INTRODUCTION
The global richness of ferns and lycophytes is estimated at approximately 12,000 species (PPG I, 2016), of which about 3,500 are found in South America (Moran, 2008), where the Andean region and the mountains of southern and southeastern Brazil are centers of diversity (Tryon, 1972). Approximately 1,300 species of ferns and lycophytes occur in Brazil, and 883 of them are present in the Atlantic Forest (Prado et al., 2015).
The Atlantic Forest is included in the Atlantic biogeographic province of the Neotropical region (Morrone, 2017), extended originally from the eastern portion of Brazil to some areas in eastern Paraguay and northeastern Argentina (Arana et al., 2017). The Atlantic biogeographic province comprised more than 1,360,000 km2 during the Middle Holocene, however, it is currently reduced to 7% of its original area (Ribeiro et al., 2009).
In southern Brazil, the best-preserved Atlantic Forest remnants are in the eastern side (Serra do Mar region) (Ribeiro et al., 2009), in montane regions where agricultural activity is limited (Moreno et al., 2003; Gasper et al., 2015). The majority of floristic inventories involving ferns and lycophytes have been carried out in the eastern portion of this region, in areas dominated by ombrophilous forests (Oliveira- Filho et al., 2015). This region is generally devoid of mountains and forest remnants are generally small and patchily distributed.
Fewer studies have considered the fern and lycophyte diversity of the Atlantic semi-deciduous forests in marginal position of southern Brazil (Bauer, 2004; Farias et al., 2014; Lautert et al.,
2015; Moraes et al., 2018). The first two and the last one performed floristic inventories in the northwest region of the state of Rio Grande do Sul and Lautert et al. (2015), performed floristic inventories in four forest areas located in the southwestern region of the state of Paraná.
Gasper & Salino (2016) presented data involving the occurrence of 73 species of ferns and lycophytes in semi-deciduous forests located in the state of Santa Catarina. This study added historical information from “Flora Ilustrada Catarinense” (Sehnem 1967a; b; c; 1968a; b; 1970a; b; 1971; 1972; 1974; 1978; 1979a - g; 1984; Fuchs-Eckert,
1986) and recent efforts of Santa Catarina forest survey. Brack et al. (1985) and Rossetto & Vieira (2013) carried out general studies on vascular flora and provided information about the occurrence of ferns and lycophytes in semi-deciduous forests in the state parks Parque Estadual do Turvo (RS) and Parque Estadual Mata dos Godoy (PR), respectively.
The size of the semi-deciduous forest remnants, many of them smaller than 50 ha (Ribeiro et al., 2009), together with the few conservation units throughout the Atlantic domain (MMA, 2003; Fonseca & Venticinque, 2018), highlight the need to exert efforts to increase our knowledge about the diversity of ferns and lycophytes associated with semi-deciduous forests, especially in southern Brazil.
Based on this scenario, the present study aimed to assess the species richness of ferns and lycophytes from 11 semi-deciduous forest remnants (of marginal position) belonging to the Atlantic biogeographic province in southern Brazil.
MATERIALS AND METHODS
Floristic survey, ecological aspects and geographic distribution
Ferns and lycophytes were surveyed in 11 areas from northwestern region of Rio Grande do Sul (Fig. 1, Table 1), between January 2015 to April 2017. The areas with semi-deciduous forest were selected through satellite imagery available on the Google Earth Pro version platform. Two of these selected areas are representing conservation units (Dois Irmãos das Missões - F1 and Parque Estadual do Turvo - F10).

Table 1 Semi-deciduous forest remants studied in the northwestern region of the State of Rio Grande do Sul (RS), Brazil.
These selected areas all have suffered from selective cutting of trees, especially during the 1980s and 1990s. In all areas, samplings were made along preexistent trails, stream margins, trunks of fallen trees, ravines, clearings and edge of the remnants, with the goal of examining different possible microenvironments. For each area, three collection surveys were carried out.
All the areas are in a range of an altitude between 150 and 580 m above sea level and the range in size from from 24 to 16,000 ha (Table 1). The climate of the region is classified as temperate, with absence of a dry season and marked by hot summers, classified as subtype Cfa in the Koppen world climate classification (Peel et al., 2007).
Specimens were collected using standard techniques (Windisch, 1992). The collected material was classified according to life-form and growth-form following Senna & Waechter (1997), and voucher specimens were sent to FUEL, FURB, RCVC, SP and VIC herbaria (Thiers, 2018). Studies carried out by Brack et al. (1985) and Bauer (2004) were considered to complement the species list of the fragment classified here as F10 (Table 1 - Parque Estadual do Turvo). In addition, specimens kept in HAS, ICN and PACA, which are the herbaria with most representative flora of the state, were also considered as complementary source for information. Species cited previously but whithout vouchers in herbaria were excluded from the list.
Species identification was performed in consultation with specialists, material deposited in herbaria and with specific bibliography, including Prado & Windisch (2000), Labiak & Prado (2008), Moran et al. (2009), Arana & Ponce (2015), Arana & Mynssen (2016), Larsen & Ponce (2016) and Dittrich et al. (2017).
The conservation status of the species was verified at www.cncflora.jbrj.gov.br (accessed on 27 Sep 2018). The arrangement and circumscription of the families and genera of ferns and lycophytes follows PPG I (2016).
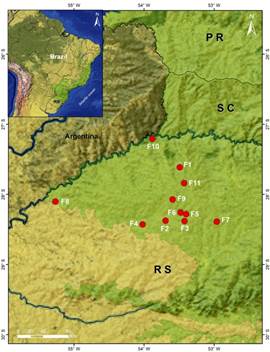
Fig. 1 Location of the study areas in northeastern region of the state of Rio Grande do Sul. See Table 1 for the full name of the areas. Color version at
Data analysis
The species were classified according to their geographical distribution pattern, adapted from Tryon (1972) and Sehnem (1977), as follows: AA-species with disjunct distribution in the Atlantic region of South America and Africa; EBN - endemic to Brazil and neighboring areas; ESS - endemic to southern and southeastern Brazil; IN - introduced species; PA - species with pantropical distribution; SA - occurrence only in South America and TA - species widely distributed in tropical and subtropical America, including the southern region of the USA.
The floristic similarity analysis was performed using Euclidian similarity index, and a dendogram was made using UPGMA. The analysis was performed with software PAST (Paleontological statistics Package for Education and Data Analysis) version 3.0 (Hammer et al. 2001). The cophenetic correlation coefficient was used to verify the degree of adjustment of the cluster; values close to 1.0 indicate a high degree of distance preservation in the cluster.
RESULTS
Floristic and ecological aspects
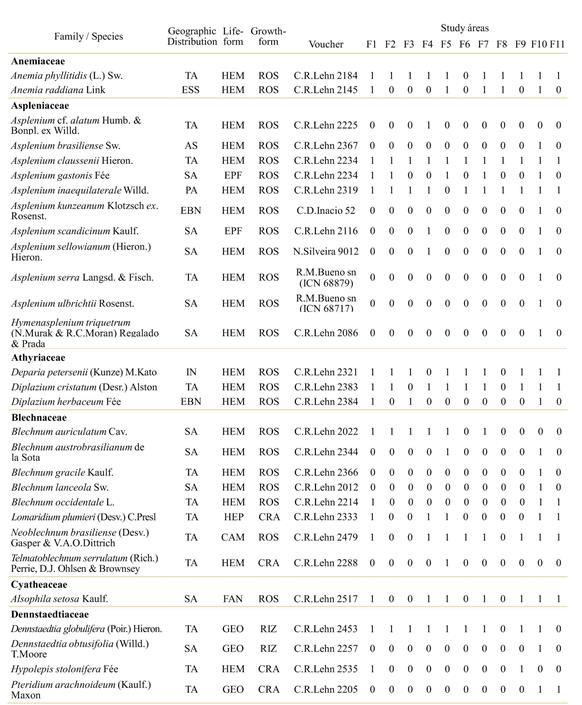
Ninety-two species (four lycophytes and 88 ferns) were recorded in the 11 studied forest remnants. Lycophytes were represented by two families (Lycopodiaceae and Selaginellaceae) and two genera, while 15 families and 44 genera of ferns were recorded (Table 2).
Pteridaceae and Polypodiaceae showed the highest richness, represented by 18 and 17 species respectively. These families, together with Aspleniaceae (11 spp.), Thelypteridaceae (9 spp.) and Blechnaceae (8 spp.), include approximately the 70% of the observed species in the studied areas. Asplenium (10 spp.) was the most diverse genus (Table 2). The number of species in the studied remnants ranged from 18 (F8) to 81 (F10), with the predominance of hemicryptophytes, followed by epiphytes (Table 2).
The species Asplenium claussenii, Microgramma squamulosa, Pecluma pectinatiformis, Pleopeltis hirsutissima, Adiantum pseudotinctum, and Doryopteris pentagona were present in all sampled areas. The species Ctenitis submarginalis, Campyloneurum nitidum, Pleopeltis minima, and Pleopeltis pleopeltifolia also showed frequentoccurrence, being absent just in one area. The Parque Estadual do Turvo (F10) harbored the largest number of exclusive species (28 spp.), including Asplenium serra, Blechnum lanceola and Tectaria incisa.
From all species surveyed, only Dicksonia sellowiana, observed just in two remnants (F1 and F10) is listed in the Brazilian Red List of Threatened Plant Species (MMA, 2008), where it is classified as endangered (EN). No endemic species of semideciduous forests were found in the studied remnants.
Floristic dissimilarity and geographic distribution
The floristic similarity analysis revealed that F10 (Parque do Turvo) is the most dissimilar area in relation to the other studied areas. All other areas formed two sets, being F8 (São Nicolau) the external group (Fig. 2). The cluster analysis showed high statistical significance and the cophenetic correlation coefficient was 0.968.
Most of the surveyed species showed a broad geographic distribution, occurring in South America (44.6%) and Tropical America (32.6%) (Table 2). Only Anemia raddiana and Polystichum platylepis are endemic to southern and southeastern Brazil and northeastern Argentina, respectively (Schwartsburd & Labiak, 2007; Morero, 2016).

Fig. 2 Similarity dendrogram of lycophyte and fern species among 11 semi-deciduous areas in the northwestern region of the Rio Grande do Sul (using Euclidian Index and UPG- MA algorithm). See table 1 for the full name of the areas.
Table 2 List of species of ferns and lycophytes associated with 11 remnants of semi-deciduous seasonal forest in the northeastern region of Rio Grande do Sul, Brazil. Geographic distribution: TA - tropical America; SA - South America; ESS - endemic to southern and southeastern Brazil; EBN - endemic to Brazil and neighboring areas; IN - introduced; AA - amphipathic; PA - pantropical. Life-form: CAM - chamephyte; EPF - epiphyte; FAN - phanerophyte; GEO - geophyte; HEM - hemicryptophyte; HEP - hemiepiphyte; TER - terophyte. Growth-form: HAN - hanging; CRA - crawling; RIZ - rhizomatous; ROS - rosulate. Locality names: see table 1.
DISCUSSION
The surveyed remnants showed intermediate species richness (Table 1: 18-43 species) in relation to other previously studied areas in the state of Rio Grande do Sul, except for Parque Estadual do Turvo (F10 - 81 species). In general, the number of species observed in most of the inventories in Rio Grande do Sul varies from 28 (Farias et al., 2014) to 77 species (Silva-Junior & Rörig 2001).
The high representativeness of Polypodiaceae and Pteridaceae (Table 2) in the region agrees with the most frequent floristic pattern in Atlantic areas of southern Brazil and neighboring areas, as shown in studies conducted in the states of Paraná (Rossetto & Vieira, 2013; Lautert et al., 2015), Santa Catarina (Gasper & Savegnani, 2010), Rio Grande do Sul (Santos & Windisch, 2008; Lehn et al., 2009; Burmeister & Schmitt, 2016), and in Argentina (Márquez et al., 2006; Torres et al., 2013). In the studied region, Polypodiaceae is represented by predominately epiphytic species, and Pteridaceae is principally represented by terrestrial taxa, apparently with little or no niche overlapping among the species of both families. This possibly could allow the high representativeness of both families in the studied areas.
Asplenium (Aspleniaceae) (Table 2) was the most diverse genus in the surveyed areas. We noted the ecological amplitude of the genus Asplenium, recording species that require wet and well-preserved terrestrial environments (e.g. Asplenium brasiliense and A. serra), while others were observed only with epiphytic habit (e.g. A. gastonis and A scandicinum) or even occurring in both shaded and exposed sites, located both on the edges and inside the remnants studied (e.g. A. claussenii and A. inaequilaterale).
Approximately 90% of the species sampled in the studied region have a broad geographical distribution (South America and Tropical America), occurring in different forest formations. These species belong to a flora with connections by the route Paraná-Uruguay rivers (Rambo 1961) and constitute relicts from an ancient flora widely distributed and fragmented with vicariant events, mainly the development of a savannah corridor during the Cenozoic era (Ponce et al., 2002; Morrone, 2006; Arana et al., 2013, 2016).
Only Dicksonia sellowiana and Crepidomanes pyxidiferum var. australe are taxa considered largely restricted to southeastern Brazil and neighbouring areas of northeastern Argentina (Ponce et al., 2017; Lehnert & Kessler, 2018).
Dicksonia sellowiana is a species with a rare occurrence in the studied region and associated to the ombrophilous forests, especially in the higher regions of the South of Brazil, being rare in the semideciduous formations located in the western region of southern Brazil (Gasper et al., 2011).
The reduced number or absence of endemic species constitute a characteristic of the semi- deciduous forests of the Atlantic domain, which was confirmed in the present study. In southern Brazil, there is an east-west gradient of endemism, which is accompanied by decreasing gradients of altitude and precipitation (Ribeiro et al., 2009), which strongly contributes to the lack of endemism of ferns and lycophytes in semi- deciduous forests located in the southern part of the country (Lautert et al., 2015; Gasper & Salino,
2016; Neves et al., 2017). In this way, the ferns and lycophytes associated to the semideciduous forests of the region are a subset of the species occurring in the ombrophilous formations of southern Brazil.
The cluster analysis revealed that F10 was the most dissimilar area, which results from the high number of species with exclusive occurrence observed in the area (28 spp.); the lack of definition in the formation of the sets, could be the result of the high number of shared species among the areas. The area F8, located in an ecotone area between the Atlantic Forest and Pampa domains, showed the lowest richness among the surveyed areas. It could be possible because the transition between semi-deciduous forest formations and Pampa formations is classically accompanied by a decreasing number of fern and lycophyte species, supporting the existence of a latitudinal gradient of diversity (Moran, 2008) promoted by the retention of specific ecological niches necessary for the occurrence of ferns. In larger areas, it is generally found that the available resources provide a more heterogeneous environment (Magurran, 2004), possibly the main explanation to the high species richness in Parque do Turvo besides that it is a protected area.
The representation of species associated with forest formations in the northwestern Rio Grande do Sul was 24.7% of the fern and lycophyte flora recognized for the entire state. Parque Estadual do Turvo (F10) represents the largest continuous area of semi-deciduous seasonal forest in northwestern Rio Grande do Sul and harbors 81 species, which corresponds more than 20% of the species of ferns and lycophytes occurring in the state. The second area with the greatest number of occurrences was the Reserva Biológica Municipal Moreno Fortes (F1) with 43 records (11.4% o f the fern and lycophyte species recognized for Rio Grande do Sul), also an integral protected conservation unit, indicating the importance of these areas for the conservation of ferns and lycophytes.
The present study represents one of the first important contributions about the richness of ferns and lycophytes occurring in semi-deciduous forests in southern Brazil. Increasing sampling effort, including of non-forest environments, should improve considerably the list of species occurring in the region, thus covering a historical gap in our knowledge of the distribution of ferns and lycophytes in southern Brazil, especially in the state of Rio Grande do Sul.












 uBio
uBio