Introduction
Threats to biodiversity, especially in the tropics, urgently require knowledge of flower biology (Endress, 1994). According to this author, the assessment for actions for the conservation of this biodiversity is done primarily through knowledge of phylogenetic history and interactions between animals and plants.
The species of Passifloraceae are widely distributed in tropical and subtropical regions of the world (Feuillet & MacDougal, 2007). There are about 630 species of Passifloraceae distributed among 18 genera (Judd et al., 2009). The genus Passiflora L. has approximately 530 species, most of which are native to the American continent and 24 species that occur in South Asia, Australia and New Zealand (MacDougal & Feuillet, 2004; Feuillet & MacDougal, 2007). The genus Passiflora stands out with high diversity in Brazil with about 157 species, of which 87 are endemic (Cervi, 1997; Bernacci et al., 2020).
Forest fragmentation resulting from human occupation causes modifications to the landscape and the loss of native vegetation, thereby changing the normal functioning of ecosystems (Fischer & Lindenmayer, 2007). The loss of genetic diversity is significant for native species of Passiflora as they are forest edge plants that directly suffer the effects of forest fragmentation (Ferreira, 2005). The species Passiflora hatschbachii Cervi, P. imbeana Sacco, P. ischnoclada Harms, P. margaritae Sacco, P. saccoi Cervi, P. setulosa Killip and P. urubiciensis Cervi are on the official list of endangered species of Brazilian flora (Brasil, 2014), while other Passiflora species are already listed as threatened in several Brazilian states, whose endemism makes them more vulnerable.
Small populations of plants resulting from the fragmentation of ecosystems are subject to processes of genetic drift and inbreeding depression, which can, in some cases, lead to the extinction of populations and, finally, species, including other species with which they interact (Ellstrand & Elam, 1993; Traveset et al., 2017). Plant-pollinator relationships present different degrees of interdependence and can be subject of cascading extinction events. This interdependence, which ranges from extremely specific to very generalist interactions, makes this mutualistic relationship an important model for ecological, evolutionary and conservation studies (Kearns et al., 1998).
Studies of floral and reproductive biology have already been carried out with several species of the genus Passiflora. The literature reports many species that present genetic self-incompatibility so they do not produce seeds when self-pollinated, such as Passiflora mucronata Lam. (Sazima & Sazima, 1978), P. vitifolia Kunth (Snow, 1982), P. miersii Mart. (Koschnitzke & Sazima, 1997), P. alata Curtis, P. galbana Mast. (Varassin et al., 2001), P. speciosa Gardner (Longo & Fischer, 2006), P. pohlii Mast. (Faria & Stehmann, 2010) and P. actinia Hook (Varassin et al., 2018). Other species, such as Passiflora amethystina J. C. Mikan (Koschnitzke & Sazima, 1997) and P. lutea L. (Holland & Lanza, 2008), show partial genetic self-incompatibility, producing fewer fruits and seeds when self-pollinated. On the other hand, other species are fully self-compatible and produce seeds and fruits when self-pollinated, such as Passiflora foetida L. (Amela García & Hoc, 1998), P. suberosa L. (Acioli, 2003) and P. capsularis L. (Faria & Stehmann, 2010). As most studied species of Passiflora are highly dependent on pollinator animals, they are more susceptible to the effects of forest fragmentation.
Passiflora morifolia Mast., a native species of Brazil, is popularly known as purple passion fruit, hairy passion fruit or curly passion fruit (Bernacci, 2003). The species also grows in Mexico, Guatemala, Venezuela, Colombia, Ecuador, Perú, Bolivia and Argentina (Deginani, 2001; Milward de Azevedo & Baumgratz, 2004). Despite its wide distribution, populations of the species are disjunct and generally with few individuals. Passiflora morifolia has several characteristics that favor its use as an ornamental plant such as intermediate size, lush foliage, showy flowers, and the capacity for growth in pots and indoor environments (Ulmer & MacDougal, 2004). In addition to being a conservation strategy, the ornamental use of native species and hybrids of the genus Passiflora represents a market with great capacity for expansion in Brazil and other Latin American countries, such as Argentina (Abreu et al., 2009; Bugallo et al., 2014).
Considering the current scenario of fragmentation of Brazilian ecosystems and the importance of species conservation, this work aimed to contribute to the knowledge of the reproductive biology of Passiflora morifolia, growing in a fragment of Araucaria Moist Forest in the state of Paraná, Brazil. For this, the phenology, floral biology, breeding system and floral visitors of P. morifolia were analyzed.
Materials and methods
Study area
Observations were carried out from January 2019 to April 2021 in an area of natural occurrence, characterized by the presence of several fragments of Araucaria Moist Forest, encompassing a total of 761.6 hectares, in the municipality of Campina do Simão (25° 05’ 42” S, 51° 49’ 48” W), located in the state of Paraná, South Brazil. According to Koppen’s classification, the area has a Cfb-type climate (Nitsche et al., 2019). Four groups of three to 11 individuals of Passiflora morifolia were identified on the edges of forest fragments.
Floral biology
Floral biology analyses included observations of the morphology of the perianth, gynoecium, androecium and corona. Floral longevity was studied by direct observation of the development of previously-marked pre-anthesis flowers (N= 20). Observed characters included the movement of sepals, petals and corona, the movement of filaments and styles, the release of pollen from the anthers, and the receptivity of stigmas before, during and at the end of anthesis.
Stigma receptivity was verified in a total of 30 emasculated flowers, 10 at the beginning of anthesis (4 a.m.), 10 at full anthesis (6 a.m.) and 10 at the beginning of senescence (12 p.m.). A 6 % hydrogen peroxide solution was applied on the stigma surface to identify the activity of peroxidase enzymes (Galen & Plowright, 1987; Kearns & Inouye, 1993; Dafni & Maués, 1998).
Pollen grain viability was determined by an adaptation of the acetic carmine staining technique (Kearns & Inouye, 1993). Evaluations were performed with pollen from anthers of 10 floral buds in pre-anthesis, 10 flowers in anthesis at 6 a.m. and 10 flowers in anthesis at 11 a.m. Percentage of viable pollen grains was obtained by counting, under a light microscope, 200 grains in three replications for each anther.
Osmophore presence was verified in 20 flowers at the time of greatest odor release, between 5 and 7 a.m., using the neutral red dye technique (Dafni, 1992).
Nectar production rate was evaluated by emptying all nectar from the same set of flowers at two-hour intervals, from 5 a.m. to 11 a.m. (N= 20). The flowers were bagged in pre-anthesis and remained so during the evaluation intervals, as proposed by Kearns & Inouye (1993). Nectar was collected with the aid of 2 and 10 µL microcapillary tubes. The concentration of total solutes in nectar was determined using a portable refractometer with a scale from zero to 50 %.
Breeding system
The reproductive strategy of the species was determined by the following treatments: free pollination (N= 44), spontaneous self-pollination (N= 36), manual self-pollination (N= 41), manual cross-pollination (N= 45) and agamospermy (N= 20). Treatments followed the procedures suggested by Dafni (1992) and Kearns & Inouye (1993).
In the days following the treatments, all tested flowers, with or without ovary development, were bagged with microperforated transparent polypropylene plastic bags (15 x 20 cm) until fruit ripening and harvesting. Fruit set was calculated as number of developed fruits / number of flowers used in each treatment. The seed set was calculated as average number of seeds per fruit / average number of ovules per flower, by each treatment. The average number of ovules was determined by cutting the ovary wall of 20 flowers and observing and counting the ovules under a stereomicroscope; this number was used in the seed set and pollen ovule ratio calculations.
Breeding system according to pollen/ovule ratio was assigned following the classification of Cruden (1977), by counting the number of pollen grains and ovules per flower. The number of pollen grains produced per flower was estimated from the collection of intact anthers from pre-anthesis flower buds (N= 10). Two anthers were removed from each flower bud for sampling; were placed in Eppendorf tubes with 500 μL of 85 % lactic acid solution and macerated to release the pollen. The number of pollen grains was counted in four samples of 1.5 µL from each tube prepared on reticulated slides for observation under a light microscope. The number of pollen grains per anther was determined by multiplying the average number of pollen of each sample with the volume of lactic acid in the solution (500 μL), and dividing this value by the product between the volume of lactic acid in the sample (1.5 μL) and the number of anthers in each tube (2).
To calculate the number of pollen grains per flower, the average pollen grain estimate per anther was multiplied by the number of anthers per flower (5) (Kearns & Inouye, 1993).
Floral visitors
Floral visitors were observed and captured throughout the period of anthesis on five non consecutive days, for a total of about 60 hours of observation. Potential pollinators were determined by observing the size, morphology and behavior of visitors in the flowers. All observed floral visitors were captured using an entomological net and a 250ml Erlenmeyer flask. Captured individuals were transferred to killing tubes containing ethyl acetate and, in the laboratory, pinned, tagged (with date and time of collection) and separated into morphotypes for identification.

Fig. 1 Passiflora morifolia flower. Abbreviations: a, androgynophore; ant, anther; c, nectar chamber; cor, corona filaments; li, limen (arrow); n, nectary annulus (arrow); op, operculum (arrow); ovr, ovary; pet, petal; sep, sepal; st, stigma; sty, style. Color version at http://www.ojs.darwin.edu.ar/index.php/darwiniana/article/view/1015/1255
Statistical analysis
The variables of pollen viability and seed set were analyzed with ANOVA and Tukey’s separation of means test. The nectar production rate and concentration variables were analyzed with repeated measures ANOVA. All tests were performed with a significance level of 0.05, using the JASP program (Love et al., 2019). In the description of measurements and values, means and standard deviations are mentioned.
Results
Flowering period
Passiflora morifolia flowered mainly during the austral summer, from January to March 2019 and from December 2020 to March 2021, but not from January to March 2020.
Floral morphology
Flowers of P. morifolia are generally solitary and axillary, but floral buds can occur in pairs, opposite on the stem and opening one flower at a time or, in some cases, both flowers opening on the same day. The positioning of the flowers is variable and can be horizontal, vertical or oblique.

Fig. 2 Anthesis of Passiflora morifolia. A, beginning of anthesis. B, flower partially open with stigmas above the ovary and the anthers, already dehiscent, facing the corona. C, fully open flower with stigmas and anthers positioned at the same level. D, flower in early closing with alteration of sepals, petals and corona position, curved filaments with anthers directed towards the androgynophore and styles beginning to move to position the stigmas above the level of the ovary. E, flower partially closed with the styles positioning the stigmas above the level of the ovary. F, flower closed at the end of anthesis. Scale bars = 1 cm. Color version at http://www.ojs.darwin.edu.ar/index.php/darwiniana/article/view/1015/1255
The flowers have a floral receptacle measuring about 0.90 ± 0.07 cm in diameter; the five sepals are white adaxially and light green abaxially and measure 1.50 ± 0.07 cm in length; the five petals are white and membranous and measure 0.90 ± 0.08 cm in length; the corona is uniseriate and possesses 55 ± 1.82 filaments white-purple-colored at the base and white tips, about 0.80 ± 0.10 cm length; the operculum is plicate and purplish; the limen, located in the basal part of the androgynophore, combines with the operculum to protect the nectariferous chamber. The androgynophore has a purplish base and supports the androecium, with five stamens, and the gynoecium, with three styles, each with a stigma (Fig. 1).
Floral biology
Anthesis started around 2 a.m. with the gradual opening of the calyx and the corolla such that it was possible to visualize the reproductive structures (Fig. 2A) at 3 a.m. and the complete opening of the flower around 4 - 5 a.m. The flowers remain in full anthesis until about 12 a.m., when the movement of the sepals, petals and corona starts to close the flower until around 5 - 6 p.m. on the same day and does not open again.
At the beginning of anthesis, the anthers were positioned vertically beside the ovary, while the stigmas were facing up and away from it. Still during flower opening, there was a deflection of the filaments and positioning of the anthers with their slits facing the base of the corona and the nectariferous chamber (Fig. 2B). Dehiscence of the anthers occurred during the movement of the filaments in some flowers, while at the end of this movement in others. About an hour after the anther positioning described (approx. at 5 a.m.), style deflection placed the stigmas at the same level as the anthers (Fig. 2C). In some flowers, the stigmas were positioned so close to the anthers that they touched them, performing spontaneous transfer of pollen grains to the stigmas. The distance between the anthers and stigmas in such position and the corona filaments was quite variable, ranging 0.4 and 0.7 cm, depending on the moments during anthesis; this is the space used by floral visitors to access the nectariferous chamber when they land on the corona. At the beginning of floral senescence, the filaments curved downwards and positioned the anthers towards the androgynophore, while the styles positioned the stigmas above the ovary again (Fig. 2D-F).

Fig. 3 Results of floral biology colorimetric tests for Passiflora morifolia. A, presence of osmophores in the sepals and filaments of the corona with the neutral red test (arrows). B, viable pollen (left) and unviable pollen grain (arrow) tested with 2% acetic carmine. Color version at http://www.ojs.darwin.edu.ar/index.php/darwiniana/article/view/1015/1255
Stigmas were receptive throughout anthesis, even before the initial movement of the styles. The hydrogen peroxide solution test was positive at all times tested, with greater intensity at 6 a.m.
The flowers presented a sweet odor at the beginning of anthesis, around 4 - 5 a.m. Odor remained intense until about 7 - 8 a.m., gradually decreasing until the end of anthesis. Neutral red solution test showed the presence of osmophores, markedly in the sepals and corona filaments and less in the petals, as intensely colored dots mainly on the margins and adaxial surface of the sepals and on the corona tips that coincided among all the evaluated flowers (Fig. 3A).
The acetic carmine test showed that the five anthers produced viable pollen grains (Fig. 3B). Pollen showed greater viability prior to anthesis (89.6 ± 4.6 %), which differed significantly from the pollen collected at the time of full anthesis (84.2 ± 4.1 %) and at the beginning of floral senescence (82.0 ± 3.4%) (F[2; 147] = 44.707; p < 0.001).

Fig. 4 Nectar characteristics throughout anthesis of Passiflora morifolia. A, volume. B, concentration of total solutes.
The volume of secreted nectar varied significantly throughout anthesis (F[1.7; 32.9] = 55.302; p < 0.001). Nectar was produced in small quantities (1.6 ± 0.7 µL) at flower opening (approx. at 5 a.m.), the greatest volume collected was between 6 - 7 a.m. (18.1 ± 7.0 µL), which differed significantly from those collected between 8 - 9 a.m. (10.8 ± 4.7 µL) and 11 a.m. (3.9 ± 3.3 µL) (Fig. 4A).
The concentration of total solutes in nectar varied significantly during anthesis (F[3; 57] = 332.820; p < 0.001). The highest concentration was detected at the beginning of anthesis (35.0 ± 1.7 %) and there was a subsequent decrease in concentration, with the lowest value at 11 a.m. (15.6 ± 3.4 %) (Fig. 4B).
Breeding system
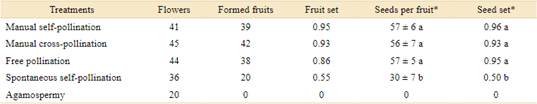
There was no fruit formation in the agamospermy treatment, only in the other treatments (Table 1).The development time, from pollination to maturation of fruits was between 28 and 42 days, regardless of treatment. Fruit abortion was recorded in all treatments, being more pronounced in spontaneous self-pollination, where the fruit set was 0.55, while in the treatments of free pollination and manual pollination, the fruit set was higher than 0.85.
Controlled pollination treatments differed significantly in the number of seeds formed per fruit (F[3; 138] = 56.728; p < 0.001). The lowest mean number of seeds was for the spontaneous selfpollination treatment, whereas the highest means were for the treatments of free pollination and manual pollination.
The seed set showed significant differences between treatments (F[3; 136] = 78.259; p < 0.001), with the lowest value for spontaneous selfpollination (0.50) and the highest for manual self-pollination (0.96), free pollination (0.95) and manual cross-pollination (0.93).
The average number of pollen grains per flower was 28,118.06 ± 5,598.93 and the average number of ovules was 59.8 ± 4.7, giving a pollen/ovule ratio of 470.2.
Floral visitors
The survey of floral visitors comprised 164 individuals, mainly of the order Hymenoptera, including three bee families (Table 2).
Ptiloglossa willinki (Colletidae) and Megommation insigne (Halictidae) were the first floral visitors (around 5 - 6 a.m.), and were seen mainly before sunrise, with a single individual of P. willinki being sampled around 9 a.m. These bees were nectar foragers, as their movements were towards the nectariferous chamber. When collecting nectar, these bees touched with their thorax and head, the anthers and/or the stigmas, which were already situated at the same level (Fig. 5A). Other relevant data regards the speed with which these bees moved within and between flowers, being able to collect resources from several flowers per minute.
Small bees of the genus Plebeia (Apidae) were the most frequent floral visitors, visiting after 9 - 10 a.m. These bees were mainly seen collecting nectar and/or pollen and walking on the corona, anthers and stigmas (Fig. 5B). Individuals of Trigona spinipes (Apidae) visited after 8 - 9 a.m. and were also seen collecting nectar and/or pollen. According to movement in the flower structures, they occasionally touched the anthers and stigmas with the thorax and head (Fig. 5C). The bees Augochlora sp., A. amphitrite and A. cupreola (Halictidae) were seen collecting nectar, but without touching the anthers and stigmas.

Fig. 5 Floral visitors of Passiflora morifolia. A, Ptiloglossa willinki. B, Plebeia sp. (arrow). C, Trigona spinipes (arrow). D, Holhymenia clavigera. Scale bars = 1 cm. Color version at http://www.ojs.darwin.edu.ar/index.php/darwiniana/article/view/1015/1255
Individuals of Holhymenia (Hemiptera, Coreidae), were nectar robbers, accessing to the nectariferous chamber externally puncturing the floral receptacle (Fig. 5D). The lepidopterans also collected nectar, but without touching the anthers or stigmas. The ants were constant floral visitors, sometimes collecting nectar, but not touching the reproductive parts.
Most floral visitors were observed when flowers were able to donate and receive pollen, with stigmas and anthers positioned at the same level (Fig. 6). They were not observed before the positioning of the anthers and stigmas, at the beginning of anthesis. As the flower closed, and the anthers were directed towards the androgynophore and the stigmas towards the position above the ovary, the number of visits decreased.
Discussion and conclusions
Passiflora morifolia presents flowers with attributes that favor pollination by nocturnal and crepuscular bees, such as opening at night, intense release of odors and production of more concentrated nectar in the first hours of anthesis, in addition to the positioning of anthers and stigmas in the path of main pollinators in the visiting time. Flowers open about an hour before the first visits of nocturnal bees (Megommation insigne) and crepuscular bees (Ptiloglossa willinki) and remain open during the following morning, when they receive visits from diurnal bees, mainly Plebeia droryana. P. remota and Trigona spinipes.
Results confirmed that P. morifolia has a self-compatible and mixed breeding system, as previously reported by Soares et al. (2015) for this species in another environment. The species possesses different adaptations for seed production: cross-pollination and self-pollination. This trait could be advantageous in conditions of uncertain pollination (Baker, 1955; Barrett, 2002), such anthropized forest fragments.
Stigmas were receptive and pollen available at the beginning of anthesis, but the positioning of the first ones resulted in temporally functionally male flowers. According to Amela García & Hoc (1998, 2011), this movement of filaments and styles develops a temporal barrier in which receptive stigmas and pollen are not in the path of pollinators at the same time for all of anthesis, characterizing a type of herkogamy. However, in P. morifolia, no floral visitors were observed during the style deflection process at the beginning of anthesis. Although herkogamy was observed, the first visits by effective pollinators occurred predominantly when flowers could donate and receive pollen (functionally hermaphroditic). Janzen (1968) and Faria & Stehmann (2010) reported that style movement in species of Passiflora may have ecological significance by hindering spontaneous self-pollination and facilitating cross-pollination in self-compatible species. This seems to be the case for P. morifolia, for which, despite its self-compatibility, the variable positioning of the stigmas resulted in a minor number of fruits and seeds in the spontaneous self-pollination treatment.
Previous studies of other species of the genus, such as P. suberosa (self-compatible) and P. speciosa (self-incompatible), showed that in addition to the spatial separation of reproductive structures, the flowers exhibit dichogamy and release pollen before stigmatic receptivity (Acioli, 2003; Longo & Fischer, 2006). These different strategies, present both in the pollination of self-compatible and self-incompatible species, demonstrate the importance of pollinators in the reproductive process of species of Passiflora. According to Barrett (2002), dichogamy and herkogamy are common among Angiosperms and are considered mechanisms to prevent selfpollination and promote cross-pollination.
Taking in account the pollen/ovule ratio classification proposed by Cruden (1977), P. morifolia is a facultative xenogamous species. This characterization agrees with the results of the breeding system experiments: high seed set were observed (with values above 0.9) in the manual self-pollination, manual cross-pollination and free pollination treatments. The absence of significant differences among these treatments, in contrast to the spontaneous self-pollination treatment, indicates that the action of a pollen vector (either a true pollinator or a simulated one) transferring either autogamous or xenogamous pollen increases seed set. The importance of biotic pollination also is evident for P. morifolia in the fruit set.
The greater volume and concentration of nectar secreted during the first hours of anthesis indicates adaptation to nocturnal and crepuscular bees. Individuals of Ptiloglossa willinki were observed always moving towards nectaries, and during the foraging period, these bees touched anthers and stigmas with its head and thorax. These bees are known for their nocturnal and crepuscular habits (Moure, 1987; Michener, 2007); they are large bees, with a body length between 15 and 20 mm (Michener, 2007). Like with P. willinki, individuals of Megommation insigne were also observed moving towards the nectaries and always touching anthers and stigmas. Megommation insigne is considered an obligatory low-light species (Wcislo & Tierney, 2009), with medium sized body, between 12 and 15 mm in length (Michener, 2007), and were able to transfer pollen during their visits to P. morifolia flowers.
According to Warrant (2008), the evolution of certain bee species to forage in flowers with nocturnal anthesis may be related to decreased competition with diurnal visitors and the avoidance of predation and parasitism.
Release of odors at night and the presence of osmophores in sepals, corona, and in lesser extant, in petals, suggest that these elements can act together as odor guides to attract to the bees to P. morifolia flowers. Odor, especially in flowers with nocturnal anthesis, is one of the main attractions for pollinators (Fægri & van der Pijl, 1979; Endress, 1994; Carvalho et al., 2012). In P. foetida, with crepuscular anthesis, the release of sweet odors and increased production of nectar coincided with visits of Ptiloglossa tarsata (Friese) (Amela García & Hoc, 1998). Passiflora pohlii flowers releasing citrus odors and also be constantly pollinated by bees of the genus Ptiloglossa (Faria & Stehmann, 2010). Different odors, produced by different combinations of volatile organic compounds, attract specific pollinators, as it was demonstrated by Varassin et al. (2001) for several Passiflora species pollinated by bees (P. alata) and by bats (P. galbana and P. mucronata). Even bees of different species respond according to the chemical compound involved in odor formation, as observed in Paullinia cupana Kunth (Sapindaceae) (Krug et al., 2018).
Bees of the meliponine genera Plebeia and Trigona may have a complementary effect on the pollination of P. morifolia due to their small-sized bodies and foraging behaviors. These bees are eusocial, living in colonies with large numbers of individuals, and their foraging is considered generalist due to the wide spectrum of plants from which they collect resources (Silveira et al., 2002). When collecting nectar, bees of the genus Plebeia do not come into contact with the anthers or stigmas of P. morifolia, but when collecting pollen, they move over the anthers and stigmas and act as occasional pollinators. Plebeia droryana contributes to the pollination of the small-sized flowers of P. suberosa, but it was considered a nectar thief in P. miersii (Koschnitzke & Sazima, 1997). For other Passiflora species, with large flowers and great spatial separation between the reproductive structures and the corona (where they land when they take nectar), visitation by these bees does not always seem to have positive effects, as in P. actinia, in which Plebeia remota was considered a nectar thief (Varassin et al., 2018). Trigona spinipes was also considered an occasional pollinator of P. morifolia because, depending on its movement in the flowers, it touched the anthers and stigmas with its thorax and head and carried pollen. In other Passiflora species, Trigona spinipes even can damage flowers and attack other floral visitors, reducing the fructification and number of seeds per fruit, as in Passiflora coccinea Aubl. (Boiça Junior et al., 2004) and in Passiflora edulis Sims (Sazima & Sazima, 1989).
Halictid bees (with the exception of Megommation insigne) occurred at low frequency, did not contribute to P. morifolia pollination and also acted as nectar thieves. Despite visiting flowers when they could donate and receive pollen, these bees are not large enough to touch anthers and stigmas when collecting nectar and were not seen collecting pollen. In Passiflora foetida (Amela García & Hoc, 1998), the observed halictids collected pollen, but did not always touch the stigmas and were also considered nectar and/or pollen thieves. Koschnitzke & Sazima (1997) considered the halictid bees observed in the flowers of P. alata as pollen thieves, different of P. suberosa, with small-sized flowers, in which one of the species of these bees contributed to self-pollination.
Passiflora morifolia flowers are adapted to pollination by nocturnal and crepuscular bees, with P. willinki and M. insigne as more important pollinators. Fruit set for the free pollination treatment were higher than 0.85 and the number of seeds formed per fruit (which in this treatment may be the result of autogamous and xenogamous pollen) was almost the total of potential seeds; this, together with the decrease in fruit and seed set when the flowers are self-pollinate spontaneously, suggests the occurrence of an efficient reproductive dynamic that benefits both plants and natural populations of pollinators. So, the reproductive biology data provided here reinforce the importance of preserving natural areas allowing the maintenance of native species, both plants and pollinators. They also complement previous descriptions and can help in the interpretation of studies on the conservation, genetic diversity and phylogenetic history of species.












 uBio
uBio