Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Darwiniana, nueva serie
versión impresa ISSN 0011-6793
Darwiniana vol.47 no.2 San Isidro ago./dic. 2009
SISTEMÁTICA Y TAXONOMÍA DE PLANTAS VASCULARES
Notes on the genus Haematomma (Ascomycota, Lecanoraceae) in Argentina
María I. Messuti & Iris N. de la Rosa
Instituto de Investigaciones en Biodiversidad y Medioambiente (INIBIOMA), CONICET-Universidad Nacional del Comahue, Quintral 1250, R8400 FRF, San Carlos de Bariloche, Río Negro, Argentina; mmessuti@crub.uncoma.edu.ar (author for correspondence).
Original recibido el 23 de junio de 2009;
aceptado el 3 de diciembre de 2009.
Abstract. New information on the taxonomy, chemistry, distribution and ecology are given for seven taxa of the genus Haematomma occurring in Argentina. Two of them, H. flexuosum and H. fluorescens var. fluorescens, are reported for the first time for Argentina. An identification key to the recognized taxa of Haematomma in Argentina is provided.
Keywords. Argentina; Chemistry; Distribution; Haematomma; Microlichens.
Resumen. Notas sobre el género Haematomma (Ascomycota, Lecanoraceae) en Argentina.
Se presentan novedades sobre la taxonomía, química, distribución y ecología de los siete taxones del género Haematomma presentes en la Argentina. Dos de ellos, H. flexuosum y H. fluorescens var. fluorescens son registrados por primera vez para el país. Se incluye una clave del género Haematomma para la identificación de los taxones reconocidos en la Argentina.
Palabras clave. Argentina; Distribución; Haematomma; Microlíquenes; Química.
INTRODUCTION
The crustose lichen genus Haematomma A. Massal. (Ascomycota, Lecanoraceae) includes ca. 35 species, all distributed in warm-temperate to tropical regions of the world.
The genus is characterized by lecanorine apothecia, blood red to orangered discs and thalluscoloured margins, epihymenium with red pigments, paraphyses somewhat branched and anastomosing, Lecanora-type asci, ascospores hyaline, oblong, fusiform to broadly acicular and transversely septate (3-25 septa) to submuriform (Brodo et al., 2001; Elix, 2004). Haematomma usually grows on the bark of trees and shrubs or on rocks.
Apothecial pigmentation and secondary chemistry play important roles for species delimitation in this genus. Notwithstanding, spore size, septa type (transverse or submuriform) and septa number are also used as important characters in separating species (Nelsen et al., 2006).
Recent studies on microlichens in Argentina, mainly from the South, North and Northeast, have provided additional information about the distri-bution and ecology of Haematomma species. The taxonomy, distribution, and ecology of Haematomma taxa were not clear before as some species mentioned in older publications had not yet been recollected (e.g. Hooker & Taylor, 1844; Crombie, 1877; Krempelhuber, 1878; Nylander, 1888; Müller, 1889; Cotton, 1915; Cengia Sambo, 1930; Räsänen, 1932; Santesson, 1942; Lamb, 1958; Ferraro, 1978; Osorio, 1980, 1981). Since the publication of a world monograph by Staiger & Kalb (1995) some records for Argentina needed correction and additional species were added to the checklist of Argentinean lichens (Grassi, 1950; Calvelo & Liberatore, 2002; Feuerer, 2007).
On the basis of available information, we propose for this work the following objectives: a) to give information of the species of the genus Haematomma in Argentina considering morphological and chemical characters, distribution and ecology and b) to establish a key to determine the species.
MATERIALS AND METHODS
This study is based on extensive collections made by the authors deposited in BCRU and material from BCRU, BCRU (ex MSC), CTES and Hb. UNP Esquel.
Chemical, morphological and anatomical techniques were used as outlined by Orange et al. (2001). The chemical constituents of most of the extracts were analyzed using the A, B´, C and E solvent systems of the high-performance thin layer chromatography method, HPTLC (Arup et al., 1993). To detect minor products or confirm the presence of certain substances, extracts were also analyzed by gradientelution high performance liquid chromatography, HPLC, by Elix according with Lumbsch (2002).
The taxa are ordered alphabetically and each taxon contains the following issue: morphological description based on the material studied, chemistry, distribution, ecology in Argentina, and besides additional observations relating to the taxonomy, chemistry, distribution and habitat of each taxa and, finally, specimens examined.
RESULTS AND DISCUSSION
We recognize six species and one variety in Argentina. Three geographical patterns of Argentinean Haematomma taxa can be distinguished: 1) endemic species: H. chilenum is confined mainly to the Nothofagus Blume forests in southern South America; 2) cooltemperate species, including H. erythromma and H. nothofagi, which are austral and/or antarctic elements; and 3) a tropical-subtropical element that includes H. fenzlianum, H. flexuosum, H. fluorescens var. fluorescens and H. persoonii.
TAXONOMIC TREATMENT Haematomma chilenum C.W. Dodge, Nova 298
Hedwigia 12: 343. 1966. TYPE: Chile, Prov. Antofagasta, Oasis de Paposo, 1961, G. Follmann 14925 (holotype KASSEL not seen). Figs. 1A and 3A.
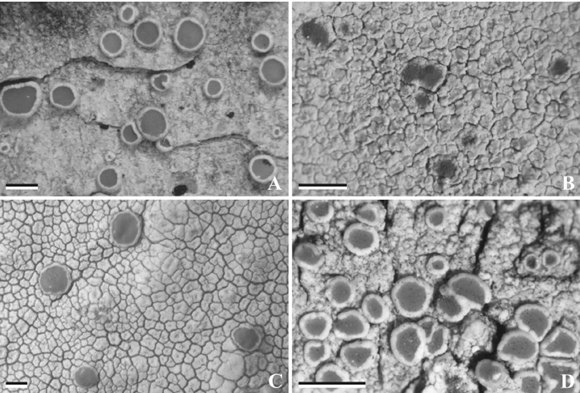
Fig. 1. Haematomma, habit of the currently known species in Argentina. A, H. chilenum (BCRU 04817). B, H. erythromma (BCRU 01100). C, H. fenzlianum (BCRU 04842). D, H. flexuosum (BCRU 04841). Scale = 1 mm.
Thallus whitish to greenish grey, rugose, rimoseareolate to areolate, continuous, 0.2-0.5 mm thick, soredia and isidia lacking. Apothecia sessile, constricted at the base, scattered, 0.35-1.45(-2) mm diam.; disc reddish orange, cinnabarred to dark purple, rarely pruinose, thalline margin well developed, entire, smooth to weakly crenulate or undulate. Ascospores ellipsoid to fusiform, straight or slightly curved, 3-6-septate, (30-)36-50 x 4-6(-7) µm. Conidia not observed.
Chemistry. Thallus K+ yellow, C-, KC-, Pd-; disc K+ violet (haematommone). By HPTLC and HPLC analyses were detected: placodiolic acid (major), atranorin (minor), usnic acid (± trace, present only in BCRU 01063), haematommone (trace). Isopseudoplacodiolic acid was detected by HPTLC in one sample (BCRU 05101).
Geographical distribution and habitat. This species is widespread in Chile; and also common in Argentina, particularly in humid areas of the temperate Southwestern forest. The species was recorded for Argentina in Neuquén Province (lago Lacar/Lácar) by Staiger & Kalb (1995), and is reported here as new for Río Negro and Chubut Provinces.
The specimens studied were found on bark and twigs of Acer pseudoplatanus L., Discaria chacaye (G. Don) Tortosa, Lomatia hirsuta (Lam.) Diels ex J. F. Macbr., Populus nigra L. and Salix fragilis L. It also grows on species of cacti and bark of Cryptocarya alba (Molina) Losser, Nothofagus spp., Oxalis gigantea Barnéoud and Salix spp. (Staiger & Kalb, 1995).
Observations. Although Haematomma chilenum is extremely variable in the apothecial morphology, the ascospores are 4- to 7-celled with rare exceptions. Most of the Chilean collections of H. chilenum reported by Staiger & Kalb (1995) contained methylplacodiolic acid, whereas in all of the Argentinean specimens this substance is absent.
It could be mistaken for two pantropical species, Haematomma accolens (Stirt.) Hillmann and H. flexuosum, by its external appearance. The distinction of H. chilenum from these two species is based mainly on chemistry, with methylplacodiolic acid present in the former species, and absent in the latter two species. Most specimens of H. chilenum studied here are congruent chemically with H. accolens (Staiger & Kalb, 1995) which contains epithecial pigment haematommone and the medullary dibenzofuran placodiolic acid. Nelsen et al. (2006) suggest that there is no reason to think that chemical characters should be more or less important in delimiting species than any other character, be it morphological or anatomical. Frequently, species are distinguished by a combination of characters, while the difference in only one character may be interpreted as infraspecific variation. Because of this, the Argentinean specimens recorded were identified as H. chilenum rather than H. accolens based on the spore size [(30-)36-50 x 4-6 (-7) µm vs. 35-55 x 3-5 µm], number of septa [3-6 vs. (4-)5-9(-11)] and the geographical distribution (endemic to southern South America vs. tropical).
Specimens examined
ARGENTINA. Chubut. El Desemboque, en el desemboque del río Epuyén, lago Puelo, 2007, M. I. Messuti s.n. (BCRU 04817); Parque Nacional Lago Puelo, Jardín Botánico en los alrededores de la Intendencia del Parque, 2009, I. N. de la Rosa s.n. (BCRU 05101). Río Negro. San Carlos de Bariloche, Península San Pedro, cerca de la costa del lago Nahuel Huapi, bajando por acceso a playa desde la Av. Campanario, 2009, I. N. de la Rosa s.n. (BCRU 05030); M. I. Messuti & I. N. de la Rosa s.n. (BCRU 05102); costa del lago Steffen, 1993, M. I. Messuti s.n. (BCRU 01063).
Haematomma erythromma (Nyl.) Zahlbr., Kungl. Svenska Vetensk. Handl. 57: 37. 1917. Lecanora erythromma Nyl., Lichenes insula-rum Guineensium (San Thomé, do Principe, das Cabras): 44. 1889. TYPE: Falkland Islands, "ad. ins. orient. Sinum Port William Stanley, 1850, W. Lechler, pl. ins. Maclovian. 58" (holotype H-NYL 24220 not seen; isotypes M, S not seen). Figs. 1B and 3A.
Thallus yellow to brownish yellow, smooth to rugose, continuous, rimose to areolate, 0.5-1.5 mm thick, soredia and isidia lacking. Apothecia immersed in the thallus or sessile, scattered, rarely in groups (2-3), (0.18-)0.2-0.6(-2.5) mm diam.; disc dark red, epruinose, thalline margin poorly developed, thin, entire, smooth to weakly crenulate. Ascospores ellipsoid to fusiform, straight or more or less curved, (2-)3-septate, 18-23(-25) x 5-7 µm. Conidia not observed.
Chemistry. Thallus K+ yellow, C-, KC-, Pd-, UV+ orange; disc K+ violet (haematommone). By HPTLC and HPLC analysis were detected: arthothelin (major), 6-O-methylarthothelin (minor), 2,4-dichloronorlichexanthone (minor), 2,5-dichloronorlichexanthone (minor), 4,5-dichloronorlichexanthone (minor), 2-chlorolichexanthone (minor), 2,5-dichloro-6-O-methylnorlichexanthone (minor), placodiolic acid (minor), lichexanthone (trace), thiophaninic acid (trace), 4,5-dichloro-6-O-methylnorlichexanthone (trace), atranorin (trace).
Geographical distribution and habitat. This uncommon Antarctic species has been previously reported from Tierra del Fuego, Antarctica and the Islas Malvinas (Falkland Islands) (Zahlbruckner, 1917; Grassi, 1950; Staiger & Kalb, 1995; Øvstedal & Smith, 2001). It has a limited distribution and is infrequently collected in the extreme south of Argentina. In Tierra del Fuego the species occurs almost exclusively within a few kilometres of the coast near Puerto Moat on the Atlantic Ocean. This is the second confirmed record of this species from continental Argentina. It occurs usually on siliceous rocks in exposed areas along the maritime coast.
Observations. The combination of 3-septate ascospores, xanthones in the thallus with a UV+ orange reaction, and epithecial haematommone make this saxicolous species one of the most distinctive taxa in Argentina.
Staiger & Kalb (1995) mentioned four chemotypes (a, b, c, d), with different combinations and concentrations of chloroxanthones and the presence or absence of placodiolic acid, pseudoplacodiolic acid, stictic acid or its derivatives and haematommone: a: lichexanthone, 2-chlorolichexanthone, arthothelin and placodiolic acid; b: lichexanthone, 2-chlorolichexanthone, arthothelin, c: lichexanthone, 2-chlorolichexanthone, arthothelin, pseudoplacodioli acid and stictic acid, and d: divers xanthones, while pseudoplacodiolic acid, placodiolic acid, and stictic acid and its derivates were not detected (Staiger & Kalb, 1995).
These authors included Fuegian material in chemotype b. In contrast, the new chemical variety (chemotype e) found here, contains secondary substances of chemotype a (placodiolic acid) and chemotype b (with the depside atranorin), although, some xanthones as thiophaninic acid may be present in minor or trace concentrations and chloroatranorin is absent.
Specimens examined
ARGENTINA. Tierra del Fuego (Antártida e Islas del Atlántico Sur). Isla Grande, Estancia Moat, junto a la costa del mar, 1993, M. I. Messu-ti & G. Vobis s.n. (BCRU 01100).
Haematomma fenzlianum A. Massal., Mem. Imp. Reale Ist. Veneto Sci. 10: 58. 1861. H. fenzlianum A. Massal., Atti Reale Ist. Veneto Sci. Lett Arti, ser. 3, 5: 253. 1860, nom. nud. TYPE: South Africa, "Caput bona spei, Reise der
Corvette Carolina 1857-58", Dr. H. Wawra (holotype VER not seen). Figs. 1C and 3B.
References. For synonyms see Staiger & Kalb (1995).
Thallus yellowish white or yellowish grey to greenish grey, rarely brownish to pale orange, ru-gose, continuous, rimose to rimose-areolate, (0.25-) 0.5-1.0 mm thick, soredia and isidia lacking. Apothecia immersed or semi-immersed in the thallus, disc level with thallus, or sessile, flat when mature, scattered, singly or in groups, (0.5-)0.8-3 mm diam.; disc dark red to dark reddish orange or reddish brown, epruinose or rarely pruinose, thalline margin developed, rather thick and prominent, entire, smooth. Ascospores fusiform, straight or more or less curved, sigmoid or sinuous, (2-)3-7-septate, 20-30(-39) x 3.5-5(-8) µm. Conidia not observed.
Chemistry. Thallus K+ yellow, C-, KC-, Pd± yel-low; disc K+ red to purplered (russulone). The following secondary compounds were detected by HPTLC and HPLC analysis: atranorin (major), chloroatranorin (minor), placodiolic acid (minor), psoromic acid (minor) and russulone (trace).
Geographical distribution and habitat. Haematomma fenzlianum occurs in Southwestern Europe, South Africa, Australia, New Zealand, the United States of America, Mexico, Chile, Paraguay, Argentina and Uruguay (Staiger & Kalb, 1995; Lumbsch et al., 1993) with a wide ecological range between warm and arid to cool and humid habitats.
In Argentina the species is uncommon, with a scattered distribution mainly in the central part of the country. It has previously been reported by Staiger & Kalb (1995) from Córdoba and Buenos Aires Provinces. Although H. fenzlianum has a pantropical to subtropical-mediterranean distribution, it is here reported for the first time from the Andean Patagonian forest in Río Negro Province. It is a species that typically grows on rocky substrates in exposed areas.
Observations. Haematomma fenzlianum appeared to be highly variable morphologically, with a great deal of variation in thallus thickness and apothecia size and development.
According to the secondary products present in H. fenzlianum, the species can presents three different chemotypes, usually with russulone, atranorin, and/or sphaerophorin, and/or isosphaeric acid (chemotype a and c) present, and sometimes with psoromic acid (chemotype b) (Staiger & Kalb, 1995). Brodo et al. (2008: 393) also detected 2´-O-demethylpsoromic acid and chloroatra-norin, without indicating to which chemotype belongs.
Regarding the chemistry of the studied specimens adds a fourth chemical variant type (chemotype d), with chloroatranorin, placodiolic and psoromic acid as minor substances, traces of russulone, and sphaerophorin and isosphaeric acid absent. The dibenzofuran, placodiolic acid, was not detected in any of the three chemotypes included in H. fenzlianum (Staiger & Kalb, 1995).
In the case of the single Patagonian collection studied by us, although presenting some chemical differentiation regarding H. fenzlianum (containing atranorin, sphaerophorin, isosphaeric acid, psoromic acid and russulone) and the distributional range does not lie within the known range of the species, the morphological and anatomical features seem to be identical. The BCRU 04842 specimen has some aborted apothecia, but contains the chemistry mentioned above.
Following the criterion proposed by Lumbsch (1998), a subspecific rank could be accepted to accommodate the chemical races when there is a correlation with major distributional differences. Nevertheless, additional examination of more Argentinean material of this taxon may be neces-sary for more morphological, ecological and distributional data in order to recognize an infraspecific rank within H. fenzlianum.
Specimens examined
ARGENTINA. Córdoba. Parque Nacional Quebrada del Condorito, camino a la picada que lleva al mirador de la quebrada, cerca de la casa de visitantes, 2009, I. N. de la Rosa s.n. (BCRU 05128 ). Río Negro. San Carlos de Bariloche, Península San Pedro, ca. 800 m s.m., 1994, M. I. Messuti et al. s.n. (BCRU 04842).
Haematomma flexuosum Hillmann, Feddes Repertorium 49: 35. 1938?-1940. TYPE: Venezuela, "In vicinitate urbium Los Teques (locus classicus) et Encanto", sine data, C. Vogel O.S.B. (holotype M not seen). Figs. 1D and 3B.
Thallus cream-coloured or pale greyish to greenish grey, more or less smooth to rugose, con-tinuous, rimose to rimoseareolate, up to ca. 0.5 mm thick, soredia and isidia lacking. Apothecia sessile or constricted at base, scattered or in groups, (0.25)0.5-1.5(-2) mm diam.; disc crimson, purplered to orangered, epruinose, thalline margin slightly prominent, smooth, crenulate to verrucose, even crenulate or flexuose. Ascospores filiform to fusiform, straight or slightly curved, (4-)5-7(-9)-septate, 35-55(-65) x 3.5-5(-6) µm. Conidia not observed.
Chemistry. Thallus K± yellow, C-, KC-, Pd± yellow; disc K+ violet. By HPTLC and HPLC (BCRU 04841) analysis were encountered: atra-norin (major), placodiolic acid (minor) isopseudoplacodiolic acid (minor) and haematommone (trace). The presence of isoplacodiolic acid was not revealed in any of the samples checked.
Geographical distribution and habitat. The pantropical H. flexuosum occurs in Southwestern Africa, Malaysia, the Southwestern United States of America, Cuba, Mexico, Costa Rica, Panama, Venezuela, Columbia, Brazil, Paraguay and Uruguay (Staiger & Kalb, 1995; Brodo et al., 2008). In Argentina it was collected in the North, in the phytogeographical area "Provincia Chaqueña" (Cabrera, 1976). Here it is reported from For-mosa and Chaco Provinces, being the first record of the species for Argentina. This infrequent species grows on little twigs of plants of leguminous plants (e.g. Acacia spp.) in xerophilic forests and gallery forests near rivers.
Observations. The chemical results observed in the examined material are not in total agreement with those founded by Staiger & Kalb (1995) and Brodo et al. (2008) in H. flexuosum, where placodiolic acid was absent and isoplacodiolic and isopseudoplacodiolic acids were always present.
It is very similar to H. accolens which overlaps morphologically and anatomically but differs mainly by having placodiolic acid as a main secondary compound. There is no a priori way to decide whether the current concept used to distinguish these species is correct. Traditionally, the presence or absence of certain dibenzofurans has been used as a taxonomical criterion to distinguish species, such as these two species. Recently, Nelsen et al. (2006) analyzed the correlation between molecular data and the geographical distribution of the H. flexuosum/H. accolens complex. Their preliminary results indicate that a species concept based on chemistry alone might not hold up in this case. Lumbsch et al. (2008), conclude that the ITS sequences support the hypothesis that H. accolens and H. flexuosum are distinct species.
Haemaotomma flexuosum is also easily confused with H. persoonii, which occurs in Argentina, but the latter, however, differs significantly in epihymenial and medullary chemistry and it is also characterized by scattered, sessile and smaller apothecia rather than crowded, more or less immersed ones. Another related species is H. chilenum but its spores are smaller [(30-)36-50 x 4-6(-7) µm] and with fewer septa (3-6-septate), and with quite different chemistry (atranorin, pla-codiolic acid and haematommone).
In the genus Haematomma, the species concepts are rather conflicting, overestimating, in some cases, the presence of certain chemical compounds to delimit the taxa. Following this, although the two specimens identified as H. flexuosum lacked isoplacodiolic acid they contain placodiolic acid, isopseudoplacodiolic acid and haematommone and also agreed with all of the morphological and anatomical characters of the species.
Specimens examined
ARGENTINA. Chaco. Dpto. La Leonesa, cercanías Arroyo Canguí Grande, 1995, L. I. Ferraro et al. 5285 (CTES 261010); 5 km después de la localidad Margarita Belén, sobre la ruta que va en dirección a Formosa, 1995, M. I. Messuti & L. I. Ferraro s.n. (BCRU 04841). Formosa. Dpto. Pil-comayo, Arroyo HéHé, interior de selva en galería, 1986, A. Krapovickas et al. 40687 (CTES 249736).
Haematomma fluorescens Kalb & Staiger var. fluorescens, Bibl. Lichenol. 59: 114. 1995. TYPE: Paraguay, "Cordillère de Peribébuy, sur les écores des arbres", 1987, B. Balansa 4154 (holotype G not seen). Figs. 2A and 3B.

Fig. 2. Haematomma, habit of the currently known species in Argentina. A, H. fluorescens var. fluorescens (CTES 75592). B, H. nothofagi (BCRU 04533). C, H. persoonii (BCRU 04882). Scale = 1 mm.
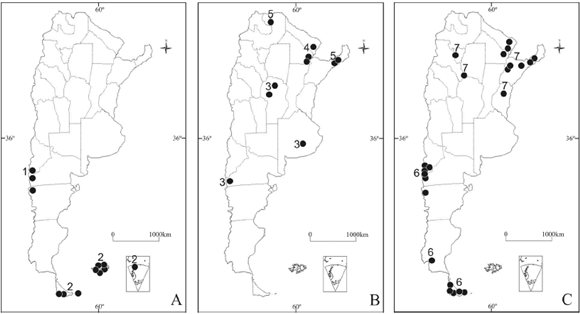
Fig. 3. Distribution of Haematomma species in Argentina. A, H. chilenum (1); H. erythromma (2). B, H. fenzlianum (3); H. flexuosum (4); H. fluorescens var. fluorescens (5). C, H. nothofagi (6); H. persoonii (7).
Thallus pale grey to yellowish white, rugose, verrucose to areolate, continuous, up to ca. 0.5 mm thick, soredia and isidia lacking. Apothecia sessile or constricted at base, scattered 0.3-0.5(-1.5) mm diam. [up to 3 mm diam., fide Brodo et al. (2008)]; disc cinnabar, scarlet to red-orange, epruinose, thalline margin slightly prominent, smooth, crenu-late to verrucose, even crenulate or flexuose. Ascospores filiform to fusiform, curved, (6-)9-13(-15)-septate, 44-80(-86) x (3)4-6(-9) µm. Conidia not observed.
Chemistry. Thallus K+ yellow, C-, KC-, Pd-, UV+ yellow (lichexanthone); disc K+ red to deep purple-red (russulone), apothecial margin UV+ yellow. In all specimens analyzed by HPTLC were detected atranorin, lichexanthone and sphae-rophorin.
Geographical distribution and habitat. This neotropical species was reported in Costa Rica, Venezuela, Bolivia, Brazil and Paraguay (Staiger & Kalb, 1995; Brodo et al., 2008).
In Argentina it is reported to be scattered in the phytogeographical "Región Neotropical" ("Provincia de las Yungas" and "Provincia Paranense") (Cabrera, 1976). Although H. fluorescens var. fluorescens has been rarely collected so far, it is probably a characteristic element of this phytogeographical region. The variety is registered here from Salta and Misiones Provinces, and is the first record for the species in Argentina. It was collected on dry branches of angiosperms.
Observations. This variety is characterized by the UV+ gold-yellow thallus and apothecia margins, an inspersed hymenium, ascospores transversally septate with 9 to 13 septa, and the presence of lichexanthone and russulone in the epihymenium.
The secondary products found in the Argentinean specimens match those recorded by Staiger & Kalb (1995). Another variety endemic to Costa Rica, Haematomma fluorescens var. longisporum Nelsen, Lücking & Navarro, differs from the nominal variety in its significantly longer ascospores (75-120 x 4-6 µm) and the number of septa (13-27). Haematomma fluorescens is readily distin-guished from the other species in the genus by its secondary lichen compounds. It can be confused with the corticolous species, Haematomma subin-natum (Malme) Kalb & Staiger, distributed in the "Región del Cerrado" in South America, which has ascospores with 5 to 7 septa and a non-inspersed hymenium (Staiger & Kalb, 1995; Nelsen et al., 2006).
Specimens examined
ARGENTINA. Misiones. Dpto. San Ignacio, Campos del Teyú Cuaré, 1981, L. I. Ferraro et al. 2375 (CTES 75592); Dpto. Candelaria, Loreto, 1981, L. I. Ferraro et al. 2355 (CTES 75707). Salta. Dpto. Orán, El Oculto, 40 km W de Orán, 5 km, 1998, C. Saravia Toledo 14689 (CTES 342739).
Haematomma nothofagi Kalb & Staiger, Bibl. Lichenol. 59: 139. 1995. TYPE: Argentina, Tierra del Fuego, Ushuaia, ca. 280 m s.m., 1940, R. Santesson 586 (holotype S not seen). Figs. 2B and 3C.
Thallus creamcoloured to yellowish, smooth to rugose, continuous, rimose to rimoseareolate, up to ca. 0.4 mm thick, soredia and isidia lacking. Apothecia immersed when young, then sessile, scattered, 0.5-1.55 mm diam.; disc dark scarlet to cinnabar-red with the color penetrating into the margin, epruinose, thalline margin thin, appearing almost granular to sorediate, evanescent with age. Ascospores fusiform, straight or slightly curved, 8-17-septate, 40-90(-94) x 4-6 µm. Conidia not observed.
Chemistry. Thallus K+ yellow, C-, KC-, Pd± yellow; disc K+ red to deep purplered (russulone). The chemistry by HPTLC and HPLC of the Argentinean collections studied here agrees with that mentioned by Staiger & Kalb (1995) containing atranorin (major), placodiolic acid (minor) and russolone (trace).
Geographical distribution and habitat. This cool-temperate species occurs in Southeastern Australia, New Zealand, southern Argentina and southern Chile (Staiger & Kalb, 1995; Elix, 2004). The species has an australantarctic distribution in southern South America. Further field studies have revealed that H. nothofagi is one of the most common and widespread species of the genus in Nothofagus forests (Staiger & Kalb, 1995; Elix, 2004).
The species was collected for the first time by Santesson in 1940 in Ushuaia, Tierra del Fuego (Staiger & Kalb, 1995). It was mentioned previously from several places in Neuquén, Río Negro, Santa Cruz, and Tierra del Fuego, Antártida e Islas del Atlántico Sur Provinces (Staiger & Kalb, 1995; Vobis et al., 1995). This is the first report of the species from Chubut Province and additional new localities for this species are reported under Specimens examined.
Its altitudinal distribution ranges from lowland to alpine elevations. The species grows on smooth bark on twigs, branches and trunks of Drymis winteri J.R. Forst. & G. Forst. as well as on Nothofagus species, such as, N. antarctica (G. Forst.) Oerst., N. betuloides (Mirb.) Oerst., N. dombeyi (Mirb.) Oerst. and N. pumilio (Poepp. & Endl.) Krasser. It was frequently collected in shaded and moist habitats.
Observations. Haematomma nothofagi is easily confused with the New Zealand endemic species Haematomma hilare Zahlbr., but the latter contains methyplacodiolic and usnic acids, and ascospores with 7-10 septa (Staiger & Kalb, 1995). It was previously recorded in Argentina as H. hilare (Calvelo & Lorenzo, 1989; Vobis et al., 1995; Calvelo & Liberatore, 2002).
Specimens examined
ARGENTINA. Chubut. Ca. 60 km de Esquel, Puerto Sagrario, Brazo Norte del Lago Menéndez, 2007, P. L. Codesal 45 (Hb. UNP Esquel). Neuquén. Cercanías de la aduana Argentina, 1993, M. I. Messuti s.n. (BCRU 01062); zona limítrofe con Chile, ruta 215, río Puyehue, 3 km antes de llegar al hito Argentino-Chileno, 1993, M. I. Messuti s.n. (BCRU 01067); desde Paso Córdoba a Villa La Angostura, mano derecha, a 5 km del lago Espejo, 1994, M. I. Messuti s.n. (BCRU 04538); lago Espejo, sobre la ruta 234, hotel Espejo, 1995, M. I. Messuti s.n. (BCRU 04539); Parque Nacional Los Arrayanes, Villa La Angostura, picada al istmo de Quetrihué, 2003, I. N. de la Rosa s.n. (BCRU 04635); Puerto Blest, picada a la laguna Ortiz Basualdo, La Heladera, 2004, M. I. Messuti s.n. (BCRU 04536); Parque Nacional Nahuel Huapi, Puerto Blest, en comienzo de la picada Paso de Los Raulíes, 2003, I. N. de la Rosa s.n. (BCRU 04537). Río Negro. Parque Nacional Nahuel Huapi, Puerto Blest, camino a Los Cántaros, 1985, S. Calvelo 74 (BCRU 00044); a 500 m de la Estación Biológica, 2005, M. I. Messuti s.n. (BCRU 04676); a 2 m de la costa del lago Frías, 2005, M. I. Messuti & I. N. de la Rosa s.n. (BCRU 04675); Brazo Tristeza, en la costa del lago Nahuel Huapi, 1994, M. I. Messuti s.n. (BCRU 03984). Tierra del Fuego (Antártida e Islas del Atlántico Sur). Isla Grande, ruta 3, km 43, Tierra Mayor, Paso Garibaldi, 1995, M. I. Messuti & G. Vobis s.n. (BCRU 04839); lago Escondido, hostería El Petrel, 1995, M. I. Messuti & G. Vobis s.n. (BCRU 04838); 54º 48´S, 65º 16'W, Bahía Buen Suceso, grove of Nothofagus behind beach at head of bay, 1971, H. A. Imshaug & K. E. Ohlsson 49959 (BCRU 03353, ex MSC); Glaciar Marcial, N von Ushuaia, Aufstieg zur oberen Seilbahnstation, ENE exponieter Hang, Nothofagus antarctica-Wald, ca. 400 m alt, 54° 48'S, 65° 22'W, 1997, R. Guderley et al. s.n. (BCRU 04585); 54° 49'S, 68° 22'W, remnants of cutover and grazed Nothofagus forest long Ushuaia-Lapataia road, 3.5 km W of Ushuaia, 1971, H. A. Imshaug & K. E. Ohlsson 55152 (BCRU 03382, ex MSC); junto a la entrada del mar del lado derecho, dirección Bahía Lapataia, cerca del cartel indicador Arch. L. C, 1995, M. I. Messuti & G. Vobis s.n. (BCRU 04840); 54° 48'S, 68° 35'W, Parque Nacional Tierra del Fuego, Nothofagus forest on N shore of lago Roca near Chilean frontier, 1971, H. A. Imshaug & K. E. Ohlsson 54973 (BCRU 03381, ex MSC); Isla de los Estados, 54° 49'S, 68° 28'W, Bahía Capitán Cánepa, open Nothofagus forest on N side of lake behind head of N arm, 1971, H. A. Imshaug & K. E. Ohlsson 53051 (BCRU 03457, ex MSC).
Haematomma persoonii (Fée) A. Massal., Atti. I. R. Istit. Veneto ser. 3, 5: 253. 1860. Lecanora persoonii Fée, Essai Crypt. Écorc. 120. 1824. TYPE: "ad corticem Clusiae albae antillarum", sine data, without collector [lecto-type G designated by Staiger & Kalb, Bibl. Lichenol. 59: 150. 1995, not seen]. Figs. 2C and 3C.
References. For synonyms see Staiger & Kalb (1995) and Brodo et al. (2008).
Thallus white or cream-coloured, pale grey to greenish grey, smooth to rugose, continuous, rimose to rimose-areolate, up to ca. 0.5 mm thick, soredia and isidia lacking. Apothecia immersed, aspicilioid, crowded, irregular in shape, rarely sessile, sometime confluent, (0.2-)0.3-1.5 mm diam.; disc dark scarletred, cinnabarred with to reddish brown, epruinose, thalline margin very poorly developed, but becoming distinct, smooth or weakly crenulate in sessile apothecia. Ascospores fusiform, straight or curved, rarely sigmoid, 5-7-septate, (26-)30-52(-62) x 3-5(-6) µm. Conidia not observed.
Chemistry. Thallus K+ yellow, C-, KC-, Pd± yel-low, UV± white or blue-white (sphaerophorin); disc K+ red to purplered (russulone). By HPTLC and HPLC analyses were dectected: atranorin (major), chloroatranorin (minor), isosphaeric acid (major), sphaerophorin (minor), russulone (trace). All of these five compounds, including the lectotypes of Lecanora persoonii Fée, Lecanora coc-cinea Fée, Haematomma puniceum var. subarthonioideum Zahlbr., were reported by Staiger & Kalb (1995). However, other species included as syn-onyms of H. persoonii may not have chloroatranorin and isosphaeric acid.
Geographical distribution and habitat. Haematomma persoonii occurs in South and East Africa, Réunion, the Phillipines, New Caledonia and North, Central and South America (Staiger & Kalb, 1995; Elix, 2004; Brodo et al., 2008). This pantropical species has a wide ecological amplitude from arid to humid areas.
In Argentina, it seems to be a characteristic species from the neotropical region (the Central and North) which includes the phytogeographical areas of "Provincia de las Yungas", "Provincia Chaqueña", "Provincia Paranaense" and "Provincia del Espinal" (Cabrera, 1976; Ferraro, 1995). This is the first report of this species from Formosa, Chaco, Misiones, Salta, Santiago del Estero and Entre Ríos Provinces. It was previously recorded from Corrientes Province (Staiger & Kalb, 1995). It grows on the tree bark often in gallery forests or in open xerophilic forest.
Observations. Haematomma persoonii has a variable morphology of the thallus and the apothecia, both within and among specimens.
The Argentinean specimens could be included within the "chemotype 2" [atranorin, isosphaeric acid (major), sphaerophorin (minor) and russulone], according with Brodo et al. (2008). However, the samples analyzed by HPTLC (E solvent) and HPLC also showed the presence of chloroatranorin.
Specimens examined
ARGENTINA. Chaco. Cercanías al arroyo Cangui Grande, camino a la provincia de Formosa, de la ruta lado izq., 1995, M. I. Messuti & L. I. Ferraro s.n. (BCRU 04845). Corrientes. Dpto. San Martín, Arrocera Drews, 8 km de Carlos Pellegrini, 1976, L. I. Ferraro & S. G. Tressens 797 (BCRU 04877). Entre Ríos. Parque Nacional El Palmar, en los alrededores de La Glorieta, en selva en galería, sobre la margen izquierda del arroyo El Palmar, 2007, M. I. Messuti s.n. (BCRU 04837). Formosa. Dpto. Pilcomayo, Arroyo Hé-Hé, interior de la selva en galería, 1986, A. Krapovickas et al. 40687 (CTES 249736); Dpto. Capital, a 10 km de la reserva Guaycolec, en dirección a Paraguay, sobre ruta 11, 1995, M. I. Messuti & L. I. Ferraro s.n. (BCRU 04843); M. I. Messuti & L. I. Ferraro s.n. (BCRU 04844); M. I. Messuti & L. I. Ferraro 5285 (CTES 261010). Misiones. San Ignacio, Pastoreo Chico, 27º 19´S, 55º 22´W, 275 m s.m., 1956, Montes 100.78.F (BCRU 04882); Dpto. Candelaria, Loreto, 1981, L. I. Ferraro et al. 2338 (CTES 76142). Salta. Dpto. Guachipas, Alemanía, km 81 a metros de la ruta, 2008, L. Pérez s.n. (BCRU 05029). Santiago del Estero. Dpto. Guasayán, Sierra de Guasayán, ruta 64, 70 km SW de Sgo. del Estero, 1981, A. Krapovickas 37487 (CTES 76221).
Key to species of Haematomma in Argentina (taxa potentially present, listed in old literature- but not yet reported, are excluded).
1.Thallus saxicolous . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2
1. Thallus corticolous . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
2(1). Epihymenium with haematommone (when treated with KOH a violet or purple cloud appears); ascospores (2-)
3-septate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . H. erythromma
2. Epihymenium with russulone (when treated with KOH the solution becomes red); ascospores (2-)3-7-septate . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .H. fenzlianum
3(1). Epihymenium with russulone (K+ red); ascospores 5-17-septate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4
3. Epihymenium with haematommone (K+ violet or purple); ascospores 3-9-septate . . . . . . . . . . . . . . . . . . . . . . . .6
4(3).Thallus and margin of apothecia UV+ gold-yellow (lichexanthone) . . . . . . . . . .H. fluorescens var. fluorescens
4. Thallus and margin of apothecia UV- (without lichexanthone) or UV+ (white or blue-white) . . . . . . . . . . . . . . .5
5(4). Apothecia sessile; ascospores 8-17-septate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .H. nothofagi
5. Apothecia usually immersed or aspicillioid, rarely sessile; ascospores 5-7-septate . . . . . . . . . . . . . . .H. persoonii
6(3). Ascospores with 3-6-septate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .H. chilenum
6. Ascospores with (4-)5-7(-9)-septate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .H. flexuosum
ACKNOWLEDGEMENTS
We thank H. S. Osorio and P. L. Codesal for sending us specimens for study. We are also grateful to J. A. Elix (Canberra) for his help with HPLC analysis and A. W. Archer (New South Wales) and H. T. Lumbsch (Chicago) for constructive discussions of the manuscript. Financial support by UNComahue (Secretaría de Investigación y Extensión, Grant N° B140) and CONICET are much appreciated.
BIBLIOGRAPHY
1. Arup, U.; S. Ekman, L. Londblom & J. E. Mattsson. 1993. High performance thin layer chromatography (HPTLC), an improved technique from screening lichen substances. The Lichenologist 25: 61-71. [ Links ]
2. Brodo, I. M.; S. D. Sharnoff & S. Sharnoff. 2001. Lichens of North America. New Haven & London: Yale University Press. [ Links ]
3. Brodo, I. M.; W. L. Culberson & Ch. F. Culberson. 2008. Haematomma (Lecanoraceae) in North and Central America, including the West Indies. The Bryologist 111: 363-423. [ Links ]
4. Cabrera, A. L. 1976. Regiones fitogeográficas argentinas. Enciclopedia argentina de agricultura y jardinería, tomo 2, fascículo 1. Segunda Edición. Buenos Aires: Acme Calvelo, S. & S. Liberatore. 2002. Catálogo de los líquenes de la Argentina. Kurtziana29: 7-170. [ Links ]
5. Calvelo, S. & L. Lorenzo. 1989. Noteworthy corticolous lichens in Nothofagus forests, North-Western Patagonia. Mycotaxon 34: 655-665. [ Links ]
6. Cengia Sambo, M. 1930. I licheni della Patagonia e di altri region Dell´Argentina. Contributi scientifici delle missioni salesiane del venerabile Don Bosco: 1-73. [ Links ]
7. Cotton, A. D. 1915. Cryptogams form the Falkland Islands, collected by Mr. Vallentin. Journal of the Linnean Society Botany 43: 137-231. [ Links ]
8. Crombie, J. M. 1877. On the lichens collected by Professor R. O. Cunningham in the Falkland Islands, Fuegia, Patagonia, and the Island of Chiloe during the voyage of H. M. S. "Nassau", 1867-9. Journal of the Linnean Society Botany 15: 222-234. [ Links ]
9. Elix, J. A. 2004. Haematommataceae. Flora of Australia 56A: 4-10. [ Links ]
10. Ferraro, L. I. 1978. Contribución a la flora liquenológica de Corrientes (Rep. Argentina). Facena 2: 167-244. [ Links ]
11. Ferraro, L. I. 1995. Comentarios sobre la distribución de los líquenes en las diferentes regiones fitogeográficas de la Provincia de Corrientes, Nordeste de Argentina, América del Sur, in F. J. A. Daniels, M. Schulz & J. Peine (eds.), Flechten Follman. Contributions to lichenology in honour of Gerhard Follmann, pp. 303-413. Germany: Geobotanical and Phytotaxonomical study group, Botanical Institute. [ Links ]
12. Feuerer, T. (ed.). 2007. Checklists of lichens and lichenicolous fungi. Version 1 February 2009. <http://www.checklists.de.> [Accessed March 2009]. [ Links ]
13. Grassi, M. M. 1950. Contribución al catálogo de líquenes argentinos, I. Lilloa 24: 5-294. [ Links ]
14. Hooker, J. D. & T. Taylor. 1844. Lichenes Antartici. Being characters and brief descriptions of the new lichens discovered in the southern circumpolar regions, Van Diemen´s Land and New Zealand, during the voyage of H. M. Discovery Ships Erebus and Terror. London Journal of Botany 3: 634-658. [ Links ]
15. Krempelhuber, A. 1878. Lichenes collecti in republica Argentina a Doctoribus Lorentz et Hieronymus, determinati et descripti a Doct. A. de Krempelhuber, Monacensi (Continuatio). Flora 61: 492-496. [ Links ]
16. Lamb, M. 1958. La vegetación líquénica de los Parques Nacionales patagónicos. Anales de Parques Nacionales (Buenos Aires) 7: 1-188. [ Links ]
17. Lumbsch, H. T. 1998. The use of metabolic data in lichenology at the species and subspecific levels. The Lichenologist 30: 357-367. [ Links ]
18. Lumbsch, H. T. 2002. Analysis of phenolic products in lichens for identification and taxonomy, in I. Kranner, R. P. Beckett & A. K. Varma (eds.), Protocols in lichenology, pp. 281- 295. Berlin: Springer. [ Links ]
19. Lumbsch, H. T.; G. B. Feige & J. M. Egea. 1993. Two lichens new to Europe. The Lichenologist 25: 303-306. [ Links ]
20. Lumbsch, H. T.; M. P. Nelsen & R. Lücking. 2008. The phylogenetic position of Haematommataceae (Lecanorales, Ascomycota), with notes on secondary chemistry and species delimitation. Nova Hedwigia 86: 105-114. [ Links ]
21. Müller, J. 1889. Lichenes Spegazziniani in Staten Island, Fuegia et in regione Freti Magellanici lecti. Nuovo Giornale Botanico Italiano 21: 35-54. [ Links ]
22. Nelsen, M. P.; R. Lücking, J. L. Chaves, H. J. M. Sipman, L. Umaña & E. Navarro. 2006. A first assessment of the Ticolichen biodiversity inventory in Costa Rica: the genus Haematomma (Lecanorales: Lecanorareae). The Lichenologist 38: 251-262. [ Links ]
23. Nylander, W. 1888. Lichenes Fuegiae et Patagoniae. Paris: A. Héloin & E. Charles. [ Links ]
24. Orange, A.; P. W. James & F. J. White. 2001. Microchemical methods for the identification of Lichens. London: British Lichen Societ [ Links ]
25. Osorio, H. S. 1980. Contribution to the lichen flora of Argentina XII. New or additional records from Buenos Aires Province. The Bryologist 83: 219-220. [ Links ]
26. Osorio, H. S. 1981. Contribution to the lichen flora of Argentina. XIII. Lichens from Misiones Province. Comunicaciones Botánicas del Museo de Historia Natural de Montevideo 63: 1-18. [ Links ]
27. Øvstedal, D. O. & R. I. Smith. 2001. Lichens of Antarctica and South Georgia. A guide to their identification and Ecology. United Kingdom: Cambridge University Press. [ Links ]
28. Räsänen, V. 1932. Zur Kenntis der Flechtenflora Feuerlands sowie der Provincia de Magallanes, Provincia de Chiloë und Provincia de Ñuble in Chile. Annales Societatis Zoolog.-Botanicæ Fennicæ Vanamo 2: 1-65. [ Links ]
29. Santesson, R. 1942. Lichens from the Nahuel Huapi National Park in Argentina. Arkiv für Botanik 30 (A): 1-12. Staiger, B. & K. Kalb. 1995. Haematomma-studien: I. Die flechtengattung Haematomma. Bibliotheca Lichenologica 59: 3-198. [ Links ]
30. Vobis, G.; S. Calvelo & M. I. Messuti. 1995. Novedades para la flora liquénica de los bosques andinopatagónicos (Argentina, Sudamérica), in F. J. A. Daniels, M. Schulz & J. Peine (eds.), Flechten Follmann. Contributions to lichenology in honour of Gerard Follmann, pp. 483-492. Germany: Geobotanical and Phytotaxonomical study group, Botanical Institute. [ Links ]
31. Zahlbruckner, A. 1917. Botanische Ergebnisse der Schwedischen Expedition nach Patagonien und dem Feuerlande 1907-1909. VI. Die Flechten. Kunglige Svenska Vetenskapsakademiens Handlingar 57: 1-62. [ Links ]














