INTRODUCTION
Epicuticular waxes represent the interaction surface between plants and biotic and abiotic factors of their environment, which determines their major ecologic importance. Functions of epicuticular waxes are mainly related to the restriction of transpiration water loss, although it does not seem to be the only factor that determines its rates (Bondada et al., 1996; Oliveira et al., 2003). However, other functions of epicuticular waxes have been proposed, such as protection against radiation (Barnes & Cardoso-Vilhena, 1996), herbivory, and natural pathogens (Jenks et al., 2010).
Wax biosynthesis in epidermal cell cytoplasm comprises complex enzymatic pathways starting from fatty acids (Jenks et al., 1995, 2010). The mechanism of wax deposition on the surface is not known in detail, although some interpretations include extrusion through microchannels and diffusion through the cuticle (Jenks et al., 2010).
Waxes crystallize in many different morphologies, from a smooth film, variable in thickness, to complex crystalloid structures, such as granules, plates, platelets, and rodlets, which have been described with different techniques (Barthlott et al., 1998; Ensikat et al., 2006). Morphology of these epicuticular structures varies in and between species with the chemical composition of the wax (Jeffree et al., 1975; Sen, 1987; Ensikat et al., 2006; Koch et al., 2006), the development stage of the leaf (Gülz et al., 1991; Jetter & Schaffer, 2001), environment conditions (Wallace et al., 1987; Bondada et al., 1996; Oliveira et al., 2003) and the way it is deposited on the surface (Anton et al., 1994).
Epicuticular wax micromorphology has been described in detail with scanning electron microscopy (Barthlott et al., 1998), and it has shown a great taxonomic value in different taxa, such as Pinus L., Huberia DC., Zanthoxylum L., Alstonia R. Br., Pilocarpus Vahl (Herbin & Sharma, 1969; Jayeola, 1998; Mimura et al., 1998; Skorupa et al., 1998; Dias-Leme et al., 2013). There are only a few extensive studies on epicuticular waxes in Fabaceae, and they are mainly focused on Caesalpinioid genera (Calliandra Benth., Acacia Mill. s.l.), although most of them have been included in more extensive studies which analysed various families (Maffei, 1996; Ramírez et al., 1997; Barthlott et al., 1998). Nevertheless, in the genus Mimosa L., which is one of the most diverse genera of Fabaceae, there are no such descriptions.
This genus comprises more than 540 species of pantropical and pan-subtropical distribution (Simon et al., 2011; Bessega & Fortunato, 2011) with relevant diversification in South America. As presently circumscribed (Barneby, 1991; Savassi-Coutinho et al., 2012; Dutra & Garcia, 2013), section Calothamnos Barneby comprises 28 species and eight varieties: M. aurivillus Mart. var. aurivillus, M. aurivillus var. calothamnoides Barneby, M. aurivillus var. calothamnos (Mart. ex Benth.) Barneby, M. aurivillus var. sordescens Benth., M. aurivillus var. warmingii Barneby, M. barretoi Hoehne, M. bathyrrhena Barneby, M. berroi Burkart, M. bonplandii Benth., M. calodendron Mart. ex Benth., M. chrysastra Mart. var. chrysastra, M. chrysastra var. itambeana Barneby, M. cylindracea Benth., M. daleoides Benth., M. eriocarpa Benth., M. flocculosa Burkart, M. furfuracea Benth., M. incana Benth., M. involucrata Benth., M. lepidorepens Burkart, M. leprosa J. F. Macbr. var. leprosa, M. leprosa var. parviceps Barneby, M. longistipula V.F. Dutra & F.C.P. Garcia, M. macedoana Burkart var. macedoana, M. macedoana var. glabrescens (Burkart) Barneby, M. myuros Barneby, M. peduncularis Bong. ex Benth., M. pilulifera Benth. var. pilulifera, M. pilulifera var. pseudincana (Burkart) Barneby, M. plumosa Micheli, M. psittacina Barneby, M. rocae Lor. & Niederl., M. roseoalba Sav.-Cout. & G.P. Lewis, M. scabrella Benth., M. taimbensis Burkart, and M. urticaria Barneby.
The subseries Dolentes Barneby (sect. Mimosa) is monotypic, comprising only M. dolens Vell., which is defined by the presence of determinate inflorescences and valvately dehiscent pods grouped in dense spheroid clusters. Barneby (1991) described five subspecies in this taxon: M. dolens ssp. callosa (Benth.) Barneby, M. dolens ssp. rigida (Benth.) Barneby with five varieties (var. rigida, var. rigescens (Benth.) Barneby, var. anisitsii (Lindm.) Barneby, var. foliolosa (Benth.) Barneby, var. deterior Barneby), M. dolens ssp. acerba (Benth.) Barneby with three varieties (var. acerba, var. latifolia (Benth.) Barneby, var. rudis (Benth.) Barneby), M. dolens ssp. eriophylla (Benth.) Barneby, and M. dolens ssp. dolens and two varieties (var. dolens and var. pangloea Barneby). The subseries Brevipedes Barneby (sect. Mimosa), on the other hand, is differentiated by its indeterminate inflorescences and craspedial dehiscence of the pod, and comprises nine species: M. brevipes Benth., M. diversipila Micheli and two varieties (var. diversipila and var. subglabriseta Barneby & Fortunato emend. M. Morales), M. fernandez-casasii Barneby & Fortunato, M. cryptogloea Barneby, M. custodis Barneby, M. sceptrum Barneby, M. longiracemosa (Burkart) Barneby, M. pseudopetiolaris Barneby, M. nitidula Barneby, and M. schininii M. Morales, Grohar & Fortunato (Barneby, 1991; Morales et al., 2019).
Therefore, our aim is to contribute to the description of the morphology of epicuticular waxes in the genus Mimosa. Due to its high morphologic variability, traditional sections based on morphological similarities do not coincide with phylogenetic analysis (Barneby, 1991; Simon et al., 2011). This is the case of sect. Calothamnos, where morphologically consistent characters do not always coincide with molecular phylogeny, in which it is polyphyletic with sect. Mimosa (Bessega et al., 2008; Coutinho, 2009; Bessega & Fortunato, 2011; Simon et al., 2011; Grohar, 2020). Furthermore, in several infrageneric groups of sect. Mimosa, intermediate forms between specific and infraspecific taxa (Morales et al., 2010, 2014, 2015; Luna-Castro et al., 2012) generates problems to delimitate infrageneric taxa, such as in the case of the taxonomic complex which comprises Mimosa subseries Dolentes and Mimosa subseries Brevipedes (Morales et al., 2010; Grohar et al., 2016). The aim of this work is to 1) study in detail the outer epidermal micromorphology in sect. Mimosa (subseries Dolentes and Brevipedes) and sect. Calothamnos, which are groups of interest because of their complex systematics and evolutionary issues, in order to describe their natural variability, and 2) determine its relevance in the differentiation of the taxa at different levels.
MATERIALS AND METHODS
Plant material. Sixteen herbarium specimens of different taxa from sect. Mimosa (subseries Dolentes and subseries Brevipedes) and 37 from sect. Calothamnos, deposited in BAB, CTES, G, HUCS, MBM, MVFA, RB, SI, and SPF (Appendix; herbarium acronyms follow Thiers, 2022) were included in the analysis. All of them were identified using Barneby (1991) taxonomic treatment.
Scanning electron microscopy (SEM). Two dehydrated mature leaflets per taxon were sampled in squares of 1 cm2 from the centre of the leaflet and sputter-coated with gold-palladium for three minutes. Later, they were examined with a Zeiss Supra 40 FESEM scanning electron microscope at the Centro de Microscopía Avanzada of Universidad de Buenos Aires, or a Philips SEM 505 at the Museo de Ciencias Naturales Bernardino Rivadavia (Buenos Aires, Argentina).
Terminology. For the description of epidermis surface, we followed the terminology used by Behnke & Barthlott (1983) and Koch et al. (2006). For epicuticular waxes’ crystals, we followed Barthlott et al. (1998).
RESULTS
In the studied taxa of genus Mimosa, the general structure of the outer surface of the leaves includes a cutin layer above the epidermis, which includes a lower cutin-pectin layer and an upper cutin-wax layer, and over the latter, a layer of epicuticular waxes (Fig. 1A-B).
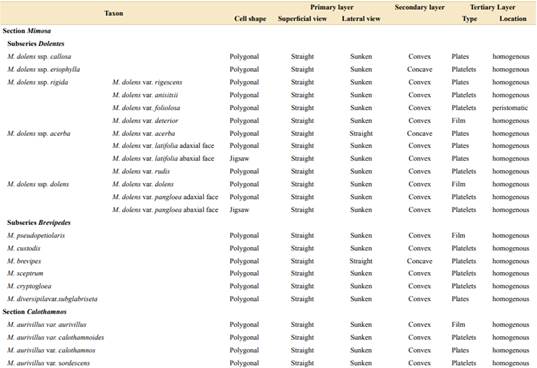
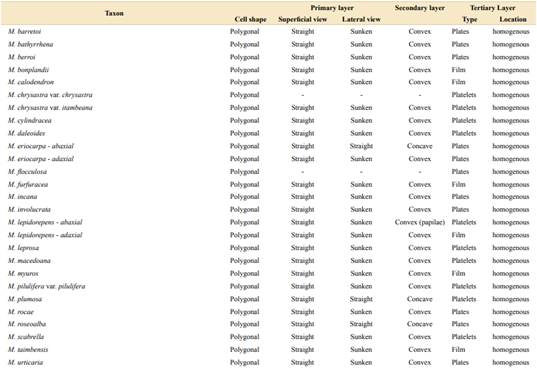
Regarding the epidermis, we described two epidermal cell shapes (Table 1, Figs. 2A-B, 3A-B). Most of the taxa of both sections show polygonal cells, while M. dolens ssp. acerba var. latifolia and M. dolens ssp. dolens var. pangloea show jigsaw shape, but both only on abaxial face. On a closer analysis, we differentiate three different layers (Fig. 1C):
Primary layer
It comprises the cell outline, that is, the connecting wall between adjoining cells. In superficial view, all considered taxa showed straight outlines (Table 1, Figs. 2A-D and 3A-D). In lateral view, however, most taxa show a sunken morphology, except for M. dolens var. acerba and M. brevipes (sect. Mimosa), and M. eriocarpa on abaxial face, M. plumosa and M. roseoalba (sect. Calothamnos).

Fig. 1 Structure of leaflet epidermis in Mimosa. A-B, SEM micrographs of leaflet anatomy. A, M. leprosa, cross-section of the leaflet. B, M. leprosa, detail of the upper epidermis in cross section. C, structure of primary, secondary, and tertiary layers; adapted from Behnke & Barthlott (1983) and Koch et al. (2008). Abbreviations: me, mesophyll; e, epidermis; cp, cutin-pectin layer; cw, cutin-wax layer; ew, epicuticular waxes. The bar indicates: A = 20 µm and B = 5 µm.
Secondary layer
It comprises the outer surface of the epidermis cell, that is, its periclinal wall. Most of the taxa of both sections show convex surfaces (Table 1, Figs. 2E-F and 3E-H). However, some taxa show concave surfaces: M. dolens ssp. eriophylla, M. dolens ssp. acerba var. acerba and M. brevipes (sect. Mimosa), and only on abaxial face of M. eriocarpa, M. plumosa and M. roseoalba (sect. Calothamnos). Besides, M. lepidorepens is distinguished from all other taxa by a distinctive secondary epidermal morphology on abaxial face, as the cell wall forms prominent papillae, densely covered by wax crystals (Fig. 3G).

Fig. 2 SEM micrographs of primary and secondary layers in Dolentes-Brevipedes taxonomic complex. A, M. dolens var. rigescens. Primary layer straight in superficial view; cell shape polygonal. B, M. dolens var. latifolia, jigsaw cell shape. C, M. dolens var. anisitsii, primary layer straight in lateral view. D, M. dolens var. dolens, primary layer sunken in lateral view. E, M. cryptogloea, secondary layer: convex. F, M. dolens var. acerba, secondary layer concave. The bar indicates: A-C = 80 µm, D = 4 µm, E-F = 100 µm.
Tertiary layer
It comprises epicuticular waxes and trichomes that are arranged over the thick layer of cutin on the outer surface of the cell wall. In both sections of Mimosa, the tertiary layer covers the entire surface, including epidermal cells and subsidiary cells, but not stomatal cells (Fig. 2C).

Fig. 3 SEM micrographs of primary and secondary layer in M. sect. Calothamnos. A-B, M. myuros. Primary layer straight in superficial view: straight; cell shape: polygonal. C, M. plumosa, primary layer in lateral view: straight. D, M. calodendron, primary layer sunken in lateral view: sunken. E-F, Secondary layer convex. E, M. rocae; F, M. bonplandii. G, M. lepidorepens, abaxial face, secondary layer papillae. H, M. plumosa, secondary layer concave. The bar indicates: A = 20 µm, B, E = 20 µm, C, H = 100 µm, D, G = 50 µm, F = 10 µm.
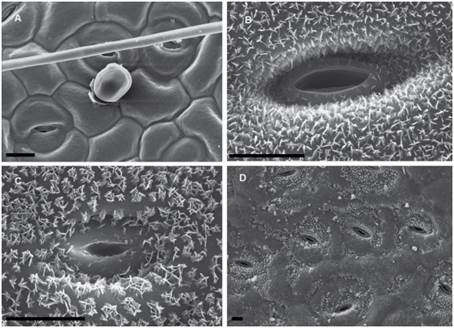
Fig. 4 SEM micrographs of epicuticular waxes in Dolentes-Brevipedes taxonomic complex. A, M. cryptogloea, abaxial face, homogeneous film of wax. B, M. dolens var. acerba, adaxial face, plate crystals. C, M. custodis, adaxial face, platelets crystals. D, M. dolens var. foliolosa, adaxial face, peristomatic platelets. The bar indicates 10 µm.
Besides, some variability has been observed in the shapes that epicuticular wax crystallizes on the foliar epidermis, varying from a fine homogeneous film to polyhedral crystalloids, and even concentrated in groups. Three distinct morphologies of wax deposition were found (Figs. 4-5, Table 1):
Film
Wax is deposited in a homogeneous layer which covers the whole leaflet surface. Due to wax flexibility, wax layer adjusts to cell surface, leading to sunken areas in epidermal or stomata cells borders (Figs. 4A, 5A). This deposition type was found in M. dolens var. dolens, M. dolens ssp. rigida var. deterior, M. cryptogloea and M. pseudopetiolaris (sect. Mimosa) and in M. aurivillus var. aurivillus, M. bonplandii, M. calodendron, M. furfuracea, on adaxial face of M. lepidorepens, M. myuros and M. taimbensis (sect. Calothamnos).
Plates
Polygonal crystalloids with distinct edges, 300-1200 nm wide, 500-920 nm long and 55-180 nm thick, are arranged in different directions, usually overlapping between them (Figs. 4B, 5B), and covering homogenously the whole leaflet surface. The plates appear isolated, although sometimes they seem to be in circular groups. In general, wax deposition adjusts to cell surface, although there are some areas where this adjustment is not observed. In sect. Mimosa, this deposition morphology was found in M. dolens ssp. callosa, M. dolens ssp. rigida var. rigescens, M. dolens var. acerba, M. dolens ssp. acerba var. latifolia and M. diversipila var. subglabriseta. In sect. Calothamnos, it was found in M. aurivillus var. calothamnos, M. barretoi, M. bathyrrhena, M. berroi, M. eriocarpa, M. flocculosa, M. incana, M. involucrata, M. rocae, M. roseoalba and M. urticaria.

Fig. 5 SEM micrographs of epicuticular waxes in M. sect. Calothamnos. A, M. bonplandii, abaxial face, homogeneous film of wax. B, M. roseoalba, adaxial face, plates. C, M. chrysastra, adaxial face, platelet rosettes. D, M. leprosa, adaxial face, wax smoothings. The bar indicates: A = 10 µm, B-D = 5 µm.
Platelets rosette
Polygonal crystalloids with entire margins but indistinct edges, 460-1200 nm wide, 650-1020 nm long y 110-170 nm thick, are arranged in circular units (Figs. 4C, 5C). Crystalloids are not radially arranged around a central axe as in rosettes, but are randomly oriented. However, these clusters can be clearly identified, and they cover the whole leaflet surface. In sect. Mimosa, this deposition morphology was found in M. dolens ssp. eriophylla, some specimens of M. dolens ssp. acerba var. acerba, M. dolens ssp. dolens var. foliolosa, M. dolens ssp. acerba var. rudis, M. dolens var. pangloea, M. brevipes, M. sceptrum, M. dolens var. anisitsii and M. custodis. In sect. Calothamnos it was found in M. aurivillus var. calothamnoides, M. aurivillus var. sordescens, M. chrysastra var. chrysastra, M. chrysastra var. itambeana, M. cylindracea, M. daleoides, M. lepidorepens, M. leprosa, M. macedoana, M. pilulifera var. pilulifera, M. plumosa, and M. scabrella.
Two distinct variations of these morphologies were also observed. In M. dolens ssp. rigida var. foliolosa, platelets are grouped in circular units, but are arranged only around stomata cells (Fig. 4D). In the rest of the leaflet surface, wax is deposited as a film adjusting to cell structure. On the other hand, in some taxa of sect. Calothamnos, epicuticular waxes crystals seem to be smoothed in some portions of the surface layer (Fig. 5D): M. aurivillus var. calothamnos, M. aurivillus var. sordescens, M. berroi, M. chrysastra var. chrysastra, M. chrysastra var. itambeana, M. cylindracea, M. daleoides, M. eriocarpa, M. furfuracea, M. leprosa, M. macedoana, M. pilulifera var. pilulifera, M. rocae and M. scabrella. However, these morphologies are occasional and seem to follow no distinct pattern neither on the leaflet nor among specimens.
The frequencies of each morphology of the primary, secondary and tertiary layers vary similarly within and between sections (Fig. 6). Regarding cell shape, primary and secondary layer, always one morphology is the dominant one, that is, polygonal, straight, sunken and convex morphology, respectively, with frequencies higher than 90%. In the tertiary layer, the most frequent type of deposition in both sections is the platelet rosette, being identified in more than half of the taxa in sect. Mimosa, and about 40% in sect. Calothamnos. It is closely followed by plates (30-40% in both sections), and by the film (15-25%). In addition, some intraspecific variability is observed in sect. Calothamnos between faces of the leaflet (M. eriocarpa, M. lepidorepens).
DISCUSSION
Wax morphology. Cell wall morphology and its epicuticular depositions observed in Mimosa sect. Mimosa and M. sect. Calothamnos are mainly in accordance with the typical morphologies described in angiosperms (Koch et al., 2006; Barthlott et al., 2017). The primary, secondary, and tertiary layers described for plants in general are all present in both sections (Behnke & Barthlott, 1983; Koch et al., 2006). It has been mentioned that the secondary and tertiary layers are mutually exclusive, that is, if one presents an elaborate morphology, the other presents a basic morphology, with very few exceptions (Behnke & Barthlott, 1983). Our results support this statement, since in most of the taxa of both sections the cell walls present a very uniform relief, with entire contours and a very slightly concave surface, but with elaborated epicuticular waxes (tertiary layer), which consists in crystalloids of different shapes and dispositions. In addition, we described here a novel combination for the genus of both secondary and tertiary layer with complex morphologies in M. lepidorepens, with prominent papillae on abaxial face of the leaflets, combined with a dense coverage of platelet rosettes. Papillae as these have already been described in genera from different plant families, such as Euphorbia L., Oryza L., and Nelumbo Adans. (Kochet al., 2006), and also in some related genera, for example, in Acacia dealbata Link (Wagner et al., 2003).
The morphology of the secondary layer, that is, the periclinal wall of the epidermis cells, can influence the interaction with water drops, determining the hydrophobicity of the surface: as the epidermis folds, the adhesion of water molecules to the surface is weaker, therefore allowing the water to drain easily (Wagner et al., 2003; Barthlott et al., 2017). Considering this, the abaxial face of M. lepidorepens would show the highest hydrophobicity of both sections, not only for the folded epidermal surface but also for the dense cover of wax crystals. This could be related to the distribution of this taxon in Serra do Quirirí (Santa Catarina, Brazil), where precipitation and air humidity levels are high throughout the year, as well as the fog is very frequent (De Souza et al., 2011).
The tertiary layer, which consists of epicuticular wax depositions, has been described in detail in different families (Jeffree et al., 1975; Barthlott, 1990; Barthlott et al., 1998) and has been taxonomically relevant in some genera from different plant families, such as Pinus, Huberia, Zanthoxylum, Alstonia, Pilocarpus and Jasminum L. (Herbin & Sharma, 1969; Jayeola, 1998; Mimura et al., 1998; Skorupa et al., 1998; Dias-Leme et al., 2013; Yohannan & Devipriya, 2017). Platelets have been identified as the typical wax deposition type in Fabales (Ditsch et al., 1995). Our results also show that this wax deposition type is also the most frequent in sect. Mimosa and sect. Calothamnos. However, we additionally found two other different deposition morphologies: film and plates, either homogeneously arranged on the whole surface or only around stomata, being both less frequent, but not rare. Our results in plate size are similar to those reported in other families (Jeffree et al., 1975, 1976; Koch et al., 2006).
The type and density of epicuticular waxes can also influence the optical effect of the leaflet surface since it can appear either pale and greyish or bright and shiny (Baker et al., 1983; Koch et al., 2006, 2008). In Mimosa sect. Calothamnos, this effect can be easily observed. On one hand, in M. calodendron and M. taimbensis, both with shiny leaflet surfaces, the wax deposition type is a film with no crystalloids, as has been described in other species (Baker et al., 1983). However, other taxa in both sections also show a wax film, but its leaflets are not shiny. Something similar was observed in M. involucrata or M. lepidorepens, which show pale and greyish surfaces, but there seem to be no taxa-specific morphology. This indicates that the wax deposition type is not the only feature that determines the leaflet’s optical properties. It has been previously suggested that more complex wax structures, like tubules, are necessary to really change the optical properties of a surface by itself (Baker et al., 1983).
Taxonomic relevance. Mimosa is a complex genus, and some of its sections based on both morphological traits and molecular phylogeny are not monophyletic (Bessega & Fortunato, 2011; Simon et al., 2011). Epidermal outer morphology could be a taxonomically relevant feature for some taxa with particular morphologies (e.g., papillae in M. lepidorepens), and are even for the distinction of varieties in some species of the genus (e.g., M. aurivillus). However, our results do not coincide with previous classifications (Barneby, 1991) at a supraspecific level, that is, between subseries and even the two sections considered here. In sect. Mimosa, other anatomical (Grohar et al., 2016; Grohar et al., 2018; Grohar et al., 2021) and also morphometric and chromosomic data (Morales et al., 2018) could help re-evaluate the supraspecific categories established only on morphological similarities (Barneby, 1991).
At a supraspecific level, the wax micromorphology by itself is not as relevant for the distinction between the sections Calothamnos and Mimosa as other anatomical (Grohar et al., 2016; Grohar et al., 2018; Grohar et al., 2021) and also complex morphometric and cytogenetic analyses (Morales et al., 2018) could help reevaluate the supraspecific categories established only on morphological similarities (Barneby, 1991).
At a supraspecific level there is high variability in both of them. However, its relevance changes at inter- and intraspecific levels. In sect. Calothamos, wax deposition types help to differentiate the varieties of M. aurivillus, since M. aurivillus var. sordescens and M. aurivillus var. calothamnoides show platelet rosettes, M. aurivillus var. calothamnos plates, and M. aurivillus var. aurivillus a wax film. These results, altogether with results from corolla micromorphology in the section (Grohar et al., 2021), questions the proposal of M. aurivillus as a single taxonomic species, which has already been suggested before based on morphological analysis (Coutinho, 2009).
In other species of the sect. Calothamnos, such as M. chrysastra, the distinction of its two varieties based on epidermal micromorphology is not observable, since they show the same deposition type, that is, platelet rosettes. Other micromorphological traits of corolla proved to be helpful in differentiating both varieties (Grohar et al., 2021).
In sect. Mimosa, it seems difficult to separate the subseries Dolentes and Brevipedes as it was proposed by Barneby (1991), because of the presence of the diagnostic traits in both subseries; it was analysed extensively by our previous works (Morales et al., 2018, 2019). This has also been observed in other traits, such as trichome micromorphology (Grohar et al., 2016). Although high variability of stomata morphology has been described at an intraspecific level in Mimosa (Montaño-Arias et al., 2018), in subser. Dolentes it is possible to associate types of wax deposition with some subspecies of M. dolens. In M. dolens ssp. callosa and M. dolens ssp. eriophylla, only one morphology is found: plates and platelets, respectively. In the other subspecies, it is not possible to define each one solely on one morphology.
However, distinction of varieties within the ssp. rigida and dolens is possible, as all varieties can be clearly differentiated by the wax deposition types. In M. dolens ssp. rigida, M. dolens var. rigescens is the only one with plates and M. dolens var. deterior is the only one with a wax film. Although M. dolens var. foliolosa and M. dolens var. anisitsii both have platelets, they also differ, as in M. dolens var. foliolosa the crystals are deposited only around the stomata guard cells, and in M. dolens ssp. rigida var. anisitsii crystals cover homogeneously the whole leaflet surface. Similarly, in M. dolens ssp. dolens, both varieties of M. dolens are clearly separated by wax deposition types, as M. dolens var. dolens shows a wax film, and in M. dolens var. pangloea, wax crystals have a platelet shape. In M. dolens ssp. acerba, there are many possible morphologies among the varieties, which fail to differentiate them. In subseries Brevipedes, we found that the main taxa are similar in wax morphology, since there is a trend to show a film or platelets in all studied taxa of the subseries, except for M. diversipila var. subglabriseta.
In addition to the wax morphology, location and epicuticular wax deposition type vary between taxa. Regarding wax spatial deposition, M. dolens ssp. rigida var. foliolosa clearly distinguishes itself from all other taxa, as it is the only taxon in the complex in which platelets are arranged only around stomata cells. In all the other taxa, epicuticular waxes cover regularly the whole leaflet surface, independently of the morphology.
Regarding crystalloid types of the tertiary layer, the most frequent morphology in both subseries is the platelet. In the subseries Dolentes, it appears only in some varieties from each subspecies, while in subseries Brevipedes, it is found only in M. custodis, M. brevipes and M. sceptrum. Other deposition types, although less frequent, are also observed only in only a few taxa. These results could be useful as taxonomically relevant features at specific and infraspecific levels, as it was described in other anatomic traits, such as stomata micromorphology (Grohar et al., 2021). In highly variable taxonomic complexes such as Dolentes-Brevipedes, the micromorphological traits could provide new relevant data for the distinction of new species, as it was recently described in M. schininii (Morales et al., 2019).
CONCLUSIONS
The analysis of epicuticular morphology in Fabaceae has been scarce at an infrageneric level, and therefore underused as a taxonomic marker. The present study represents the most detailed analysis of epicuticular morphology in the genus Mimosa, and accounts for a huge variation in micromorphological features of epicuticular waxes within Mimosa sect. Calothamnos and Mimosa sect. Mimosa (subseries Dolentes and Brevipedes). These data could be a potential tool of great taxonomic value within the complex, as they clearly separate individual taxa or groups at a specific and infraspecific level. For instance, M. lepidorepens shows a unique micromorphology of papillae, covered with platelets, on the abaxial face of the leaflets. This type of analysis is particularly helpful in the differentiation of varieties of M. aurivillus, M. dolens ssp. rigida and M. dolens ssp. dolens. However, it is not relevant at a supraspecific level by itself; at that level, it is necessary to solve taxonomic conflicts with other tools. This problem leads to the requirement of multidisciplinary studies including exo-morphological, anatomical, chromosome and molecular data.












 uBio
uBio