated.
MATERIALS AND METHODS
Study area, types of soil
The study area was a dry forest dominated by Celtis tala and Scutia buxifolia 20 km south east from Magdalena city (35° 11 S, 57° 17 W) in the Province of Buenos Aires, Argentina. The climatic characteristics of this forest have been described by Arturi et al. (1996).
Table 1 shows the main characteristics of the three types of soils sampled.
Table 1. Physical and chemical properties of the forest soils used in this study.

Fungal isolation
Ascomycota were isolated from soil collected from the described area by the washing method (Parkinson & Williams, 1961) on malt extract agar [MA: 10g of malt extract powder (Difco), 2.5g of peptone, 20g of agar, 1 L of distilled water] which initial pH was adjusted after autoclaving with buffers according to Nagai et al. (1998) to the values 8, 9, 10, and 11. Twenty plates were prepared for each pH and four soil particles transferred. After incubation for 5-7 days at 24° C, the growing fungi were observed under a light microscope. Eight species of Ascomycota were isolated from alkaline pH.
Growth rates of isolates at various pH
One strain was selected at random as a representative from each species. The strains were inoculated onto malt extract agar MA which initial pH was adjusted after autoclaving to 5.0, 6.0, 7.0, 8.0, 9.0, 10.0 and 11.0. Growth patterns of the different species were obtained by measuring twice the colony diameter of the representative strains after incubation for one to two weeks.
Strains that could not grow at pH 10 were considered as alkalophobic; those that grew at pH 10 as alkali- tolerant; and those that could grow at pH over 10, but not at pH 5, as alkalophilic.
RESULTS
Table 1 shows the characteristics of the profiles of the 3 soils sampled. All of them presented silt to clayey silty texture. The pH ranges varied from acid soils (Argialbol) to alkaline (Rendoll and Natracualf). In Natracualf soil, the increment of pH was due to the elevated contents of sodium at the two depths sampled (5.39 and 13.9 meq100g-1, respectively), while in Rendoll, the increment of pH was due to the high contents of calcium carbonate (48 and 94%, respectively).
Table 2 shows 8 species of Ascomycota isolated from the 3 different soil types at two depths and various pH.
Table 2. Species of Ascomycota isolated from the three soils at two depths and various pH.
Taxonomy
BIONECTRIACEAE
1. Emericellopsis minima Stolk, Trans. Brit. Mycol. Soc. 38: 419. 1955. (Fig. 1A-B). For synonyms see Domsch et al. (1993).
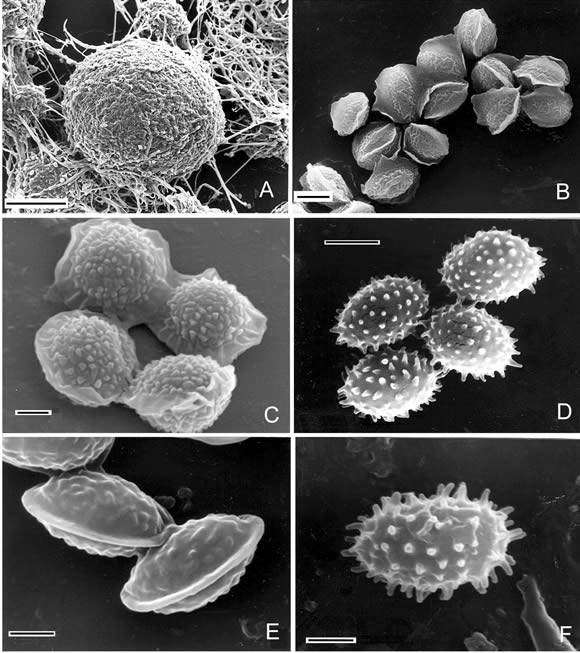
Fig. 1. A-B, Emericellopsis minima, A, ascomata (SEM). Scale bar = 5 µm. B, ascospores (SEM). Scale bar = 2 µm. C, ascospores of Neosartorya stramenia (SEM). Scale bar = 1 µm. D, ascospores of Talaromyces flavus var. flavus (SEM). Scale bar = 2 µm. E, ascospores of Talaromyces stipitatus (SEM). Scale bar = 1 µm. F, ascospores of Talaromyces trachyspermus (SEM). Scale bar = 1 µm.
Observations. During development of the colonies of E. minima, the first observed phase of its cycle was the anamorph belonging to Acremonium sp., then the culture developed ascomata Our specimen agrees with the original description and with that by Wright et al., (1971) from Argentina.
Material examined
ARGENTINA. Buenos Aires. Magdalena, IX-2003, Elíades s.n. LPS culture N° 839 isolated from superficial horizons from Argialbol at pH 11.
TRICHOCOMACEAE
2. Neosartorya stramenia(Novak & Raper) Malloch & Cain, Can. J. Bot. 50: 2622. 1972. (Fig. 1C).
Aspergillus stramenius Novak & Raper, in Raper & Fennell, The genus Aspergillus: p 260. 1965 (nom. holom.).
Colonies on MEA attaining a diameter of 20-23 mm in 7 days, velvety. Teleomorph: Ascomata scattered on the basal mycelium, non-ostiolate, globose to subglobose, 30-150 µm in diameter. Asci 8 spored, globose to subglobose or pyriform, 12-17 x 10-12 µm, evanescent at maturity. Ascospores hyaline to pale yellow, lenticular 6-7 x 3-5 µm, with two equatorial crests which are somewhat widely separated, with convex surfaces finely roughened (spinulose under SEM).
The anamorph showed the characteristics of the genus Aspergillus Micheli ex Link. Conidial heads loosely columnar, 60-100 µm long. Conidiophores 40-120 x 3-7 µm., smooth and fairly thick walled; vesicles flask-shaped, 8-14 µm diam, Aspergillia uniseriate; phialides cylindrical, 4,5-6 x 2-3 µm, covering the upper one-half to two-thirds of the vesicle. Conidia hyaline to pale-colored, globose to subglobose, 2-3 µm diam, delicately roughened.
Observations. This species is recorded for the first time in Argentina.
Material examined
ARGENTINA. Buenos Aires: Magdalena, II-2005 Elíades. LPS culture N° 833 isolated from Rendoll at 20cm deep horizon and Natracualf soils, at pH 6, 8 and 11.
3. Talaromyces flavus(Klocker) Stolk & Samson var. flavus, Stud. Mycol. p 2. 1972. (Fig. 1D).
Gymnoascus flavus Klöcker, Hedwigia 41: 80. 1902. For more synonyms see Stolk & Samson 1972.
Anamorph: Penicillium vermiculatum Dangeard, Botaniste 10: 23. 1907.
Observations. Our specimens are in agreement with those described from Argentina by Bertoni et al. (1973).
Material examined
ARGENTINA. Buenos Aires: Magdalena, IX-2003, Elíades s.n. LPS culture Nº 838 from Argialbol superficial pH 7; LPS culture Nº 832 from Argialbol, 20 cm deep, pH 9.
4. Talaromyces stipitatus (Thom) C. R. Benjamin, Mycologia 47: 684. 1955. (Fig. 1E).
Penicillium stipitatus Thom, Mycologia 27: 138, 1935. (Diagnosis Latina status perfecti).
Anamorph: Penicillium stipitatum Thom ex C. W. Emmons, Mycologia 27: 138. 1935.
Observation. Our species agree with that described by Stolk & Samson (1972). This fungus was described in our country by Wright et al., (1971).
Material examined
ARGENTINA. Buenos Aires: Magdalena, IX-2003; Elíades s.n. LPS culture N° 836 from Natracualf superficial isolated at pH 6; LPS culture Nº 835 from Natracualf superficial pH 8; LPS culture Nº 840 isolated from Argialbol superficial pH 7.
5. Talaromyces trachyspermus (Shear) Stolk & Samson, Stud. Mycol. 2: 32. 1972. (Fig. 1 F).
Arachniotus trachyispermus Shear, Science N.Y. II 16 :138. 1902
Talaromyces spiculisporus (Lehman) Benjamin, Mycologia 47: 683. 1955.
Anamorph: Penicillium spiculisporum Lehman, Mycologia 12: 268. 1920.
Observation. This species has been previously cited by Godeas (1972).
Material examined
ARGENTINA. Buenos Aires: Magdalena, IX-2003 Elíades s.n. LPS culture N° 831 isolated from superficial horizon from Argialbol at pH 7.
SPORORMIACEAE
6. Westerdykella dispersa Clum, Mycologia 47: 899, 1955. (Fig. 2A-B).
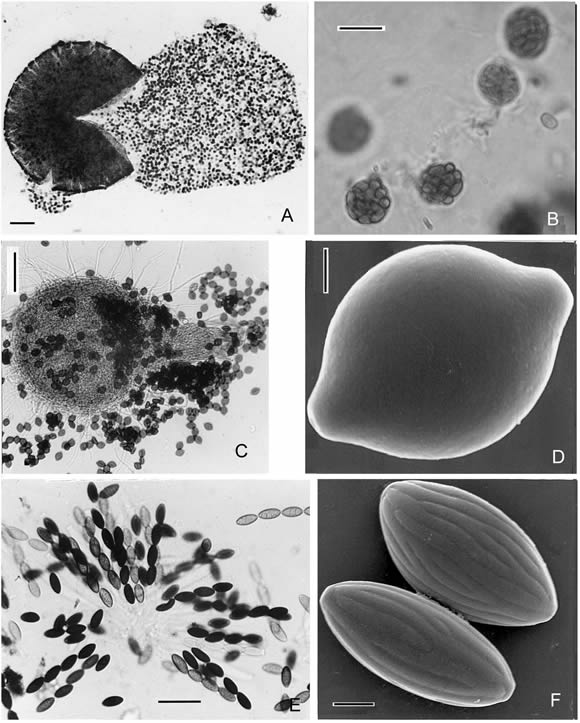
Fig. 2. A-B, Westerdykella dispersa. A, ascomata. Scale bar = 100 µm. B, asci with ascospores. Scale bar = 5 µm. C-D, Melanospora zamiae. C, ascomata. Scale bar = 100 µm. D, ascospore (SEM). Scale bar = 2 µm. E-F, Neurospora tetrasperma. E, asci with ascospores. Scale bar = 100 µm. F, ascospores (SEM). Scale bar = 5 µm.
Mycelia homotallic, profusely branched, the hyphae 1-6 (m in diameter; Teleomorph: ascomata 60-580 (m in diameter; asci globose, 10-14.5 µm in diameter. Ascospores 2.0-3.0 x 2.5-5 µm, hyaline when young, later becoming light brown.
Anamorph: the conidial state corresponds with the species of Fungi Imperfecti Phoma Saccardo. Pycnidia varying from globose to irregularly-elongated in shape, glabrous; the lower portion colorless and the walls of the cells around the slightly papillate ostiole light brown, 26-75 x 26-158 µm . Conidiophores simple, extremely short. Conidia hyaline, unicellular, oblong, 1.5-3.5 x 2.6-5 µm.
Observation. This is the first record from our country.
Material examined
ARGENTINA. Buenos Aires: Magdalena. IX-2003,. Elíades s.n. LPS culture N° 830 isolated from Argialbol superficial horizon at pH 8; LPS culture Nº 834, Argialbol superficial horizon, pH 9.
CERATOSTOMATACEAE
7. Melanospora zamiae Corda, Icones Fungorum I: 27. 1837. (Fig. 2C-D).
For synonyms see Calviello 1973.
Observation. During development of the colonies of M. zamiae, both stages of the life cycle - anamorphic and teleomorphic- appeared at the same time. The anamorphic state belonging to the mitosporic genus Acremonium. M. zamiae was isolated and described in our country by Calviello (1973).
Material examined
ARGENTINA. Buenos Aires: Magdalena, VI-2004, Elíades s.n. LPS culture N° 841, isolated from the calcareous soil Rendoll at pH 10.
SORDARIACEAE
8. Neurospora tetrasperma Shear & Dodge, J. Agric. Res. 34: 1027 (1927). (Fig. 2 E-F).
For synonyms see García et al. (2004).
Colonies brown or orange. Teleomorph: Ascomata ostiolated, 180-570 µm. Asci 4- spored. Ascospores ellipsoidal or elongated, 25-46 x 13-25 µm; wall with 16-22 sometimes branched ribs and ornate epispore, one germ pore at each end. Anamorph: Chrysonilia tetrasperma (Shear & Dodge) Arx (Garcia et al., 2004). Conidia subglobose to obovoid, smooth, 5-10 (-15) x 8-10 µm, orange in mass.
Observation. This is the first record from our country.
Material examined
ARGENTINA Buenos Aires: Magdalena, XI-2004, Elíades s.n. LPS culture N° 837 isolated from alkaline soil Natracualf at pH 6.
Growth patterns of the isolates
Fig. 3 shows the growth patterns of 8 Ascomycota isolated. Members of the Family Trichocomaceae, namely Neosartorya stramenia, Talaromyces flavus var flavus, T. stipitatus and T. trachyspermus showed different patterns of growth. It was found that N. stramenia could grow at all the pH tested. All the species of Talaromyces developed mycelial growth up to pH 8 and 9.
Although we were able to isolate T. flavus var. flavus from soil particles at pH 9 and 11, this species did not show the same ability to keep growing at these pH after isolation.
Emericellopsis minima isolated from Argialbol at pH 11 was able to grow in all the pH tested. The restricted development observed corresponds to the intrinsic characteristics of the species. Westerdykella dispersa showed optimal growth from pH 5 to pH 8, while Melanospora zamiae, isolated from Rendoll at pH 10, showed optimal growth from pH 5 to 10 and a decrease in mycelial development at pH 11. Neurospora tetrasperma increased its growth when the pH in the culture medium was increased.
It is important to point out that all the Ascomycota tested developed ascomata in various culture media and at the pH tested.
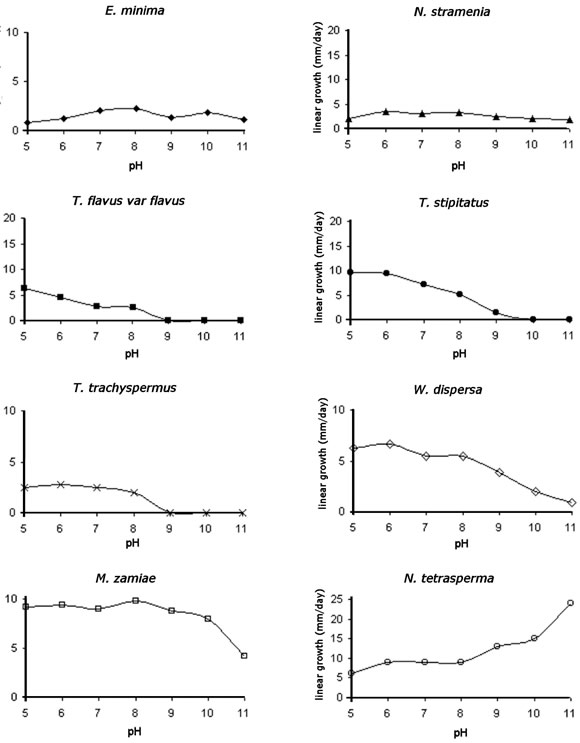
Fig. 3. Growth patterns of 8 Ascomycota isolated.
DISCUSSION
The distribution of Ascomycota in soils is generally low when compared with other fungal groups (Cabello et al., 2003). This distribution can be higher and can reach up to 49% of the total taxa isolated if the species belong to fungal communities developed on organic debris (Van Ryckegem & Verbeken, 2005).
In the present work, 8 species of Ascomycota, isolated from soils with silt to clayey silty texture and from pH 6 to pH 11, are reported. All the isolated Ascomycota are either alkali-tolerant or alkalophilic species according to Nagai et al. (1998). In previous works about alkaline soils, the richness of isolated species of Ascomycota has been much lower (Cabello & Arambarri, 2002; Vardavakis, 1990). Neosartorya stramenia, Neurospora tetrasperma and Westerdykella dispersa are reported for the first time in Argentina.
Emericellopsis minima, Neosartorya stramenia and Melanospora zamiae were isolated at pH 10 to11. These species are alkalophilic according to the pH isolation medium. Of these three species, E. minima was always found associated with its anamorph Acremonium sp, which is the first phase of the cycle of the fungus that appears in the culture medium. It developed ascomata after a few days.
Besides Acremonium, M. zamiae also showed its anamorphic stages in Proteophiala sp. (Ciferri, 1957) and Phialophora sp. (Ito & Yokoyama, 1987) The presence of the complete cycle (anamorph + teleomorph) is a relevant fact and the appearance of the anamorphic phase is consistent with the classification of the genus Acremoniumas alkali-tolerant (Nagai et al., 1998; Cabello & Arambarri, 2002).
Among the members of the family Trichocomaceae, only Neosartorya stramenia can be considered an alkalophilic species because it can keep growing up to pH 11. The low values (measured in mm/day) correspond to intrinsic characteristics of the species (Yaguchi et al., 1994). Its anamorphic stage is found in Aspergillus stramenius. Cabello & Arambarri (2002) have previously observed that the species of the genus Aspergillus predominated in alkaline isolation media. Due to the fact that there are few reports on N. stramenia in the literature, this species is probably a rare fungus (Raper & Fennell, 1965; Yaguchi et al., 1994).
Other members of the family Trichocomaceae are the three species of Talaromyces.
All the species here described belonging to this genus are homothallic, and they have their conidial states in the genus Penicillium. Among all the isolated Ascomycota, Talaromyces species were the most sensitive to high pH, although we were able to isolate them at pH 6 to pH 11. Their anamorphs belonging to Penicillium preferred acid pH (Gams, 1992). Although some members from the genus Talaromyces reported here were isolated at pH 11, they can not be strictly considered as alkaline-tolerant since the growth patterns showed that they were not able to grow at high pH. The fact that they were isolated at a high pH could be due that these species were over-represented in the soil.
Although Neurospora tetrasperma has been frequently isolated from burnt vegetation (Jacobson et al., 2004), in this study this species was isolated from the alkaline Natracualf soil. Taking into account the great myceliar development at pH 11, we can state that this species preferred alkaline conditions.
The Ascomycota isolated from these soils at different pH ranges were different from these species reported in other soils in Argentina (Cabello et al., 2003). The soil fungal Ascomycota need further analysis and physiological studies in order to know how Ascomycota fungal strains live in alkaline conditions.
ACKNOWLEDGEMENTS
The authors are indebted to W. Gams for critical review of the manuscript, to A. M. Bucsinszky, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), who was in charge of the culture collection at the Spegazzini Institute, to N. Tedesco for improving use of English language and to anonymous reviewers who greatly contributed to improve this manuscript. Elíades, L. is a recipient of a scholarship from CONICET, Cabello, M. N. is a researcher from Comisión de Investigaciones Científicas (CIC) Bs. As. Province, Voget, C. E. is researcher from CONICET. This research is part of N411UNLP and CYTED, Red Iberoamericana sobre Diversidad, Ecología y uso de hongos microscopicos (REDEMIC) projects. Grant from CIC, and ANPCyT No 13404 BID 1201/OC-AR Argentina supported this research.
BIBLIOGRAPHY
1. Arturi, M., M. D. Barrera & A. D. Brown. 1996. Caída y masa de hojarasca en bosques de Celtis tala Gill ex Planch y Scutia buxifolia Reiss del este de la provincia de Buenos Aires, Argentina. Revista Fac. Agron. Univ. Nac. La Plata. 101: 151-158. [ Links ]
2. Bertoni, M. D., A. M. Godeas, M. E. Loewenbaum & J. E. Wright. 1973. Micoflora del suelo de la Argentina. IV Algunas formas ascospóricas adicionales de la Región Chaqueña. Bol. Soc. Argent. Bot. 15: 93-105. [ Links ]
3. Cabello, M. N. & A. M. Arambarri. 2002. Diversity in soil fungi from undisturbed and disturbed Celtis tala and Scutia buxifolia forests in the eastern Buenos Aires province (Argentina). Microbiol. Res. 157: 115-125. [ Links ]
4. Cabello, M. N., M. A. Aon & M. S. Velázquez. 2003. Diversity, structure, and evolution of fungal communities in soils under different agricultural management practices. Bol. Soc. Argent. Bot. 38: 225-232. [ Links ]
5. Calviello, B. O. 1973. Contribución al estudio de Ascomycetes Argentinos. I. Comun. Mus. Argent. Ci. Nat. "Bernardino Rivadavia". Ci. Bot. 2, 7: 31-39. [ Links ]
6. Ciferri, R. 1958. Osservazioni intorno a tre fungilli dell Ólivo. Sydowia 11: 282-289. [ Links ]
7. Domsch, K. H., W. Gams & T. Anderson. 1993. Compendium of soil fungi. IHV-Verlag. [ Links ]
8. Elíades, L. A., A. M. Bucsinszky & M. N. Cabello. 2004. Micobiota alcalofilica y alcalino-tolerante en suelos de bosques xéricos en una localidad de la Provincia de Buenos Aires, Argentina. Bol. Micol. 19: 41-47. [ Links ]
9. Gadd, M. G. 2004. Mycotransformation of organic and inorganic substrates. Mycologist. 18: 60-70. [ Links ]
10. Gams, W. 1992. The analysis of communities of saprophytic microfungi with special reference to soil fungi, pp 183-223, in W. Winterhoff (ed), Fungi in vegetation Science. Kluwer Academic Publishers, Dordrecht. [ Links ]
11. Garcia, D., A. M. Stchigel, J. Cano, J. Guarro & D. L. Hawks-worth. 2004. A synopsis and re-circumscription of Neurospora (syn. Gelasinospora) based on ultrastructural and 28S rDNA sequence data. Mycol. Res. 108: 1119-1142. [ Links ]
12. Godeas, A. M. 1972. Micoflora del suelo de la Argentina. I Algunas formas ascospóricas de la Región Chaqueña. Mycopath. Mycol. Appl. 46: 189-204. [ Links ]
13. Ito, T. & T. Yokohama. 1987. Descriptive catalogue of IFO fungus Collection X. IFO Res. Comm. 13: 83-85 (Annual Report 1985-1986) Institute for Fermentation, Osaka, Japan. [ Links ]
14. Jacobson, D. J., A. J. Powell, J. R. Dettman, G. S. Saenz, M. M. Barton, M. D. Hiltz, W. H. Dvorachek Jr., N. L. Glass, J. W. Taylor & D. O. Natvig. 2004. Neurospora in temperate forests of western North America. Mycologia 96: 66-74. [ Links ]
15. Nagai, K., T. Sakai, T. Ratiatmodjo, R. M. Suzuki, K. Gams, W. & G. Okada. 1995. Studies on the distribution of alkalophilic and alkali-tolerant soil fungi I. Mycoscience 36: 247-256. [ Links ]
16. Nagai, K., K Suzuki & G. Okada. 1998. Studies on the distribution of alkalophilic and alkali-tolerant soil fungi II: Fungal flora in two limestone caves in Japan. Mycoscience 39: 293- 298. [ Links ]
17. Parkinson, D. & S. T. Williams. 1961. A method for isolating fungi from soil microhabitats. Pl. Soil 13: 347-355. [ Links ]
18. Rai, J. N., S. C. Agarwal & J. P. Tewari. 1971. Fungal microflora of usar soils of India. J. Indian Bot. Soc. 50: 63-74. [ Links ]
19. Raper, K. B. & D. I. Fennell. 1965. The genus Aspergillus. Williams and Wilkins, Baltimore. 686 pp. [ Links ]
20. Sánchez, R. O., J. A. Ferrer, O. A. Duymovich & M. A. Hurtado. 1976. Estudio pedológico integral de los Partidos de Magdalena y Brandsen (Provincia de Buenos Aires). Anales del LEMIT, serie II N° 310. Ministerio de Obras Públicas de la Provincia de Buenos Aires. 1-123. [ Links ]
21. Stolk, A. C. & A. Samson. 1972. The genus Talaromyces. Studies on Talaromyces and related genera II. Stud. Mycol. 2: 1-65. [ Links ]
22. Van Ryckegem, G. & A. Verbeken. 2005. Fungal diversity and community structure on Phragmites australis (Poaceae) along a salinity gradient in the Scheldt estuary (Belgium). Nova Hedwigia 80: 173-197. [ Links ]
23. Vardavakis, E. 1990. Seasonal fluctuations of soil microfungi in correlation with some soil enzyme activities and VA mycorrhizae associated with certain plants of a typical calcixerol soil in Greece. Mycologia 82: 715-726. [ Links ]
24. Wright, J. E., A. M. Godeas & M. D. Bertoni. 1971. Micoflora del suelo de la Argentina. II Algunas formas ascospóricas de la Provincia de Buenos Aires. Bol. Soc. Argent. Bot. 14: 43-56. [ Links ]
25. Yaguchi, T., A. Someya & S. Udagawa. 1994. Fennellia flavipes and Neosartorya stramenia, two new records from Japan. Mycoscience 35: 175-178. [ Links ]














