INTRODUCTION
The current global extinction crisis presents a great ecological challenge, and the loss of biodiversity is one of the most critical ongoing environmental problems (Dirzo & Raven, 2003; Ceballos et al., 2015). Global warming, coupled with habitat destruction and degradation, are causing substantial species-range shifts, contractions and local extirpations, which can be major causes of biodiversity loss (Brook et al., 2008; Chen et al., 2011; Urban, 2015). However, the need for conservation actions sometimes contrasts with the considerable uncertainty about the status of threats (Corlett, 2016; Bachman et al., 2018) and the spatial distribution of diversity (Whittaker et al., 2005; Bini et al., 2006). Species extinction risk assessment following the IUCN Red List categories and criteria (International Union for Conservation of Nature, www.iucn. org) is widely recognized as one of the most useful approaches for identifying conservation priorities and targeting government decisions to mitigate impacts on biodiversity (Ricketts et al., 2005; Rodriguez et al., 2006; Brooks et al., 2015; Bennun et al., 2018; Nic Lughadha et al., 2020). Although IUCN Red List Category data are produced on a global scale, is also essential to establish IUCN threat categories at the regional level in order to implement concrete conservation actions (Gärdenfors et al., 2001).
Argentina includes a high number of ecoregions due to its latitudinal and altitudinal diversity (Lean et al., 1990; Morello et al., 2018). Likewise, the Argentinean Flora, distributed along 13 biogeographical provinces sensu Cabrera & Willink (1980) or 17 ecoregions and six biomes sensu Olson et al. (2001), presents the greatest number of species in the Southern Cone region, comprising ca. 9237 species, of which 1731 (~18%) are endemic to the country (Zuloaga et al., 2019). Within it, the mustard family (Brassicaceae) is well represented by eleven tribes, 35 genera, 160 species, and two subspecies that are distributed mainly along the Andes, but with representatives in all biogeographic provinces and biomes. During the last 50 years, taxonomic revisions and systematic studies, several regional floras and checklists have focused on the distribution of the family in the country (Boelcke 1967; 1987; Boelcke & Romanczuk 1984a; 1984b; Boelcke & Martínez-Laborde, 1994; Prina, 1995a; 1995b; Martínez-Laborde, 1999; Al-Shehbaz, 2008), but it was not until the publication of the flora of Argentina (Al-Shehbaz, 2012a) that the family had its most comprehensive treatment. Nevertheless, the study of the species geographic ranges, their threat categorization, the association with the different ecoregion/ biomes, and an integrative analysis of its distribution in the country are still pending.
Argentinean crucifers seem to be mainly distributed along the central and southern Andean highlands (~ 22º to 55ºS latitude), inhabiting a variety of habitats in the Altoandina, Puna, and Prepuna biogeographical provinces (sensu Cabrera & Willink, 1980). These highland ecosystems are considered highly sensitive to climatic change and global warming (Halloy & Mark, 2003; Gonzales, 2009; IPCC, 2014; Cuesta et al., 2017). Furthermore, the restricted altitudinal range that many species occupy within these highland ecosystems, enhances their vulnerability to climatic change (Urban, 2015). On the other hand, endemics of this family are also present in lowlands of Patagonia and Central-Eastern Argentina [e.g., Chilocardamum patagonicum, Lepidium parodii, Lepidium hickenii, Mostacillastrum subscandens, Mostacillastrum ventanense, Physaria lateralis, and Trichotolinum deserticola] (Al-Shehbaz, 2012a). These areas are generally exposed to high human impact and land-use pressures, which can have a direct effect on the habitat degradation and biodiversity loss (Grau et al., 2005; Paruelo et al., 2006; Volante et al., 2012; Newbold et al., 2015; Vallejos et al., 2015). Additionally, several Argentinean endemic genera, such as Delphinophytum Speg., Litodraba Boelcke, and Zuloagocardamum Salariato & Al- Shehbaz, are micro-endemics with very restricted distribution ranges (Al-Shehbaz, 2012a; Salariato & Al-Shehbaz, 2014). Therefore, disturbances of their habitats expose these species to a high level of vulnerability (Gaston, 1994).
Based on these observations for the Brassicaceae family, and since urgent conservation strategies need to be implemented in order to preserve the native flora of Argentina (Morea, 2014), here we explored the geographic distribution of Argentinean crucifers using georeferenced species distributional data, to preliminary classify the species according to the IUCN threat categories, and analyzing patterns of richness, endemism, and threat in the different ecoregions and biomes of the country. Furthermore, elevation, annual mean temperature, annual precipitation, and aridity were compared for endemic vs. non-endemic and threatened vs. non- threatened species. Geographical range maps for all Argentinean native crucifers are also provided. This work seeks to contribute to the knowledge of the geographical distribution of the Argentinean flora and its conservation, complementing the Brassicaceae treatment of the Argentinean Flora (Al-Shehbaz, 2012a).
MATERIALS AND METHODS
Species occurrence data and threat assessments
Extensive botanical surveys of the Brassicaceae family were conducted in Argentina by the authors between 2008 and 2020. Collection sites were geographically referenced using the WGS 84 geodetic datum, and specimens collected were deposited in SI, with duplicates in several herbaria (herbarium acronyms following Thiers, 2020). Additionally, we studied specimens deposited in different herbaria, mainly from BA, BAA, BAB, BCRU, CORD, CTES, LIL, LP, MERL, MO, SF, and SI. Based on the most recent taxonomic treatments and phylogenetic analyses (Al-Shehbaz, 2012a; Salariato et al., 2013a; 2014; 2015a; 2015b; 2018; 2019; 2020a; 2020b; 2020c; Salariato & Al-Shehbaz, 2014; Salariato & Zuloaga, 2015), we included 162 taxa representing 160 species and two subspecies, corresponding to Menonvillea scapigera and Xerodraba patagonica, since the differentiation of these subspecies with the type subspecies has been supported by both morphological and molecular data (Salariato et al. 2012, 2015a). Records were also checked in the Flora of Argentina (Al-Shehbaz, 2012a) and the Documenta Florae Australis database www.darwin.edu.ar/iris) (last accessed March 2020), which represents the most up-to-date and comprehensive database of the Argentinean Flora (Zuloaga et al., 2019). For species and subspecies author names see Table 1. Occurrences were mapped using QGIS v3.14.16 ‘Pi’ (Quantum GIS Development Team, 2016) for visual inspection, and in cases of specimens with no GPS coordinates but exact locality names, records were georeferenced using Google Earth Pro v7.3.3.7786 (https://www.google.com/intl/en/earth/). We obtained 5258 data points corresponding to 35 genera and 162 species/ subspecies present in Argentina (species occurrences: min = 1, max = 285, mean=32) (Fig. S1, supplementary material).
Preliminary threat assessments for Argentinean species were based on the IUCN Red List categories and criteria v3.1 (IUCN, 2012) following the IUCN guidelines v14 (IUCN, 2019). Conservation status were determined under criterion B, which is based on geographic ranges (IUCN, 2012; 2019) and suitable for estimating conservation status when the distribution of taxa is only known from georeferenced herbarium specimens with limited information on abundance and potential continuing decline (Schatz, 2002; Nic Lughadha et al., 2018). For each species we used its extent of occurrence (EOO) (subcriterion B1; IUCN, 2019) calculated with a minimum convex polygon around occurrence points and clipped to the extent of Argentina. Area of occupancy (AOO) (subcriteria B2) was not used for categorization because it can lead to overestimation of extinction risk (when herbarium specimens are used occurrences are rarely sufficient to support calculation of AOO using the recommended 4 km2 grid cell; Nic Lughadha et al., 2018; IUCN, 2019). However, for species with less than three unique occurrences, for which EOO cannot be computed, AOO was reported. To estimate the number of locations for each species (condition “a”, a location is defined as a geographically or ecologically distinct area in which a single threat can rapidly affect all individuals of the taxon present; IUCN, 2019) we registered the number of cells of 0.1 arc-degrees (~100 km2) occupied by the occurrences. For condition “b” we evaluated vulnerability and potential deterioration of the habitat where species grow (case iii), considering ecoregions and biomes, and using data from field observations, herbarium labels, and literature, to characterize the environment where they inhabit (Brown et al., 2006; Brooks et al., 2006; 2015; INTA, 2009; Chehébar et al., 2013; Morello et al., 2018; Nanni et al., 2020). All IUCN parameters needed for assessments of taxa under criterion B were calculated using the package ConR v1.3.0 (Dauby et al., 2017; Dauby, 2020) implemented in R v4.0.2 (R Core Team, 2020).
Spatial analyses
To study the geographic patterns of richness and threat in a biogeographic context, we analyzed the distribution of four variables: a) number of species (richness), b) number of threatened species (considering as threatened species all species under CR, EN, and VU categories), c) number of endemic species (species only present in Argentina), and d) number of threatened endemic species. First, we registered numbers of these variables for political provinces, ecoregions, and biomes of Argentina; these last two eco-geographical classifications sensu Olson et al. (2001) and included in a shapefile provided by the WWF (https://www.worldwildlife.org/publications/terrestrial-ecoregions-of-the-world). Although these values were calculated at family level, they were also estimated for the different tribes (Al-Shehbaz, 2012b). In addition, the similarity of ecoregions in terms of their species composition was studied using Jaccard distances and agglomerative hierarchical clustering with the unweighted pair- group average (UPGMA) algorithm in the R packages vegan v2.5-6 (Oksanen et al., 2019) and cluster v2.1.0 (Maechler et al., 2019).
Alternatively, we explored spatial patterns for the four variables (richness, threat, endemics, and threatened endemics). Because the use of small cells would result in a finer and more detailed resolution, but at the same time increase the number of artificially empty cells where species occur but have not been recorded (Linder, 2001), we used grids at two different cell sizes, 1 and 0.5 arc-degrees (approx. 100 × 100 km and 50 × 50 km, respectively), to explore both distribution patterns at different scales and the robustness of the results to changes in the grid size. Analyses were conducted using the R packages raster v3.1.5 (Hijmans, 2020) and sp v1.4.2 (Pebesma & Bivand, 2005; Bivand et al., 2013). The protection status of the hottest cells was evaluated overlaying shapefiles of the protected areas in Argentina provided by the Biodiversity Information System of the National Parks Administration (SIB-APN) (https://sib.gob.ar/cartografia).
We also analyzed differences in elevation, annual mean temperature (BIO1), annual precipitation (BIO12), and aridity (AI) for endemic vs. non-endemic and threatened vs. non-threatened species. These variables can represent limiting climatic factors that constrain species distributions in the face of global warming and desertification (Jezkova & Wiens, 2016). After removing occurrences closer to 30 arc-seconds (~1 km), we extracted values of BIO1 and BIO12 for each occurrence point from the CHELSA v1.2 climatic dataset (Karger et al., 2017a; 2017b; climate.org/) at a resolution of 30 arc-seconds (~1 km2), while values of the annual aridity (AI) were obtained from the CGIARCS v2 database (Trabucco & Zomer, 2019) at the same resolution. Elevation data was extracted from specimen vouchers and by georeferencing. Values obtained for each variable were then used to calculate the mean of each species, and the Mann-Whitney U test was conducted to assess for differences between (1) endemic vs. non-endemic species and (2) threatened vs. non-threatened species, for elevation, BIO1, BIO12, and IA. Despite the fact these variables may exhibit phylogenetic signal, this is an explicitly non- phylogenetic test, since rather than an evolutionary signal, we are interested in whether the association exists at time zero given current conditions.
Finally, environments where species grow were categorized following the climate classification scheme for Aridity Index values (UNEP, 1997) (< 0.03: hyper Arid, 0.03 - 0.2: arid, 0.2 - 0.5: semi-arid. 0.5 - 0.65: dry sub-humid, > 0.65 humid). For each species we used the AI values corresponding to the 5th and 95th percentile to capture the niche breadth.
RESULTS
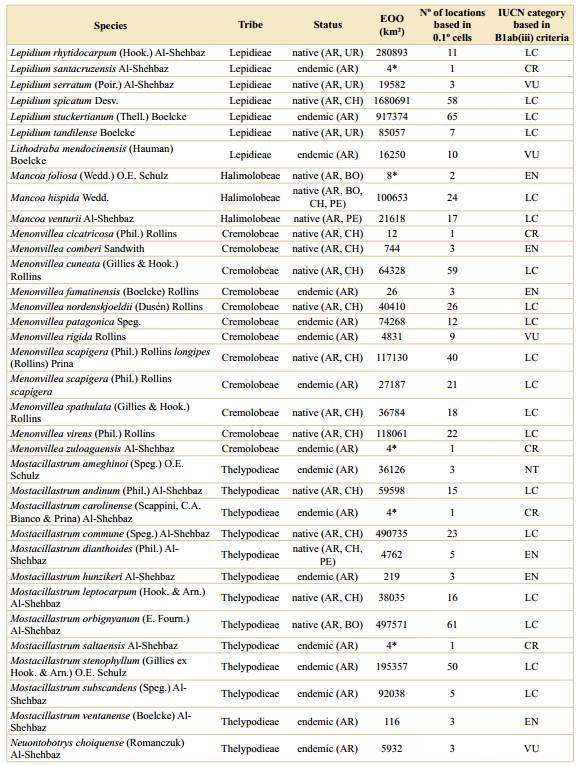
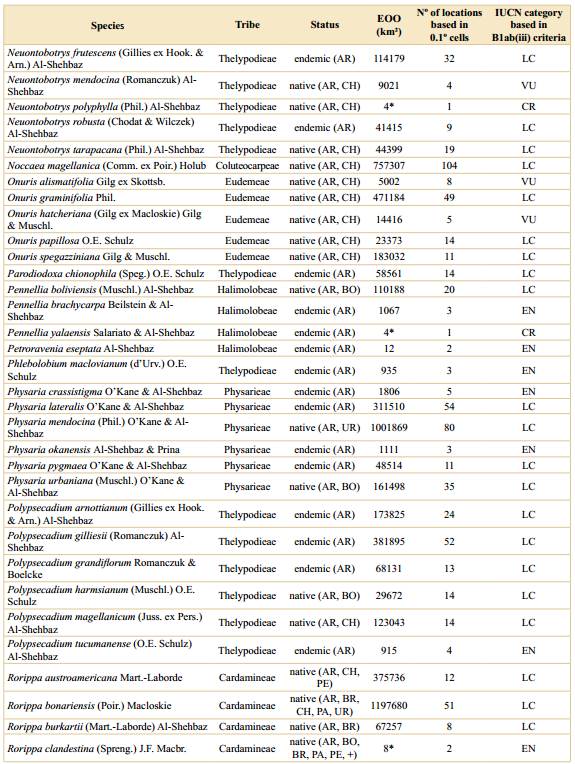
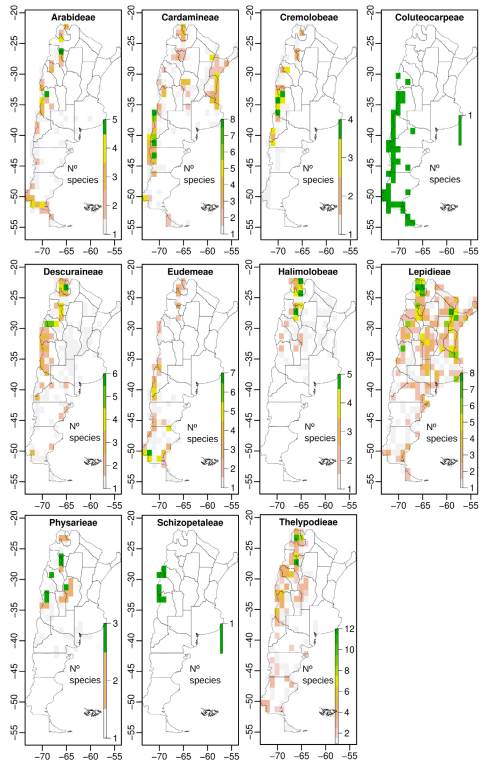
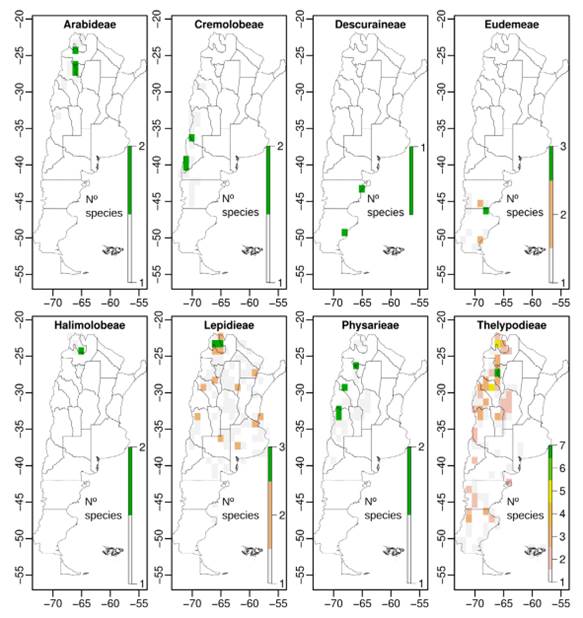
We compiled a total of 5258 presence records of Brassicaceae for Argentina, representing eleven tribes, 35 genera, 160 species, and two subspecies (Table 1). After the preliminary IUCN threat categorization we classified 97 species (60%) under Least Concern (LC), 7 (4%) as Near Threatened (NT), 19 (12%) as Vulnerable (VU), 25 (15%) as Endangered (EN), and 14 (9%) as Critically Endangered (CR) (Table 1, Fig. 1); counting a total of 58 species under threatened categories (36%) (geographic range maps for each species and the rationale for the threat categories are supplied in Appendix 1 http://www.ojs.darwin.edu.ar/index.php/darwiniana/article/view/922/1200 and Appendix 2 http://www.ojs.darwin.edu.ar/index.php/darwiniana/article/view/922/1201, respectively). Argentinian crucifers were present in all political provinces, ecoregions, and biomes (Tables S1, S2, and S3, Supplementary Material), with 58 species (~ 36%) and eight genera endemic to Argentina (Chilocardamum O. E. Schulz, Delpinophytum, Lithodraba, Parodiodoxa O. E. Schulz, Petroravenia Al-Shehbaz, Phlebolobium O. E. Schulz, Trichotolinum O. E. Schulz, and Zuloagocardamum) (Fig. 2) (Table 1). Of these 58 Argentinean endemics, 33 species (~ 57%) are classified in some threat category (Table 1).
When we analyze the number of endemics and threatened species by tribe, Thelypodieae presented the highest values, followed by Lepidieae and Eudemeae (Table S4, Supplementary Material).
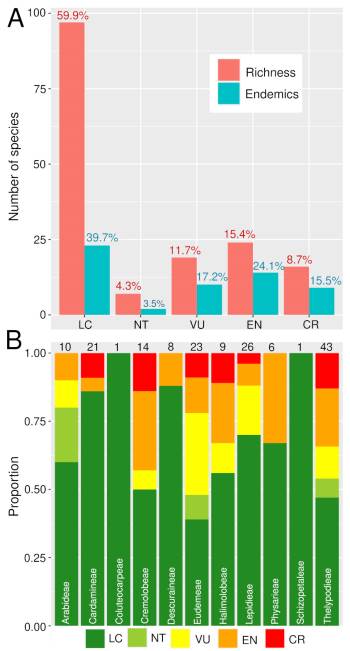
Fig. 1 Preliminary threat assessments for Argentinean crucifers were based on the IUCN red list categories and criteria. A, Barplots showing number of species (red) and endemics (blue) for each IUCN category. Numbers above the boxes correspond to the percentage of species included in each category with respect to the total number of species (red) and endemics (blue). B, Barplots showing the proportion of species of each tribe classified in the different categories. Numbers above the boxes correspond to the total number of species for each tribe. Color version at http://www.ojs.darwin.edu.ar/index.php/darwiniana/article/view/922/1199

Table 1 Preliminary threat assessments for Argentinean species based on the IUCN red list categories and criteria. EOO (extent of occurrence) was calculated with a minimum convex polygon around occurrence points and clipped to the extent of Argentina. *For species with less than three unique occurrences (EOO cannot be computed) area of occupancy (AOO) was reported. For the geographical ranges: AR= Argentina, BO= Bolivia, BR= Brazil, CH= Chile, PA= Paraguay, PE= Peru, UR= Uruguay, += other countries in northern South America. Geographic range maps for each species and the rationale for the preliminary threat categories are supplied in Appendix 1 and Appendix 2, respectively.
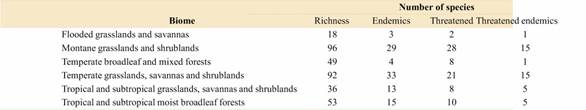
Overall, the province Jujuy included the greater numbers of species, threat categories, and Argentinean endemics (Table S1, Supplementary Material), followed by the provinces of Salta, Mendoza, Chubut, Santa Cruz, and Tucumán. A high number of endemics were also found in La Rioja and Catamarca. Regarding the species distribution along the Argentinean ecoregions, the Patagonian steppe and the Central Andean Puna were the regions with the highest number of species, threat categories, endemics, and even threatened endemics (Fig. 3, Table S2). Other ecoregions associated with different Andean environments, such as the Southern Andean steppe, the High Monte, and the Southern Andean Yungas, also showed high values for these variables (Fig. 3, Table S2). When we analyzed similarity of ecoregions in terms of their species composition using Jaccard distances we recovered three main clusters (Fig. 4): a cluster composed by the ecoregions associated with the Southern Andes (sensu Luebert & Weigend, 2014) (Patagonian steppe, Magellanic Subpolar forest, Valdivian Temperate forest, and Southern Andean steppe ecoregions), a cluster including ecoregions associated with the central Andes (Central Andean Puna, High Monte, Southern Andean Yungas, and Central Andean Dry Puna ecoregions), and a cluster including ecoregions from central-eastern Argentina (Low Monte, Dry Chaco, Espinal, Humid Chaco, Paraná flooded savanna, and Humid Pampas ecoregions)

Fig. 2 Some endemic genera of Argentina. A, Chilocardamun patagonicum. B, Delpinophytum patagonicum. C, Lithodraba mendocinensis. D, Parodiodoxa chionophila. E, Petroravenia eseptata. F, Zuloagocardamum jujuyensis. Except for Chilocardamum, all other genera are monospecific. Photos by Fernando O. Zuloaga (A, F), Diego L. Salariato (B, C, E), and Soledad Cuello (D). Color version at http://www.ojs.darwin.edu.ar/index.php/darwiniana/article/view/922/1199
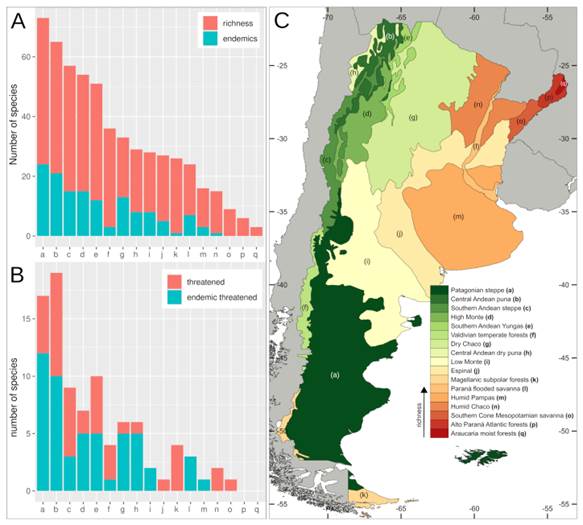
Fig. 3 Crucifer biodiversity throughout the ecoregions of Argentina. A, Barplot showing number of species (red) and endemics (blue) for the different ecoregions of Argentina. B, Number of threatened species (red) and threatened ende- mics (blue) for the different ecoregions of Argentina. C, Argentinean ecoregions colored according to their richness of crucifer species. Abbreviations: (a) Patagonian steppe, (b) Central Andean Puna, (c) Southern Andean steppe, (d) High Monte, (e) Southern Andean Yungas, (f) Valdivian Temperate forests, (g) Dry Chaco, (h) Central Andean Dry Puna, (i) Low Monte, (j) Espinal, (k) Magellanic Subpolar forests, (l) Paraná Flooded Savanna, (m) Humid Pampas, (n) Humid Chaco, (o) Southern Cone Mesopotamian Savanna, (p) Alto Paraná Atlantic forests, (q) Araucaria Moist forests. Color version at http://www.ojs.darwin.edu.ar/index.php/darwiniana/article/view/922/1199
Some species that characterize ecoregions of the Southern Andes and Patagonia belong to Alshebazia Salariato & Zuloaga, Cardamine L., Chilocardamum, Delpinophytum, Draba L., Litodraba, Menonvillea DC., Onuris Phil., Sarcodraba Gilg & Muschl., Sibara Greene, Stenodraba O. E. Schulz, Trichotolinum, and Xerodraba Skottsb. Alternatively, species of Central Andean ecoregions mainly belong to Alshehbazia, Aschersoniodoxa Gilg & Muschl., Brayopsis Gilg & Muschl., Cremolobus DC., Dictyophragmus O. E. Schulz, Mancoa Wedd., Menonvillea, Neuontobotrys O. E. Schulz, Mostacillastrum O. E. Schulz, Parodiodoxa, Pennellia Nieuwl., Polypsecadium O. E. Schulz, Physaria (Nutt. Ex Torr. & A. Gray) A. Gray, Weberbauera Gilg & Muschl., and Zuloagocardamum. Ecoregions from central-eastern Argentina were mainly characterized by species of Cardamine, Descurainia Webb & Berthel., Exhalimolobos Al-Shehbaz & C. D. Bailey, Lepidium L., Mostacillastrum, Physaria, and Rorippa Scop.; while humid ecoregions of northeastern Argentina were mainly occupied by species of Cardamine, Lepidium, and Rorippa. Regarding the species distribution across biomes, the “montane grasslands and shrublands” biome (Table S3) exhibited the greater numbers of species, followed by the “temperate grasslands, savannas and shrublands” biome, the latter including the greater number of Argentinean endemics.
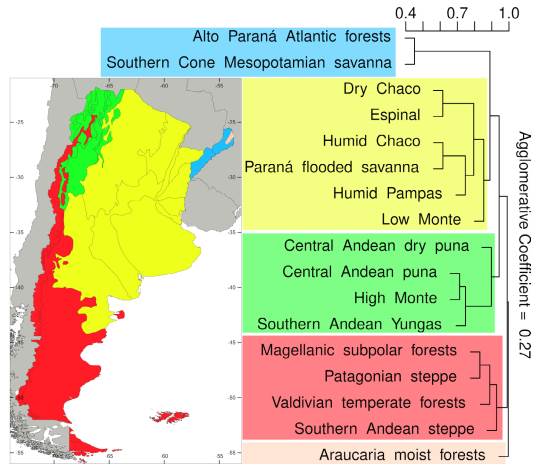
Fig. 4 Agglomerative hierarchical clustering for Argentine ecoregions using the UPGMA method and Jaccard distances based in their species composition (presence-absence data). The colored boxes denote the different clusters obtained, which are represented in the map on the right. Color version at http://www.ojs.darwin.edu.ar/index.php/darwiniana/article/view/922/1199
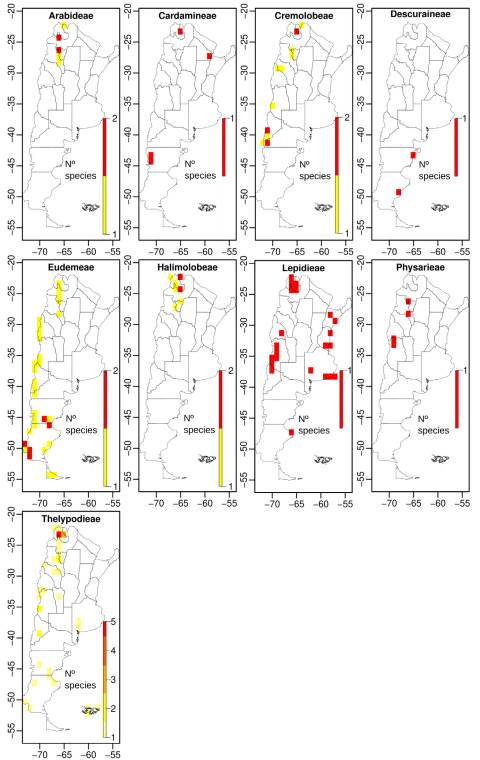
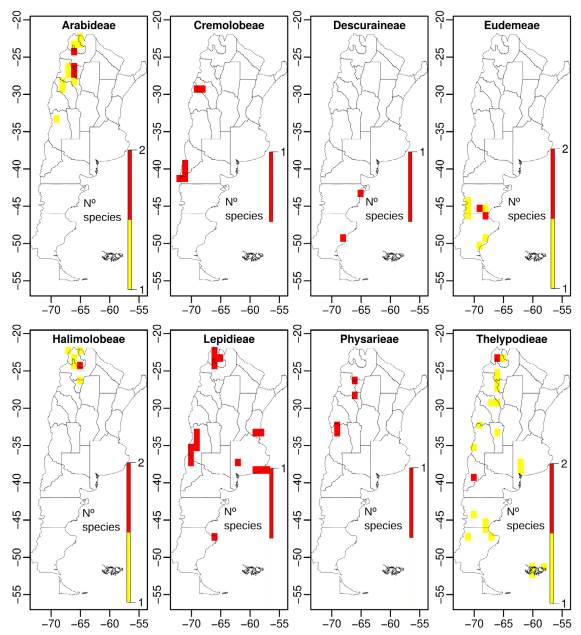
Spatial analyses using grids of 1 arc-degrees showed that diversity of the Argentinean Brassicaceae is concentrated along elevations of the Andes, the Precordillera, the internal mountain ranges (e.g., the “Sierras Pampeanas” and “Sierras transpampeanas” mountain systems), and lowlands of the Patagonian steppe (Fig. 5A). Species richness at tribal level showed that, except for tribes Cardamineae and Lepidieae, their distribution was mainly associated with the Andean regions and the Patagonian steppe (Figs. S2-S5, Supplementary Material).
Species richness was higher in cells located in: (c1) Jujuy province, Cochinoca and Humahuaca departments (~ 23.1ºS, 65.8ºW) along the Central Andean Puna ecoregion, and mainly associated with the highlands of “Sierra del Aguilar” and other secondary localities, such as “Tres Cruces, “Esquinas Blancas”, and “Humahuaca” (Figs. 5A, S6 from Supplementary Material); (c2) Tucumán province, Tafi del Valle department (~ 26.5ºS, 65.8ºW), primarily along the Central Andean Puna ecoregion and associated to the highlands of the “Cumbres Calchaquíes” mountain system (e.g., “Cerro Negrito”); (c3) Tucumán (depts. Tafi del Valle and Chicligasta) and Catamarca (dept. Andalgalá) provinces (~ 27.0ºS, 65.8ºW), along the Central Andean Puna, Southern Andean Yungas, and High Monte ecoregions, and related to elevations of the “Sierra del Aconquija” mountain system (e.g. “Cerro el Bolsón”, “Cerro Yutuyaco”); (c4) Catamarca province, Ambato department (~ 28.2Sº, 66.0Wº), in the High Monte and Dry Chaco ecoregions, and mainly associated with the highlands of the “Sierra del Manchao”; (c5) La Rioja province, Famatina department (~29.0ºS, 67.8ºW), within the High Monte ecoregion and associated with the mountain slopes of the “Sierra de Famatina”; (c6) north of the Mendoza province, Las Heras, Luján de Cuyo, Tupungato, and Tunuyán departments (~33.6S, 69.5W), along the Southern Andean steppe ecoregion, and mainly associated with the Andean highlands in “Cordón del Plata” (e.g., “Cerro del Plata”) and “Portillo de Piuquenes”; (c7) south of Mendoza province, Malargüe department (~35.1Sº, 70.2Wº), along the Southern Andean steppe ecoregion, and related to the Andean slopes along “Valle Hermoso” and the highlands of “Cerro Campanario” and “Paso Pehuenches”; (c8) Río Negro (dept. Bariloche) and Neuquén (dept. Los Lagos) provinces (~41.0Sº, 71.4Wº), along Valdivian Temperate forest and Patagonian steppe ecoregions, associated with the mountain slopes in the “Nahuel Huapi” national park (e.g., “Cerro Catedral”, “Cerro Negro”, “Cerro López”, “Cerro Ventana”, and “Cerro Ñireco”); (c9) South of Santa Cruz province, Lago Argentino and Güer Aike departments (~50.5Sº, 72.5Wº), along the Magellanic Subpolar forest and Patagonian steppe ecoregions, associated with elevations in the “Los Glaciares” National Park (e.g., “Cerro Buenos Aires” and “Cordon de los Cristales”), “El Calafate” (e.g., “Cerro Calafate” and “Cerro Huyliches”), and the “Vizcachas” river basin (e.g., “Cerro de la Virgen”, “Cerro Tridente”, “Cerro Pináculo”, and “Cerro Pan de Azúcar”) (Fig. 5A). Endemic species were more represented in cells from “Sierra del Aguilar” and “Humahuaca” (c1), “Cumbres Calchaquíes” (c2), “Sierra del Aconquija” (c3), “Sierra de Famatina” (c5), “Cordón del Plata” (c6), and a new cell located in the east of the Chubut (dept. Escalante) - Santa Cruz (dept. Deseado) provinces (45.9Sº, 68.4ºW) (c10), along the Patagonian steppe and primarily associated with the “Pampa del Castillo” locality (Fig. 5B).
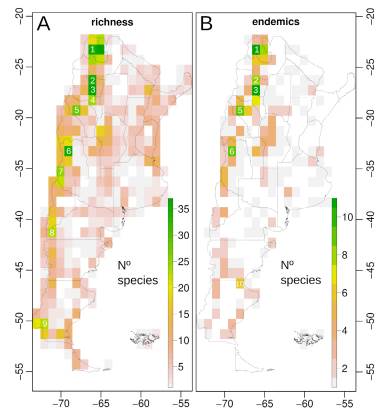
Fig. 5 Spatial patterns of biodiversity for Brassicaceae family in Argentina based on a spatial grid of 1 arc-degrees. A, Number of species (richness). B, Number of endemics (endemism). Cells with numbers are discussed in the text (see Results and Discussion sections). Color version at http://www.ojs.darwin.edu.ar/index.php/darwiniana/article/view/922/1199

Fig. 6 Spatial patterns of threat for Brassicaceae family in Argentina based on a spatial grid of 1 arc-degrees. A, Number of threatened species. B, Number of threatened endemic species. Cells with numbers are discussed in the text (see Results and Discussion sections). Color version at http://www.ojs.darwin.edu.ar/index.php/darwiniana/article/view/922/1199
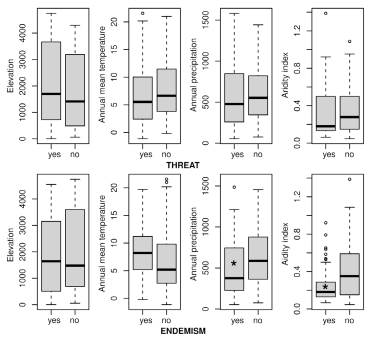
Fig. 7 Box-and-whisker plots showing values for elevation, annual mean temperature (BIO1), annual precipitation (BIO12) and aridity index (AI) between threatened vs. non-threatened species (above) and endemic vs. non-endemic species (below). Median (dark line), first and third quartile (boxes), and 95% confidence interval of median (whiskers). Boxes denoted by an asterisk indicate p ≤ 0.01 for the Mann-Whitney U test.
When analyzing the number of threatened species, cells from the Central Andean Puna of Jujuy (“Sierra del Aguilar” and “Humahuaca”) (c1) plus highlands along “Cerro Tuzgle”, “Abra Chorrillos” (Susques department) and “San Antonio de los Cobres” in Salta Province (Los Andes department) (~24.2ºS, 66.5ºW) (c11); “Cumbres Calchaquies” (c2), “Sierra del Aconquija” (c3), and “Valle Hermoso” (c7), exhibited the highest values. (Fig. 6A). Alternatively, when examining number of threatened endemics, cells of “Sierra del Aguilar” (c1), “San Antonio de los Cobres” (c11), “Cumbres Calchaquíes” (c2), and “Pampa del Castillo” (c10) presented the highest values (Fig. 6A). Overall, cells from “Sierra del Aguilar” (c1) and “Cumbres Calchaquíes” (c2) showed the highest values for all variables. In addition, the cells with the least coverage of protected areas (including national, provincial and biosphere reserves) were those corresponding to the localities of “Sierra de Famatina” in La Rioja (c5) , “Valle Hermoso” and “Paso Pehuenches” in southern Mendoza (c7); the “Vizcachas” river basin in Southern Santa Cruz (c9), and “Pampa del Castillo” (c10) in southeastern Chubut (Fig. S7, Supplementary Material).
Spatial patterns using cells of 0.5 arc- degrees recovered a greater richness for the same localities as the one-degree cells, but at a finer scale (Fig. S8A, Supplementary Material). Threatened species were concentrated in “Sierra del Aguilar” (c1), “Cumbres Calchaquíes” (c2), and “Sierra del Aconquija” (c3) (Fig. S9A, Supplementary Material), while endemic species were mostly recovered in these localities together with “Sierra de Famatina” (c6), and “Cordón del Plata” (c7) (Fig. S8B). Threatened endemics were concentrated in “Sierra del Aguilar” (c1) and “Cumbres Calchaquíes” (c2) (Fig. S9B).

Fig. 8 Niche preference associated with aridity levels for different species of Argentinean crucifers. A, Bar plots showing number of species (red), number of endemics (green), and number of threatened species (blue) that inhabit different aridity regimes. Environments where species grow were categorized following the climate classification scheme for Aridity Index values (UNEP 1997) (< 0.03: hyper Arid, 0.03 - 0.2: arid, 0.2 - 0.5: semi-arid. 0.5 - 0.65: dry sub-humid, > 0.65 humid). For each species we used the AI values corresponding to the 5th and 95th percentile to capture the niche breadth. B, Geographic distribution of aridity categories in Argentina. Color version at http://www.ojs.darwin.edu.ar/index.php/darwiniana/article/view/922/1199
Analysis using specimen occurrence values for elevation, annual mean temperature (BIO1), annual precipitation (BIO12), and aridity (AI) showed that threatened and non-threatened species were not differentiated for any of these variables (elevation: p=0.271, BIO1: p=0.118, BIO12: p=0.584, AI: p=0.126) (Fig. 7). Endemic and non-endemic species also did not show significant differences in elevation (p=0.207) and annual mean temperature (p=0.058), but they were differentiated for annual precipitation (p=0.004) and aridity (p<0.001), the endemic species showing lower mean annual precipitation and aridity (higher aridity) than native non- endemic species (Fig. 7). Finally, environment classification using the AI values (Fig. 8) showed that 97 species (out of 162 spp./subspp. present in Argentina; 60%), 42 threatened species (out of 58; 72%), and 48 endemic species (out of 58; 83%) occupy exclusively arid to semi-arid environments. Furthermore, and following this classification, all localities identified in the spatial analyses (see above, Fig. 5A) corresponded to arid-semi arid environments, except those included in the “Nahuel Huapi” and “Los Glaciares” national parks (cells C8 and c9, respectively) (Fig. 8).
DISCUSSION
Crucifers are well represented in South America by ca. 406 native species (ca. 10% of the family). These species inhabit a variety of different habitats along the biogeographical provinces of the North Andean Paramo, Puna, Prepuna, Altoandina, Yungas, and Subandean Patagonia (Cabrera & Willink, 1980; Morrone, 2018). These regions, together with the Atacama-Sechura Desert, the Chilean Matorral, and the Patagonian steppe, provide a high diversity of habitats for the diversification of numerous plant groups (Luebert & Weigend, 2014), including several lineages of this family (e.g., Toro-Núñez et al., 2013; Salariato et al., 2015b; 2016; 2018; 2020c). Events that occurred during the Neogene (e.g., Mid Miocene climatic optimum, uplift of the Andes, marine ingressions into the continent, changes in the Amazonian drainage system) and later during the Pleistocene (e.g., climatic oscillations, glacial- interglacial cycles, aridification, changes in the sea level and seashores) had enormous effects on the diversification of the local biodiversity (Hoorn et al., 2010; Antonelli & Sanmartín, 2011; Rull, 2011; Hazzi et al., 2018).
Regarding the Southern Cone of South America (Argentina, southern Brazil, Chile, Paraguay, and Uruguay), Argentina has the greatest number of vascular plant species (Zuloaga et al., 2019). Within the Brassicaceae, ca. 40% of the South American crucifers (160 species and two subspecies) are present in Argentina. Of these, 58 species were categorized here as threatened (VU, EN, or CR) (36%), however, when considering only endemism, more than half of the endemic species (57%, 33 spp.) are threatened. Species inhabit all environments and biogeographical regions of the country, although areas associated with the Andes and the Patagonian steppe contain most of the species. Arid to semi-arid environments in ecoregions of the Central Andean Puna, the Southern Andean steppe, the High Monte, and the Patagonian steppe harbor most of endemic and threatened species. These regions, corresponding to the “montane grasslands and shrublands” and “temperate grasslands, savannas and shrublands” biomes, are mostly associated with the South American Arid Diagonal (SAAD), a band of drylands that stretch diagonally across South America in a north-west to south-east direction (Garleff et al., 1991; Abraham et al., 2000; 2020). While humid regions in the country such as the Southern Andean Yungas or the Paraná Atlantic forest present high richness for vascular plants, main areas of endemism for vascular plants are found in the arid and semi-arid habitats of the Andes and the Patagonian steppe, with little endemism in the species rich humid forests (Zuloaga et al., 1999; Aagesen et al., 2012; Elías & Aagesen, 2019). Nevertheless, crucifer species were nearly absent in the unique hyper arid region of the country located in western Catamarca and Salta (the only species present was Neuontobotrys tarapacana), in contrast to the high number of endemic species that grow in the Atacama Desert of Chile (Al-Shehbaz, 2010; Salariato et al., 2013b; Toro-Núñez et al., 2013; 2015).
In our analyses, Central Andean Puna in northwestern Argentina (NOA) showed the highest values of richness, endemism, and threatened species, matching hotspots for richness and endemism. Hotspots of endemic vascular plants were reported for the arid ecoregions of NOA (Zuloaga et al. 1999; Aagesen et al., 2012; Godoy-Bürki et al., 2014), even though it has been reported that global hotspots of species richness are not congruent with endemism and threat (Orme et al., 205). Spatial congruence between richness and endemism has been detected in the NOA at a local scale and with specific plant families (Godoy-Bürki et al., 2014), including Poaceae (Aagesen al., 2009), Cactaceae (Ortega-Baes et al., 2012) and Brassicaceae (this study). Collections from localities from the NOA (e.g., Sierra del Aguilar in Jujuy and Cumbres Calchaquíes in Tucumán) correspond mostly to the Puna (between approx. 3500-4500 m) and the Altoandina (above 4500 m) biogeographical provinces, characterized by the montane shrublands (in the Puna) and grasslands (in the Altoandina) (Cabrera & Willink, 1980). Some of the native “non-endemic” threatened species in these regions are Alshehbazia friesii, Aschersoniodixa cachensis, Mostacillastrum dianthoides, Weberbauera densifolia, and Weberbauera herzogii. On the other hand, threatened endemics are Dictyophragmus punensis, Draba burkartiana, Lepidium jujuyanum, Pennellia brachycarpa, Pennellia yalaensis, Petroravenia eseptata, Physaria okanensis, Polypsecadium tucumanense, and Zuloagocardamum jujuyensis.
Mountains of Sierra de Famatina in La Rioja also exhibited high richness for crucifers, being this family the fifth in number of species after Asteraceae, Poaceae, Solanaceae, and Fabaceae (Barboza et al., 2016). Highlands of this area, included in the High Monte ecoregion and mainly associated with the Puna and Altoandina biogeographical provinces, encompassed many of the species found in the Central Andean Puna of Jujuy, Tucumán, and Catamarca, and also species distributed to the South such as Draba magellanica, Menonvillea scapigera subsp longipes, and Neuontobotrys frutescens. Highlands of Sierra de Famatina are one of the main diversity hotspots of Argentina, but also one of the most deficient areas in terms of conservation; the need to include protected areas to conserve its biodiversity has been reported previously (Godoy-Bürky et al., 2014; Barboza et al., 2016). In this region Menonvillea famatinensis is the only threatened endemic.
Another area recovered with a high number of species and Argentinean endemics corresponds to the Andean highlands in Northern Mendoza (e.g., Cordón del Plata). This region, located in the southern portion of the Southern Andean steppe ecoregion, includes species mainly from the Cuyan High Andean district (Cabrera, 1971) as Cardamine volckmannii, Menonvillea cuneata, M. scapigera, M. spathulata, Mostacillastrum leptocarpum, and Neuontobotrys robusta. Mountains in this region were identified as a center of endemism of the genus Senecio L. (Asteraceae) and a potential cradle for its speciation (Elías & Aagesen, 2019). Alternatively, along lowlands and valleys of this region, species from the Low Monte and Dry Chaco ecoregions, or Monte and Chaqueña biogeographical provinces (Cabrera & Willink, 1980), are also present, including Physaria lateralis, P. mendocina, Exhalimolobos pazense, and E. weddellii. Threatened endemics in this area are Lithodraba mendocinensis, Physaria crassistigma, and Sibara mendocina.
The southernmost highlands of the Andes also present a high diversity of crucifer species. These areas, included in the Altoandina biogeographical province (Cabrera & Willink, 1980) extend from central Mendoza southward to northern Tierra del Fuego, where high- altitude environments are found as islands on the mountain peaks of the Andes (Cabrera & Willink, 1980; Padró et al., 2020). Southwards, species are generally shared with the Chilean Andes, so the number of endemics is lower than in the Central Andes. Mountains at Southern Mendoza and Northern Neuquén exhibits high species richness, and threatened species in this region are Lithodraba mendocinensis, Menonvillea cicatricosa, Neuontobotrys mendocina, and Stenodraba chillanensis. Eastern mountains of the Nahuel Huapi National Park (~ 41ºS latitude) also harbour a high number of species and two threatened endemics: Menonvillea comberi and M. rigida. Since precipitation decreases eastward, eastern mountains usually have a greater richness associated with their greater aridity. Eastern peaks have a higher diversity of environments, from dry slopes to humid forest spots, allowing the growth of both xeric and montane species, whereas western slopes lack arid environments. Furthermore, the snow cover is thinner and less persistent on the eastern mountains, while the higher amount of persistent snow covering some western peaks reduces the available area for plants (Ferreyra et al., 1998; Speziale et al., 2010).
High richness was also recovered in mountains of southern Santa Cruz, especially those located in the Vizcachas river basin. In this region, the Altoandina biogeographical province begins around 500-1000 m a. s. l., while in northern Patagonian Andes Altoandina begins above 2000 m a. s. l. (Cabrera & Willink, 1980). Threatened species in this region are Alshehbazia hauthalii, Onuris hatcheriana, and Sarcodraba dusenii.
Arid lowlands in the southern portion of the Patagonian steppe also harbors a high number of species, but unlike species of the southern Andean slopes, most Patagonian species are endemic, with the steppe including even more endemics than the Central Andean Puna in the NOA. The Patagonian steppe, which acquired their current extensions at the end of the Neogene (Iglesias et al., 2011), is characterized by its dry and temperate-cold climate, with strong winds, snow during the winter, and frost almost all year (Cabrera & Willink, 1980). Threatened endemics are mainly distributed along southeastern Chubut and eastern Santa Cruz, being some of these areas identified of conservation priority (Chehébar et al., 2013). Among threatened endemics are Chilocardamum longistylum, C. onuridifolium, Delpinophytum patagonicum, Lepidium santacruzensis, Sarcodraba subterranea, Trichotolinum deserticola, Xerodraba colobanthoides, X. glebaria, and X. monantha.
Other extra-Andean ecoregions, i.e. the Low Monte, the Dry Chaco, the Espinal, or the Humid Pampas showed considerably lower richness and endemism for the family; nevertheless, these regions also harbor threatened endemic species. For example, humid pampas in Entre Rios province includes Lepidium burkartii, while in Buenos Aires Lepidium hickenii and Mostacillastrum ventanense grow, the latter is distributed along the Ventania Mountain System. In the Comechingones biogeographical province in San Luis inhabits Mostacillastrum carolinense. This biogeographical province, located in the Dry Chaco ecoregion, is characterized by moderate-altitude grasslands located in the Dry Chaco ecoregion, which, due to present a high number of endemics, has its own biogeographical categorization (Martínez et al., 2017).
Knowledge about biodiversity at a global level remains incomplete either because there is still uncertainty about the identity of the species living on Earth and their phylogenetic relationships (the Linnean and the Darwinian shortfalls, respectively), and because geographical distributions and ecological niches of most species are poorly understood and contains many gaps (the Wallacean and Hutchinsonian shortfalls, respectively) (Whittaker et al., 2005; Bini et al., 2006; Oliveira et al., 2016). These shortfalls are scale dependent, both on spatial and temporal dimensions, revealing different patterns of diversity by varying the scale of analysis (Whittaker et al., 2005). Using herbarium data, richness can be biased for some localities because they were more intensively sampled (due for example, to easier access to high mountain areas) generating a pattern of spatially biased sampling effort (Oliveira et al., 2016). Some poorly explored areas, as the high peaks of the southernmost Andes or the southern portion of Patagonia, need greater sampling effort in future field trips. Alternatively, most of the Argentinean species were relatively recently reviewed in taxonomic revision and in the Argentinean Flora (Al-Shehbaz, 2012a). However, for some species complexes, [e.g. Cardamine, Descurainia, Lepidium, Physaria] the taxonomic status of some populations and species limits are still unclear (e.g., Salariato & Zuloaga, unpublished). Molecular studies with species-delimitation analyses that update and review species’ geographic distribution, coupled with their taxonomic status, are necessary to provide basic information for biogeographic, ecological, systematic, and conservation studies (Riddle & Hafner, 1999; Agapow et al., 2004; Isaac et al., 2004; Dayrat, 2005; Sukumaran & Knowles, 2017). Furthermore, molecular phylogenies including nearly all South American species are urgently needed in order to estimate useful metrics for conservation, as the evolutionary distinctiveness (ED) (Isaac et al., 2007; Tucker et al., 2012) and the evolutionary distinct and globally endangered (EDGE) (Isaac et al., 2007; Redding et al., 2010) indices for species, or the phylogenetic diversity (PD) indices (Swenson, 2014; Tucker et al. 2017) for different regions of Argentina.
Mountain ecosystems of Argentina, along with the Patagonian steppe, are important centers of regional diversity and endemism (Zuloaga et al. 1999). Arid and semi-arid environments in the Andean region are home to many endemic and threatened species (Zuloaga et al., 1999; Aagesen et al., 2012; Godoy-Bürky et al., 2014; Elías & Aagesen, 2019). However, and despite the relevance of habitat quality for biodiversity and ecosystem service policies, mountain environments are poorly known, making it difficult to establish rational conservation priorities on political agendas. Global warming together with cattle grazing, erosion, industrial activities, mining, and contamination of water supplies, are major threats in the region (Dinerstein et al., 1995; Aagesen, 2000; Gonzales, 2009). Therefore, effective conservation strategies are urgently needed to adequately preserve the endemic flora. Global and regional classification of species using the IUCN Threatened Species Categories, together with the analysis of spatial patterns of biodiversity constitute the first step to propose conservation strategies (Nic Lughadha et al., 2018; Rejmánek, 2018; Humphreys et al., 2019; Stévart et al., 2019). Results presented here on spatial patterns and the threat status of the Argentinean crucifers seeks to complement the information published in the flora of Argentina (Al-Shehbaz, 2012a), and hopes to be a useful contribution for future analyses of plant biodiversity patterns in the Southern Cone, as well as for the development of conservation plans.












 uBio
uBio