Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Darwiniana, nueva serie
Print version ISSN 0011-6793On-line version ISSN 1850-1699
Darwiniana, nueva serie vol.6 no.1 San Isidro July 2018
http://dx.doi.org/10.14522/darwiniana.2018.61.775
SISTEMÁTICA Y TAXONOMÍA DE ALGAS Y HONGOS
10.14522/darwiniana.2018.61.775
New species, new combination, and notes on Clitocella and Rhodocybe (Entolomataceae) from Paraná state, Brazil
Nuevas especies, nueva combinación y notas sobre Clitocella y Rhodocybe (Entolomataceae) del estado de Paraná, Brasil
Alexandre Gonçalves dos Santos Silva-Filho1, Márcia de Araújo Teixeira-Silva2 & Vagner G. Cortez3
1 Universidade Federal do Paraná, Programa de Pós-Graduação em Botânica, Caixa Postal 19031, CEP 81531-980 Curitiba, Paraná, Brasil; silvafilhoags@gmail.com (author for correspondence).
2 Faculdade Meta, Estrada Alberto Torres, 947, Bairro da Paz, Rio Branco, Acre, Brasil.
3 Universidade Federal do Paraná, Departamento de Biodiversidade, Rua Pioneiro 2153, Jardim Dallas, CEP 85950-000, Palotina, Paraná, Brasil.
Original recibido el 25 de octubre de 2017, aceptado el 19 de abril de 2018
Editor Asociado: Mario Saparrat
Abstract. In a survey of Agaricales fungi in Seasonal Semidecidual Forest remnants from Paraná State, southern Brazil, two Clitocella and two Rhodocybe species were identified. Based on morphological data, Clitocella pallescens is described as a new species, and a new combination is proposed to Clitocella himantiigena. Rhodocybe galerinoides and C. himantiigena are new records from Brazil. Rhodocybe caelatoidea, already registered in Paraná State, is also described, illustrated and discussed.
Keywords. Agaricoid fungi; Atlantic Forest; Mycobiota; Morphology; Taxonomy.
Resumen. En un estudio de los hongos del orden Agaricales, en los remanentes del bosque estacional semideciduo en el estado de Paraná, al sur de Brasil, fueron identificadas dos especies de Clitocella y dos de Rhodocybe. Como resultado de los análisis morfológicos, se describe Clitocella pallescens como nueva especie para la ciencia; además, se propone una nueva combinación: Clitocella himantiigena. Las especies Rhodocybe galerinoides y C. himantiigena son nuevos registros para Brasil. Rhodocybe caelatoidea, ya conocida en el estado de Paraná, es también descrita, ilustrada y discutida.
Palabras clave. Bosque Atlántico; Hongos agaricoides; Micobiota; Morfología; Taxonomía.
INTRODUCTION
AgaricfungibelongingtotheRhodocybe-Clitopilus clade are placed in Entolomataceae Kotl. & Pouzar and groups mushroom species recognized by the presence of attached lamellae and basidiospores that appear angular in face and profile views, with pinkish color in deposit (Baroni, 1981; Singer, 1986; Largent, 1994; Co-David et al., 2009). The classification of this group has been subject of recent discussion: Co-David et al. (2009), based on basidiospore ultra-structure and molecular analysis, proposed to include members of Rhodocybe-Clitopilus clade, into a single genus, Clitopilus (Rabenh.) P. Kumm.; based on larger subset analysis of specimens of the Rhodocybe-Clitopilus clade, Baroni & Matheny (2011) recognized and proposed four major clades: Clitopilus-Rhodocybe p.p., Clitopilopsis, Rhodocybe s. str. and Rhodophana; Kluting et al. (2014), presented a new approach based on a multigene phylogeny and proposed a new topology for the phylogenetic tree with five monophyletic clades, splitting Clitopilus in five genera: Clitopilus, Clitocella Kluting, T. J. Baroni & Bergemann, Clitopilopsis Maire, Rhodocybe s.str. Maire, and Rhodophana Kühner. In a more recent paper, Morgado et al. (2016) reached similar phylogenetic results, indicating a more established classification for this clade.
In spite of numerous species reported from Europe and North America, in Brazil only 12 species belonging to the genus Rhodocybe were recorded (Maia et al., 2015). Singer (1973, 1989) described three species: R. crepidotoides, R. angustispora and R. conica. Raithelhuber (1990) described R. oenocephala Raithelh., but this species requires revision (Baroni & Halling, 1992). Recently, de Meijer (2008) described R. levispora de Meijer, and recorded R. cf. caelata (Fr.) Maire, R. caelatoidea Dennis, R. aff. conchata E. Horak, R. aff. mellea T. J. Baroni & Ovrebo. R. cf. mycenoides Singer, and R. pseudonitellina Dennis (de Meijer, 2006). On the other hand, Clitocella was, so far, an unknown genus in Brazil.
In a survey of the agaricoid fungi in areas of Seasonal Semideciduous Forest from the western Paraná State (Silva-Filho et al., 2016; Silva-Filho & Cortez, 2017), specimens of Clitocella and Rhodocybe were gathered and are reported here, aiming to improve the knowledge of south Brazilian mycobiota.
MATERIALS AND METHODS
Fieldwork was conducted from January to December 2015 in two fragments of seasonal semideciduous forest, belonging to the Atlantic Forest Biome, in the western region of Paraná State, southern Brazil (Fig. 1): Parque Estadual São Camilo (abbreviated as PESC), in the city of Palotina (24°18'26"S and 53°54'29" W), and Reserva Particular do Patrimônio Natural Fazenda Açu (abbreviated as RPPN Fazenda Açu), situated in the city of Terra Roxa (24°11'54" S and 53°58'4" W). Collected specimens from 2010-2014 at PESC were also examined and considered in the present survey.
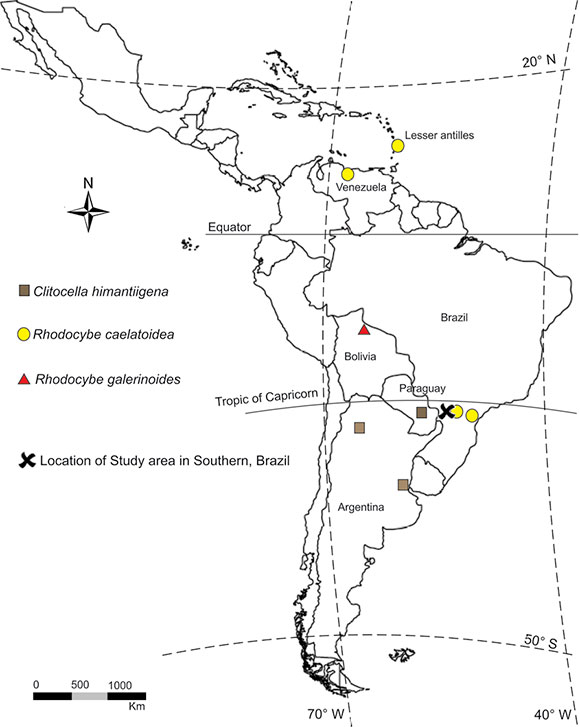
Fig. 1. Distribution map of Clitocella and Rhodocybe species recorded in western of Paraná State.
All specimens were analyzed both macro-and micromorphologically following standard procedures (Baroni, 1981). Color names and codes used in the macroscopical descriptions are according to Kornerup & Wanscher (1978); colors for the
microscopic features are under 3% KOH (potassium hydroxide), although Congo red dye was added to preparations later. Microscopic measurements and photographs were made under an Olympus CX31 optical microscope with a Toup Cam FMA050 digital camera, and measurements were taken through software Toup Tek, Toup View 3.7. In the basidiospores description, Q is the quotient between the length and width, Qm is the medium value of Q, and n is the number of measured basidiospores/number of analyzed basidiomata/number of collections.
Scanning electron microscopy (SEM) studies followed the modified procedure by Baroni (1981) and were performed at the Center of Electron Microscopy of the Universidade Federal do Paraná (UFPR) at Curitiba, under a Jeol JSM-6360LV. Specimens are preserved at the Herbarium of Campus Palotina (HCP), except the types, housed at the Herbarium of Department of Botany (UPCB) in Curitiba. Generic taxonomical concepts are following Kluting et al. (2014).
RESULTS
Clitocella himantiigena (Speg.) Silva-Filho & Cortez, comb. nov. Basionym: Clitocybe himantiigena Speg., Bol. Acad. Nac. Ci. Republ. Argent. 23: 373 (1919). Rhodocybe
himantiigena (Speg.) Singer, Lilloa 22: 609 (1951). Clitopilus himantiigenus (Speg.) Noordel. & Co-David, Persoonia 23: 162 (2009). MycoBank MB 825225. Figs. 2, 6A.
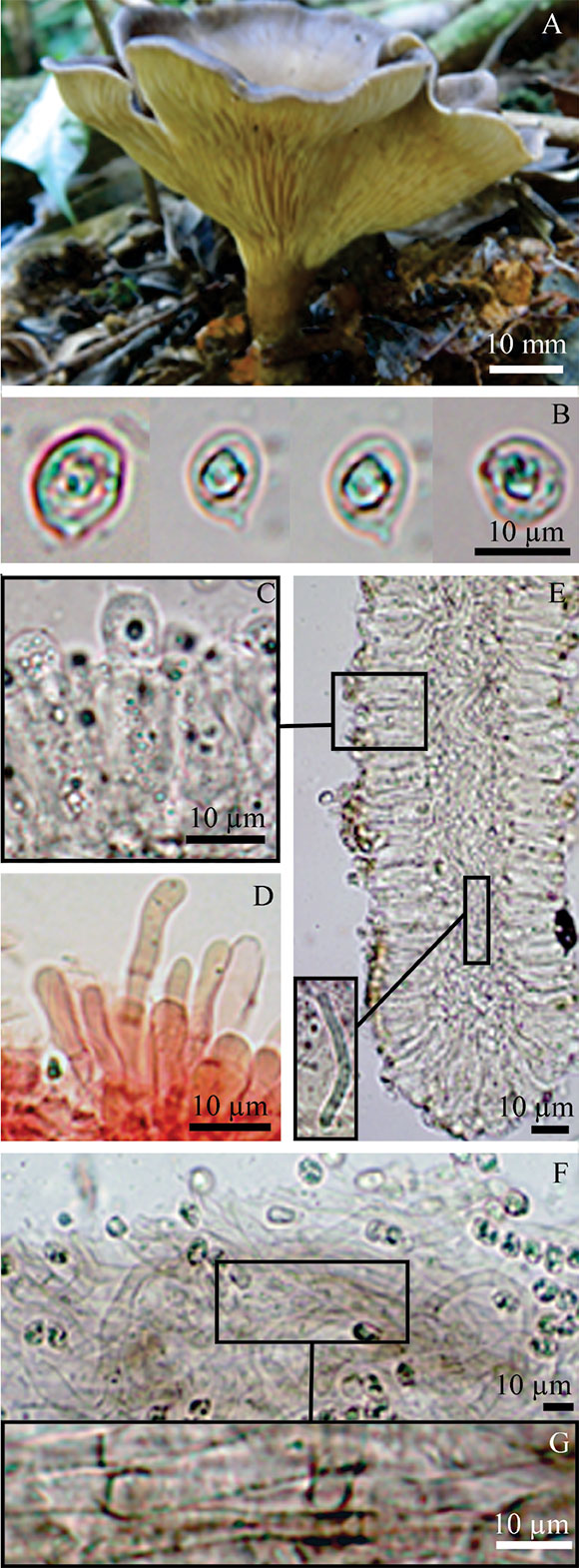
Fig. 2. Clitocella himantiigena. A, bsidiomata. B, basi-diospores. C, basidium. D, pseudoparaphyses E, section of lamellar trama with oleiferous hyphae. F, pileipellis. G, smooth and encrusted hyphae of pileipellis. All photos from A. G. S. Silva-Filho 394.
Pileus 67-81 mm diam., at first plane to slightly umbonated at the disc, infundibuliform in mature specimens, then slightly depressed at disc, surface smooth to slightly pruinose, margin involute, non-striate, rimose, yellowish brown (5D5) to greyish brown (6F3) at the center, dark brown (6F5) toward the margin. Lamellae decurrent, crowded, with five sizes of lamelullae, margin even to wavy, concolor with the sides, fleshy, greyish yellow (1B2) to brownish grey (6D2). Stipe 31-33 × 8-13 (apex) 8-9 (base) mm, central, terete, at first equal in young species, becoming tapered at the base, surface striated, fleshy, brownish grey (5C2), with white (1A1) rhizomorphs (Fig. 2A). Context thin (2.5 mm thickness), pale grey (1A2). Spore print not observed. Spot test: not reacting (reddish) with KOH 3% at pileus of dried specimens.
Basidiospores 5-8 × 3.5-5.5 µm, (n=53/5/5, Q=0.83-1.77, Qm=1.40), short-broadly ellipsoid in profile view, globose in polar view, with obscure pustules, appearing almost smooth or with minute to obscure angles in polar view, hilar appendix conspicuous, thin-walled, hyaline, inamyloid (Fig. 2B, 6A). Basidia 23-33 × 6.5-7.5 µm, clavate, tetrasporic, rarely mono-or bisporic, hyaline (Fig. 2C). Pleurocystidia and cheilocystidia absent (Fig. 2E). Lamella edge fertile. Pseudoparaphyses on lamella edge, 15-30 × 3-9 µm, filiform, cylindroclavate, rare branched at apex, septate, hyaline (Fig. 2D). Lamellar trama irregular with hyphae 3-6 µm diam., smooth, hyaline in KOH (Fig. 2E). Pileus trama with interwoven hyphae, 4.5-10.5 µm diam., smooth, hyaline. Pileipellis a cutis of interwoven hyphae, 3-5 µm diam, composed of two layers: the upper layer with hyphae smooth and hyaline, the lower layer with hyphae brown incrusted, hyaline and brown (Fig. 2F-G). Stipitipellis composed of a cutis of subparallel hyphae 2-7 µm diam., incrusted, hyaline. Stipititrama regular, with hyphae 2-4 µm diam., slightly incrusted, hyaline. Caulocystidia 13.6-36 × 3-6.5 µm, catenulate, clavate, cylindro-clavate, hyaline. Clamp connections absent. Oleiferous hyphae (thrombopleurous) observed only in the lamella and stipe trama.
Distribution and habitat. Solitary in the forest, terricolous, among litter fall, possibly arising from a buried wood. Known only from Paraguay and Argentina (Singer, 1949) and now in Brazil (Fig.1).
Observations. Clitocybe himantiigena was described by Spegazzini (1919) based on a collection from Paraguay, which Singer (1949) transferred to Rhodocybe and provided a more detailed description, including microscopic features. Baroni (1981), on the other hand, synonymized the South American R. himantiigena with R. mundula (Lasch) Singer, a known species in North America and Europe. After molecular studies, Co-David et al. (2009) recombined this species to Clitopilus. However, based on the above cited features and conclusions by Kluting et al. (2014), C. himantiigena is considered a good member of Clitocella, reason for which we propose the combination to that genus.
Our specimens were identified based on Singer's (1949) description, who reported the following features: robust basidiomata with dark colored pileus, basidiospores size (4.3-8 × 3.7-5 µm), absence of pleuro-and cheilocystidia, presence of versiform pseudoparaphyses and pileipellis a cutis of incrusted brown pigments.
Clitocella himantiigena and C. mundula (Lasch) Kluting, T. J. Baroni & Bergemann are similar species and, as well as in C. obscura (Pilát) Vizzini, T. J. Baroni, E. Sesli & Antonín and C. popinalis (Fr.) Kluting, T. J. Baroni & Bergemann, all these react positively to reddish under 3% KOH on the dried pileal surface (Baroni 1981). In addition, C. himantiigena and C. mundula present similar basidioma stature, pileus size and surface, lamellae insertion, stipe size, color and surface, but the pileus color of R. himantiigena is darker, when compared with European and North American specimens of C. mundula, which also range from pale to dark grey (Baroni, 1981). The basidiospores of C. himantiigena are longer (5-8 × 3.5-5.5 µm, in our specimens; 4.3-8 × 3.7-5 µm in Singer, 1949) and predominantly short to broadly ellipsoid in profile view, while in C. mundula they are shorter (5-6.5 × 4-5 µm), subglobose to obovoid in profile and face view (Pegler & Young, 1975). Singer (1949) reported filiform, cylindro-clavate, rarely branched pseudoparaphyses, which also were observed in our collections (Fig. 2D) and are not reported in C. mundula (Baroni, 1981).
By the set of the above cited features and by the collecting area close to type localitiy (Paraguay, Spegazzini, 1919) and Argentina (Niveiro & Albertó, 2014), we consider that C. himantiigena is a South American species, distinct of C. mundula, which is distributed in the northern hemisphere, especially North America and Eurasia (Noordeloos & Gates, 2012).
Specimens examined
BRAZIL. Paraná. Palotina. PESC, 09-VI-2010; A. J. Ferreira & D. Souza 3-2 (HCP 1019); 22-I-2015, M. A. Teixeira-Silva 058 (HCP 1018), 11-V-2015, A. G. S. Silva-Filho 394 (HCP 1144); RPPN Fazenda Açu. Terra Roxa, 20-IV-2015, A. G. S. Silva-Filho 282 (HCP 1016); 04-V-2015, A. G. S. Silva-Filho 361 (HCP 1017).
Clitocella pallescens Silva-Filho & Cortez sp. nov. MycoBank MB 825224. TYPE: Brazil. Paraná. PESC, 03-II-2015, A. G. S. Silva-Filho 172 (holotypus UPCB). Figs. 3, 6B-C.

Fig. 3. Clitocella pallescens. A, basidiomata. B, basidios-pores. C, basidia. D, section of lamellar trama. E, section of pileipellis, pileus and lamella trama, highlighting be-low terminal hyphae of the pileipellis. All photos from A. G. S. Silva-Filho 172.
Diagnosis: Basidiomataclitocyboid, pileus 13-30 mm diam., infundibuliform to plano-convex, white to pastel grey, lamellae decurrent, stipe cylindrical, with mycelial pad and rhizomorphs, basidiospores 4-5 × 3-4.3 µm, globose to subglobose, smooth to slightly angled in polar view, cystidia absent, pileipellis a transition between trichocutis and a trichoderm, clamp connection absent.
Pileus 13-30 mm diam., infundibuliform when young, plane to plano-convex or depressed when mature, surface smooth, margin smooth, abrupt, lobed, rimose, pastel grey (1C1) to pale grey (1B1) at the center and pale grey (1B1) to white (1A1) at the margin. Lamellae decurrent, crowed, with two-sized lamellulae, margin even to eroded, concolor with the sides, consistency fleshy to coriaceous, white (1A1), pale grey (1B1) to greenish yellow (1C2). Stipe 12-15 × 1-3 (apex) 3-6 mm (base), central, terete, tapered at the apex, pale grey (1B1) to yellowish white (1A2), consistency fleshy, smooth to slightly striated, with white mycelial pad and rhizomorphs (Fig. 3A). Context thin (<1mm thickness), pale grey (1B1). Spore print not observed. Spot test: not producing a reddish color reaction under 3% KOH on pileus.
Basidiospores 4-5 × 3-4.3 µm (n=50/3/1, Q=1.42-1.45, Qm=1.16), globose to subglobose in profile view, globose in polar view, with obscure pustules, almost smooth or with minute and obscure angles in polar view, hyaline, thin-walled, inamyloid (Fig. 3B, 6B-C). Basidia 17-26 × 4.5-6 µm, clavate to cylindro-clavate, uni-, bi-and tetrasporic, hyaline (Fig. 3C). Pleurocystidia and cheilocystidia absent. Lamella edge fertile. Lamellar trama irregular, with hyphae 2.5-5.5 µm diam., smooth, hyaline (Fig. 3D). Pileus trama with interwoven hyphae, 2-6 µm diam., prostrate, smooth, hyaline. Pileipellis a transition between cutis and trichoderm (or trichocutis), formed of subparallel hyphae, 2.5-6.5 µm diam., slightly gelatinized, smooth and hyaline (Fig. 3E). Stipitipellis is a cutis of parallel hyphae 1.6-3.6 µm diam., smooth, hyaline. Stipititrama irregular, composed of hyphae 1.5-5 µm diam., smooth and hyaline. Caulocystidia absent. Clamp connections absent. Oleiferous hyphae (thrombopleurous) observed in the lamella trama.
Etymology. Latin reference to the pale color of the basidiomata.
Distribution and habitat. Solitary or gregarious on rotten wood, in the forest. Known only from type locality.
Observations. Clitocella pallescens is proposed as a new species, based in the singular morphology of the basidioma. The centrally stipitate basidiomata, pileus whitish, and decurrent lamellae, place it in Rhodocybe sect. Decurrentes sensu Baroni (1981), whose species are briefly discussed as follows.
According to Baroni (1981, under Rhodocybe): C. mundula, C. obscura and C. popinalis produce a reddish reaction in 3% KOH, and for this reason they differ from C. pallescens; on the other hand, the European Rhodocybe hirneola (Fr.) P. D. Orton does not become red in 3% KOH, but has a grayish to dark grayish pileus, and presents cheilocystidia; R. heterospora (Murrill) T. J. Baroni present interwoven hyphae in lamella trama and the basidiospores are larger (5.5-7 × 5.5-6.5 µm); R. porcellanica (Dennis) E. Horak, R. semiarboricola T. J. Baroni and R. mairei T. J. Baroni have parallel to sub-parallel hyphae in lamella trama, but these species have grayish to dark grayish color at the pileus and basidiospores are subglobose to short-broadly-ellipsoid and longer (≥ 5µm).
Clitocella fallax (Quél.) Kluting, T. J. Baroni & Bergemann does not react under 3% KOH, the basidiomata is white to whitish and the lamellae trama is parallel, as well as C. pallescens. However, C. fallax has more robust basidiomata, the pileus is larger (30-40 mm diam.) and with a mammilate central umbo, rhizomorphs are absent, basidiospores are amygdaliform to ellipsoid and larger (6.5-8 × 4-6.5 µm), the pileipellis is a loosely entangled layer of ascending cylindrical hyphae (Baroni, 1981). In addition, C. fallax is distributed along the northern hemisphere (Europe and North America, Kluting et al. 2014).
Thus, we consider that our sample collection present unique features, as white to off-white pileus (Fig. 3A), not producing a reddish reaction in KOH 3%, absence of pseudocystidia and small (≤ 5 µm long) globose to subglobose basidiospores (Fig. 3B). Based on the combination of these features, we propose it as a new species of Clitocella. In spite of type collection is the only collected material, it was composed of seven basidiomata, which were in perfect condition when gathered, allowing a full and detailed examination of their macro-and microscopical features, which we considered enough to prospose it as a new taxon.
Specimens examined
BRAZIL. Paraná. PESC, 03-II-2015, A. G. S. Silva-Filho 172 (UPCB Holotypus; HCP Isotypus). Rhodocybe caelatoidea Dennis, Kew Bull. 15: 154 (1961). Clitopilus caelatoideus (Dennis) Noordel. & Co-David, Persoonia 23: 161 (2009). Figs. 4, 6D.

Fig. 4. Rhodocybe caelatoidea. A, Basidiomata. B, ba-sidiospores. C, pseudocystidia. D, basidium. E, section of lamellar trama and subhymenium. F, pileipellis. G, oleiferous hyphae of pileipellis. H, terminal hyphae of pileipellis. All photos from A. G. S. Silva-Filho 167.
Pileus 12-25 mm diam., convex, slightly depressed at the disc, surface smooth, margin smooth to slightly translucent striate, lobed, orange (6B7) to brownish orange (7C8). Lamellae adnate to decurrent, close to subdistant, with up to three sizes of lamellulae, margin slightly wavy, concolor with the sides, membranous, orange grey (6B3), greyish orange (6B4). Stipe 23-31 × 2-4 mm, central, slightly compressed, surface smooth from the bottom to centre, and velutinous at the apex, consistency fleshy to coriaceous, brownish orange (7C8), greyish orange (5B4), with basal mycelium (Fig. 4A). Context thin (1-3 mm thickness), light orange (6A5). Spore print not observed.
Basidiospores 7-8.5 × 5-6.5 µm, (n= 50/3/3 Q= 1.09-1.36, Qm= 1.21), subglobose to short ellipsoid in profile view, globose to subglobose and angular in polar view, undulate-postulate in all views, hilar appendix evident, thin-walled, hyaline, inamyloid (Fig. 4B-6D). Basidia 25-29 × 6.5-8 µm, cylindro-clavate to clavate, bi-and tetrasporic, hyaline (Fig. 4D). Pleurocystidia and cheilocystidia as pseudocystidia, 32-46 × 3-6 µm, ventricose-rostrate, ventricose to lageniform, with brightly yellowish content, granulated or coagulated, scattered, non-abundant, little projecting from the hymenium (Fig. 4C). Lamella edge fertile. Lamellar trama subregular, with hyphae 4-5 µm diam., smooth, hyaline. Pileus trama irregular, with hyphae 3-5.5 µm diam., hyaline. Pileipellis composed of a layer of entangled hyphae, 2.5-6 µm diam., slightly incrusted, hyaline, terminal elements filiform, forming a nearly a trichodermium, 26-43 × 4.5-8.5 µm (Fig. 4F-H). Stipitipellis a cutis, formed by hyphae 2.5-3.5 µm diam., light brown, incrusted, with fusiform and incrusted terminal elements, 46 × 4 µm, thin walled, hyaline. Stipititrama subregular, with hyphae 2-6 µm diam., smooth, with brightly yellowish contents. Caulocystidia absent. Oleiferous hyphae (thrombopleurous) scarce, present at the pileipellis. Clamp connections absent.
Distribution and habitat. Gregarious or solitary on decaying wood. Neotropical species, known only from South and Central America (Dennis, 1961; Horak, 1978; Pegler, 1983). In Brazil, only de Meijer (2006) reported the species from Paraná state (Fig. 1).
Observations. Rhodocybe caelatoidea belong to Rhodocybe sect. Rhodocybe sensu Baroni (1981), due to presence of hymenial pseudocystidia (Fig. 4C), centrally stipitate basidiomata (Fig. 4A) and absence of clamp connections. This species was collected in this survey and can be confounded in the field with R. galerinoides due the small reddish basidiomata. However, as discussed below, R. caelatoidea has non-hygrophanous and larger pileus (12-25 mm), convex, slightly depressed at the disc (Fig. 4A), while R. galerinoides is hygrophanous with smaller pileus (7-14 mm), conical to convex, umbonate to papillate at the disc (Fig. 5A). Microscopically, the basidiospores of R. caelatoidea are larger (7-8.5 × 5-6.5 µm, Fig. 4B) than those of R. galerinoides (4.5-6.5 × 4-5 µm, Fig. 5B), and the pileipellis has incrusted and oleiferous hyphae (Fig. 4G-H), in contrast to the smooth hyphae of the pileipellis of R. galerinoides (Fig. 5F).
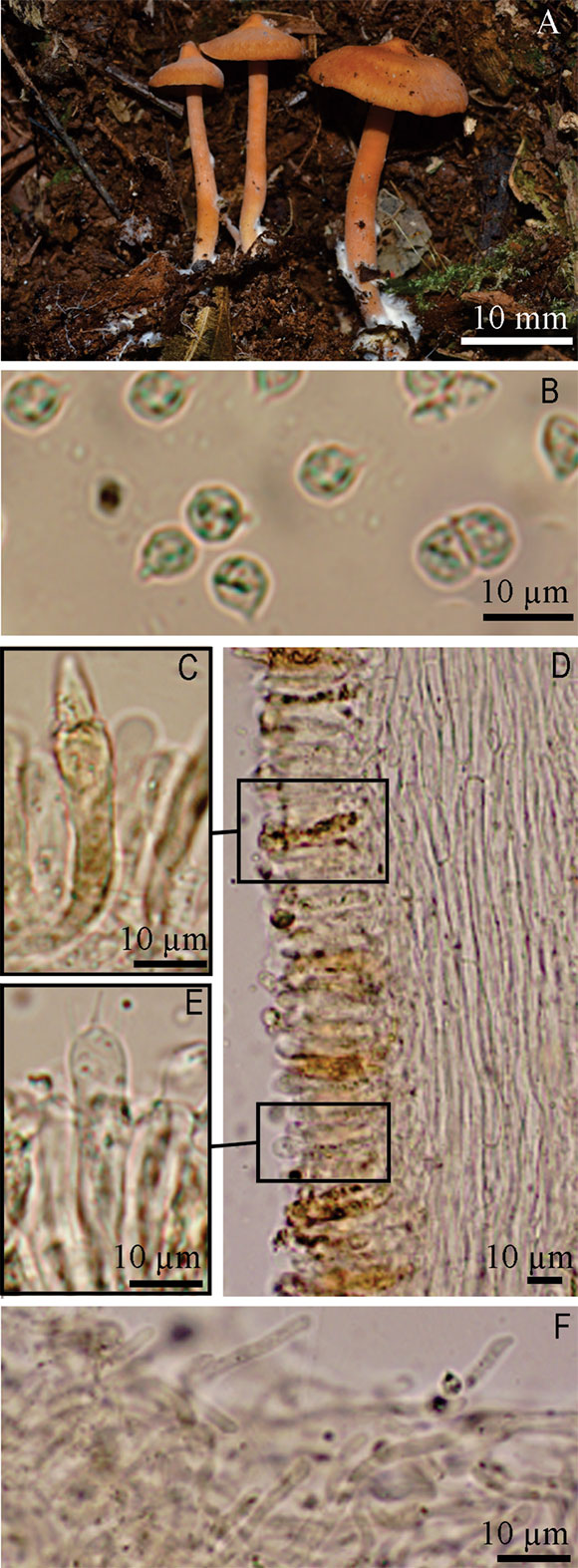
Fig. 5. Rhodocybe galerinoides. A, basidiomata. B, basidiospores. C, pseudocystidia. D, section of lamellar trama. E, basidium. F, pileipellis. Photo A, from M. A. Teixeira-Silva 060.

Fig. 6. SEM micrographs of the basidiospores. A, C. himantiigena. B-C, C. pallescens. D, R. caelatoidea – whithout treatment. E, R. galerinoides.
Baroni (1981) related R. caelatoidea in the group with terrestrial basidiomata, however the Antillean collections by Pegler (1983) were lignicolous, as well our sample collections, which were gathered on very decayed wood and litter fall.
Rhodocybe retroflexa (Berk. & Broome) Pegler, from Sri Lanka, is similar to R. caelatoidea, but differs by the cream-buff pileus and the pileipellis formed by an undifferentiated cutis of radial hyphae, without anticlinal terminal elements (Pegler, 1986). Rhodocybe caelatoidea was originally described based on a type collection from Venezuela, which later was reviewed by Horak (1978) and Pegler (1983), who recorded and illustrated specimens from the Lesser Antilles. From Brazil, R. caelatoidea is known only from Paraná state, reported by de Meijer (2006) in his checklist of macromycetes, but no descriptions or illustrations were provided by the author. According the reported collections by de Meijer (2006), R. caelatoidea is well distributed along the State of Paraná, being reported from the eastern (Curitiba) central (Fênix) and also western region (Altônia).
Specimens examined
BRAZIL. Paraná. PESC, 2-III-2015, A. G. S. Silva-Filho 167 (HCP 1023), 11-V-2015, A. G. S. Silva-Filho 372 (HCP 1025), 1-VI-2015, A. G. S. Silva-Filho 513 (HCP 1024).
Rhodocybe galerinoides Singer, Sydowia 15: 81 (1962). Clitopilus galerinoides (Singer) Noordel. & Co-David, Persoonia 23: 161 (2009). Figs. 5, 6E.
Pileus 7-14 mm diam., conical to convex, umbonate to papillate at disc, surface velutinous to slightly fibrillose towards the margin, margin irregular, incurved, non-striated, orange (6A6) to brownish orange (6C7). Lamellae subdecurrent, abundant, close, with 3-5 sized lamelullae, semicircular, margin entire and slightly wavy, concolor with the sides, membranous, greyish orange (6B3-6B4). Stipe 19-27 × 1-2 mm, central, terete, equal, velutinous near the apex, coriaceous, concolor to pileus, with basal mycelium (Fig. 5A). Context thick (up to 5mm at the disc), pale grey (1B1). Spore print not observed.
Basidiospores 4.5-6.5 × 4-5 µm, (n= 50/2/2, Q= 1.09-1.36, Qm= 1.21); globose to subglobose in profile view, globose and angular in polar view, undulate-postulate in all view, thin walled, hilar appendix evident, hyaline, inamyloid (Fig. 5B-6E). Basidia 24.5-35 × 5.5-6.5 µm, cylindro-clavate to clavate, tetrasporic, hyaline (Fig. 5E). Pleurocystidia and cheilocystidia as pseudocystidia, 32-76 × 4-7 µm, ventricose, ventricose-rostrate to lageniform, with brightly yellowish contents, granulated or coagulated, scattered, a little projecting from the hymenium (Fig. 5C). Lamella edge fertile. Lamellar trama regular, with hyphae 3.5-6.5 µm diam., slightly incrusted, hyaline and light brown (Fig. 5D). Pileus trama regular, with hyphae 8.5-11 µm diam., some slightly incrusted, light brown. Pileipellis composed of a layer of entangled hyphae, 2-5 µm diam., with some anticlinal terminal elements, 13.5-35 × 3-5 µm, forming a nearly a trichodermium, hyphae septate, smooth to slightly incrusted, light brown to brown (Fig. 5F). Stipitipellis a cutis, composed of hyphae 3-6 µm diam., hyaline and light brown, thin walled, hyaline. Stipititrama subregular, with hyphae 1.5-4.5 µm diam., incrusted, with brightly yellowish contents.
Caulocystidia absent. Clamp connections absent.
Distribution and habitat. Gregarious on decaying wood. Previously known only from Bolivia (Singer, 1962), it is now reported for the first time in Brazil (Fig. 1).
Observations. Rhodocybe galerinoides and R. caelatoidea are both classified in Rhodocybe sect. Rhodocybe sensu Baroni (1981) as mentioned earlier. It was named by virtue of small basidiomata (7-14 mm diam), as well as the lignicolous habitat among mosses, as several Galerina Earle species.
Rhodocybe nitellinoides E. Horak from Papua New Guinea presents basidioma similar in size and color, but differ in the non-hygrophanous pileus, ovoid basidiospores, and pileipellis with incrusted yellow hyphae (Horak, 1978).
Rhodocybe pruinosistipitata T. J. Baroni, Largent & Aime, from Guyana, is also comparable to R. galerinoides by the virtue of similar basidiomata, basidiospore shape and presence of pseudocystidia, but differs in the larger pileus (15-30 mm diam.) and stipe (35-57 × 3-3.5 mm), presence of white pruina over stipe surface, larger basidiospores (6.8-8.1 × 4.7-6.8 µm), and incrusted hyphae of stipe trama (Henkel et al., 2010).
Rhodocybe galerinoides was described and is only known from Bolivia (Singer, 1962), thus it is the first record from Brazil and the second for this species.
Specimens examined
BRAZIL. Paraná. Palotina, PESC, 22-I-2013, M. A. Teixeira-Silva 060 (HCP514); 11-V-2015, A. G. S. Silva Filho 393 (HCP 1020); Terra Roxa, RPPN Fazenda Açu, 12-XI-2015, A. G. S. Silva-Filho 643 (HCP 1021).
ACKNOWLEDGEMENTS
This research was supported by funds of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Proc. 483455/2013-3), a grant by Fundação Araucária (Convênio 675/2014) to VGC, and student fellowship to AGSSF and MATS from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). We thank the two anonymous reviewers and André de Meijer for comments on the earlier version of this manuscript.
BIBLIOGRAPHY
[other standard="nd" count="27"]1. Baroni, T. J. 1981. A revision of the genus Rhodocybe Maire (Agaricales). Beihefte Nova Hedwigia 67: 1-194. [ Links ]
2. Baroni, T. J. & R. E. Halling. 1992. New species of Rhodocybe from South America with a key to species. Mycologia 84: 411-421. [ Links ]
3. Baroni, T. J. & P. B. Matheny. 2011. A re-evaluation of gasteroid and cyphelloid species of Entolomataceae from Eastern North America. Harvard Papers in Botany 16: 293-310. DOI: 10.3100/0.25.016.0205 [ Links ]
4. Co-David, D.; D. Langeveld. & M. E. Noordeloos. 2009. Molecular phylogeny and spore evolution of Entolomataceae. Persoonia 23: 147-176. DOI: 10.3767/003158509X480944 [ Links ]
5. de Meijer, A. A. R. 2006. Preliminary list of the macromycetes from the Brazilian state of Paraná. Boletim do Museu Botânico Municipal 68: 1-59. [ Links ]
6. de Meijer, A. A. R. 2008. Notable Macrofungi from Brazil's Paraná Pine Forests. Colombo: Embrapa. [ Links ]
7. Dennis, R. W. G. 1961. Fungi venezuelani: IV. Agaricales. Kew Bulletin 15: 67-156. [ Links ]
8. Henkel, T. W.; M. C. Aime, D. L. Largent. & T. J. Baroni. 2010. The Entolomataceae of the Pakaraima Mountains of Guyana III: New species of Rhodocybe. Mycoscience 51: 23-27. [ Links ]
9. Horak, E. 1978. Notes on Rhodocybe Maire. Sydowia 31: 58-80. [ Links ]
10. Kluting, K. L.; T. J. Baroni. & S. E. Bergemann. 2014. Toward a stable classification of genera within the Entolomataceae: a phylogenetic re-evaluation of the Rhodocybe-Clitopilus clade. Mycologia, 106: 1127-1142. DOI: 10.3852/13-270 [ Links ]
11. Kornerup, A. & K. H. Wanscher. 1978. Methuen Handbook of Colour. 3 ed. London: Eyre Methuen. [ Links ]
12. Largent, D. L. 1994. Entolomatoid Fungi of the Pacific Northwest and Alaska. Eureka: Mad River. [ Links ]
13. Maia, L. C.; A. A. Carvalho Júnior, L. H. Cavalcanti, A. M. Gugliotta, E. R. Drechsler-Santos, A. L. M. A. Santiago, M. S. Cáceres, T. B. Gibertoni, A. Aptroot, A. J. Giachini, A. M. S. Soares, A. C. G. Silva, A. C. Magnago, B. T. Goto, C. R. S. Lira, C. A. S. Montoya, C. L. A. Pires-Zottarelli, D. K. A. Silva, D. J. Soares, D. H. C. Rezende, E. D. M. N. Luz, E. L. Gumboski, F. Wartchow, F. Karstedt, F. M. Freire, F. P. Coutinho, G. S. N. de Melo, H. M. P. Sotão, I. G. Baseia, J. Pereira, J. J. S. Oliveira, J. F. Souza, J. L. Bezerra, L. S. Araujo Neta, L. H. Pfenning, L. F. P. Gusmão, M. A. Neves, M. Capelari, M. C. W. Jaeger, M. P. Pulgarín, N. Menolli Junior, P. S. Medeiros, R. C. S. Friedrich, R. S. Chikowski, R. M. Pires, R. F. Melo, R. M. B. Silveira, S. Urrea-Valencia, V. G. Cortez. & V. F. Silva. 2015. Diversity of Brazilian fungi. Rodriguésia 66: 1033-1045. DOI: 10.1590/2175-7860201566407 [ Links ]
14. Morgado, L. N.; M. E. Noordeloos. & A. Hausknecht. 2016. Clitopilus reticulosporus, a new species with unique spore ornamentation, its phylogenetic affinities and implications on the spore evolution theory. Mycological Progress 15: 26. DOI: 10.1007/s11557-016-1165-0 [ Links ]
15. Niveiro, N. & E. Albertó. 2014. Checklist of the Argentine Agaricales 7. Cortinariaceae and Entolomataceae. Check List 10: 72-96. [ Links ]
16. Noordeloos, M. E. & G. M. Gates. 2012. Entolomataceae of Tasmania. Dordrecht: Springer. [ Links ]
17. Pegler, D. N. 1983. Agaric flora of the Lesser Antilles. Kew Bulletin Additional Series 9: 1-668. [ Links ]
18. Pegler, D. N. 1986. Agaric flora of Sri Lanka. Kew Bulletin Additional Series 9: 1-520. [ Links ]
19. Pegler, D. N. & T. W. K. Young. 1975. Basidiospore form in the British species of Clitopilus, Rhodocybe and Rhodotus. Kew Bulletin 30: 19-32. [ Links ]
20. Raithelhuber, J. 1990. Die Gattung Clitocybe ss. lat. in den ABC-Staaten. Metrodiana 18: 1-72. [ Links ]
21. Silva-Filho, A. G. S. & V. G. Cortez. 2017. Hohenbuehelia (Pleurotaceae) in western Paraná, Brazil. Acta Biológica Paranaense 46: 23-38. [ Links ]
22. Silva-Filho, A. G. S.; G. Coelho. & V. G. Cortez. 2016. Further notes on the morphology and distribution of Neopaxillus echinospermus (Agaricales, Basidiomycota) in Southern Brazil. Check List 12: 1834. [ Links ]
23. Singer, R. 1949. The Agaricales in modern taxonomy. Lilloa 22: 1-832. [ Links ]
24. Singer, R. 1962. Diagnosis fungorum novorum Agaricalium. II. Sydowia 15: 45-83. [ Links ]
25. Singer, R. 1973. Diagnoses fungorum novorum agaricalium III. Beihefte zur Sydowia 7: 1-106. [ Links ]
26. Singer, R. 1986. The Agaricales in Modern Taxonomy. 4 ed. Koenigstein: Koeltz. [ Links ]
27. Singer, R. 1989. New taxa and new combinations of Agaricales (Diagnoses fungorum novorum agaricalium IV). Fieldiana Botany 21: 1-133. [ Links ]
28. Spegazzini, C. 1919. Reliquiae mycologicae tropicae. Boletín de la Academia Nacional de Ciencias 23: 365-541. [ Links ]














