INTRODUCTION
Zuccagnia punctata Cav. (Fabaceae) is a monotypic species, commonly known as jarilla pispito, puspus, lata, jarilla poposa, jarilla blanca, jarilla del cerro. It is an Argentine endemic shrub widely distributed in the biogeographic region of “Monte de Sierras y Bolsones” from western Argentina (provinces of Jujuy, Salta, Tucumán, Catamarca, La Rioja, San Juan, Mendoza, and San Luis) between 700 and 2700 m a.s.l. (Burkart et al., 1999). This species in used locally as medicinal and it shows resistance to diseases and pests. Zuccagnia punctata is a part of the “jarillal species” together with Larrea cuneifolia Cav. and Larrea divaricata Cav. (Morello, 1958). Zuccagnia punctata is a glutinous and aromatic shrub (1-5 m) with alternate pinnate resinous leaves. The plants bloom from August to March and show bisexual, zygomorphic flowers with calyx of yellow color and red-brown capsule-like indehiscent fruits (Morello, 1958; Burkart et al., 1999; Zuloaga & Morrone, 1999; Ulibarri, 2005; Moreno et al., 2015a).
It is known that morphological variations i.e. disposition of leaves, flowers colour, size and colour of the fruits, etc. could be found within the same plant species in natural environments, which could be the result of local adaptation processes of the species to the different climates and environments conditions (phenotypic plasticity) (Oliva et al., 1993; Moreno & Bertiller, 2012; Isla et al., 2022; Montanari Beltrame et al., 2022). Aridity, for example, is related to increasing diversity of shrub morphotypes differing in traits such as plant height, rooting depth, leaf mass per unit area, leaf longevity, and physiological adjustment, among others (Campanella & Bertiller, 2009; Saraví Cisneros et al., 2013). There may be a high correspondence between the morphological and genetic characters or a disconnect between them. Genetic divergences between morphotypes may be associated with differences in their chemical composition as type and level of constituents. For example, pigments, alkaloids, polyphenols, and other compounds that may be involved in multiple defensive systems, i.e. UV absorption, tolerance to drought, thermal protection (low-high temperature), defence against herbivores or pathogens or plants, soil microbial activity, soil organic matter decomposition and mineralization, ability of shrubs to capture soil water and to leaf turnover, among others (Oliva et al., 1993; Hartley & Jones, 1998; Souto et al., 2000; Saraví Cisneros et al., 2022).
The chemical composition of aerial parts of Z. punctata with red-brown fruits (RBF Morphotype) was previously reported. Flavanones, flavones, chalcones and caffeoyl esters were found, being the chalcones the major bioactive components with demonstrated pharmacological properties, i.e. antioxidant, antibacterial, antifungal, antiulcerous, anti-inflammatory, antigenotoxic, chemopreventive, antihypertensive, nematicide, anti-age, inhibitor of drug resistance mechanisms (Quiroga et al., 2001; De la Rocha et al., 2003; Svetaz et al., 2004, 2007; Zampini et al., 2005, 2008, 2012; Morán-Vieyra et al., 2009; Agüero et al., 2010; Chieli et al., 2012; Nuño et al., 2014, 2018; Moreno et al., 2015a, b; Isla et al., 2016, 2021a, b; Carabajal et al., 2017, 2019, 2020; Roco et al., 2017, 2018; Gomez et al., 2020; Orqueda et al., 2020). The aerial parts (stems and leaves) of Z. punctata with and without flowers or fruits have been used extensively as a traditional medicine in Argentina for the treatment of bacterial and fungal infections, asthma, gastrointestinal and inflammatory diseases according to ethnobotanical data (Ratera & Ratera, 1980; Toursarkissian, 1980; Carabajal et al., 2020). According to the Z. punctata pharmacological properties, this plant species would have the potential to obtain phytotherapeutic, food and cosmetic products (Moreno et al., 2018; Orqueda et al., 2020; Isla et al., 2021b). In accordance with the Argentine Regulations by the “Administración Nacional de Medicamentos, Alimentos y Tecnología Médica” (ANMAT, 2015), based on its long history of medicinal use in Argentina by different communities, Z. punctata could be considered as an herbal medicine of traditional use (Art. 2). Considering the EMA guidelines (2010), the dry extracts of aerial parts from Z. punctata could be used in herbal medicinal products, as standardized extracts, declaring the quantity of main bioactive compounds or marker compounds (chalcones) (Isla et al., 2022). This species was cited as a plant species of economic interest in Argentina (Cantero et al., 2019). Nevertheless, it is very important to note that Z. punctata has been included in the preliminary red list of endangered plants in category 3 (Norma RE-84-2010-SADS). Thus, the knowledge on Z. punctata morphological diversity is the first step towards the evaluation of genetic diversity and to develop strategies for the conservation, preservation, and sustainable use of genetic resources (Boero, 2010; Shilpashree et al., 2021). The present research focuses on the description of a new morphotype of Zuccagnia punctata with yellow fruits (YF) found in wild populations from Valles Calchaquíes in Tucumán (Argentina) and the comparison of karyotypes and chemical profile (phenolic compounds) of the two morphotypes of Zuccagnia punctata, the red-brown fruits and the yellow fruits morphotypes.
MATERIALS AND METHODS
Plant Material
Aerial vegetative parts (leaves and stems), seeds and fruits of two morphotypes of Z. punctata (RBF and YF) were collected in different places of Valles Calchaquíes, Tucumán, Argentina (Fig. 1, Table 1). Zuccagnia punctata was authenticated by PhD Soledad Cuello, INBIOFIV (CONICET). Voucher specimens were deposited at the Herbarium of Fundación Miguel Lillo, Tucumán, Argentina (LIL; Thiers, 2022): Ampimpa (LIL 618078 (RBF) and LIL 618077 (YF)), Los Zazos o Sasos (LIL 618072 (RBF) and LIL 618071 (YF)), Fuerte Quemado (LIL 618076 (RBF) and LIL 618075 (YF)) and Tío Punco (LIL 618074 (RBF) and LIL 618073 (YF). Samples from three individuals from each morphotype (RBF and YF) from each one of the different populations were analyzed.

Fig. 1 Map showing the distribution of the populations where both morphotypes of Zuccagnia punctata were identified. Color version at http://www.ojs.darwin.edu.ar/index.php/darwiniana/article/view/1083/1292
Karyotype determination of two morphotypes of Z. punctata
The karyotype analysis was carried out according to Levan et al. (1964). Ten seeds of each morphotypes of Z. punctata were disinfected by immersion in a 15% sodium hypochlorite solution for 20 min. Then, the seeds were washed three times with sterile distilled water. Aseptic seeds were placed in Petri dishes containing filter paper soaked in distilled water and kept for five days to obtain roots. Roots tips were cut and pretreated in 8-hidroxyquinoline 0.002 M for 24 h at 4 °C and then fixed in Carnoy’s fluid (v:v:v, 6:3:1, absolute ethanol: chloroform: acetic acid), for 48 h. The roots were transferred to 70% alcohol and stored at -4 °C. Then, they were hydrolyzed in 1 N hydrochloric acid and placed on slides in a drop of 2% propionic hematoxylin and squashed. The measures were to take in seven somatic cells for each sample of each morphotype. Chromosomes were organized in decreasing length. An ideogram was assembled by arranging the chromosomes in homologous pairs in order of their length and arm ratio. Mean length was considered for each chromosome pair and these values were then used to calculate the total chromatin length of each karyotype. The parameters used for morphological descriptions such as chromosome length (C), long-arm length (L), short-arm length (S), centromeric index (Ci) and chromosome type were determined. Karyotype asymmetry was estimated according to the intrachromosomal asymmetric index (A1) and interchromosomal asymmetric index (A2) established by Romero-Zarco (1986).
All chromosome plates were observed and photographed by a Q-Color 5 camera (Ontario, Canada) attached to a Nikon Eclipse E200 microscope (Tokyo, Japan). For karyotyping software MicroMeasure 3.3 (Reeves, 2001) and for pictures software CorelDRAW X3 were used.
Chemical profile of two morphotype of Z. punctata
Extraction and determination of polyphenolic compounds in aerial vegetative parts and fruits
Aerial vegetative parts and fruits of two morphotypes collected in four sites (Table 1) were separated according to morphotype. A pool of each organ and morphotype was made from collected plants and each pool was dried and grounded. Then, 1 g of each pool were extracted with 60° ethanol (20 mL) and ultrasound assistance for 1 h at 25 °C by triplicate. Then, the extracts were filtered. Each extraction was done by triplicate. Total phenolic compound content was determined by Folin-Ciocalteu reagent, according to Singleton et al. (1999) in each of them. Results were expressed as g gallic acid equivalents per g of dry plant material (g GAE.g-1). The flavonoid content was measured according to Lamaison & Carnet (1990) using AlCl and NaNO2. Results were expressed as mg quercetin equivalents per g of dry plant material (mg QE. g-1).
Extraction and determination of anthocyanins in fruits and aerial vegetative parts
Zuccagnia punctata yellow and red-brown fruits and aerial vegetative parts were extracted overnight at 5 °C with acidified methanol containing 1% HCl. Anthocyanins enriched extracts were filtered to remove particles before use (Abdel-Aal et al., 2006). Total anthocyanins were evaluated by the differential pH method (Lee et al., 2005). Methanolic extract in 25 mM potassium chloride solution (pH 1.0) and 400 mM sodium acetate buffer (pH 4.5) was measured simultaneously at 520 nm and 700 nm, respectively. The content of total anthocyanins was expressed as mg cyanidin-3-glucoside equivalents per g of plant material (mg C3GE.g-1).
Identification of phenolic compounds
Zuccagnia punctata extracts were analyzed by HPLC-DAD and HPLC-ESI-MS/MS. The HPLC-DAD system consisting of a Waters 1525 Binary HPLC Pumps system with a 1500 Series Column Heater, a manual injection valve with a 20 µL loop (Rheodyne Inc., Cotati, CA) and a Waters 2998 photodiode array detector (PDA). A BridgeTM C18 column (4.6 × 150 mm, 5 µm; Waters Corporation, Milford, MA) were used to analyze the extracts. The solvent system for the separation of components from extracts was composed of solvent A (0.1% acetic acid in water) and solvent B (0.1% acetic acid in methanol) (conditions: 10-57% B from 0 to 45 min and kept at 100% B from 45 to 60 min) was used. The flow rate was set at 0.5 mL/min. Solutions of 4 mg DW.mL-1 were used. Data collection was carried out with EmpowerTM 2 software. The identification of phenolic compounds present in the extract was carried out by comparing the retention times and spectral data (220-600 nm) of each peak with those of standards from Indofine SRL. The quantification of polyphenols was based on external calibration curves from available standards. Plots were built by comparation of area and concentration in the range of 1-500 ppm.
Mass spectra were recorded using an Agilent 1100 (Agilent Technologies Inc., CA, USA) liquid chromatography system connected through a split to an Esquire 4000 Ion Trap LC/MS(n) system (Bruker Daltoniks, Germany). Ionization was performed at 3000 V assisted by nitrogen as a nebulizing gas at 50 psi and as a drying gas at 365 °C and a flow rate of 10 L.min-1. Negative ions were detected using full scan (m/z 20-2200) and normal resolution (scan speed 10,300 m/z/s; peak with 0.6 FWHM/m/z). The trap parameters were set in an ion charge control (ICC) using manufacturer default parameters, and maximum accumulation time of 200 ms. The mass spectrometric conditions for analysis were electrospray needle = 4000 V, endplate offset = -500 V, skimmer 1 = 56.0 V, skimmer 2 = 6.0 V, capillary exit offset = 84.6 V, capillary exit = 140.6 V. Collision induced dissociation (CID) spectra were obtained with a fragmentation amplitude of 1.00 V (MS/MS) using helium as the collision gas and was automatically controlled through SmartFrag option. The sample was analyzed by using a MultoHigh 100 RP 18-5 μ (250×4.6 mm) column (CS-Chromatographie Service GmbH, Langerwehe, Germany) kept at 25 °C. The HPLC-MS analyses were performed using a linear gradient solvent system consisting of 1% formic acid in water (A) and acetonitrile as follows: 15% to 25% B over 15 min, increasing to 30% B at 30 min, changing to 40 % B at 37 min, 48 % to 51% B from 40 to 70 min, 53% to 55% B from 75 to 78 min, 60 % to 100% B from 81 to 85 min, maintained to 100% B from 90 to 95 min and returning to 15% B from 95 to 105 min. The flow rate was 0.5 mL.min-1 and the volume injected was 20 μL. Compounds were monitored at 254 nm.
Determination of anthocyanin profile by HPLC-MS/MS.
Data were recorded on a previously described HPLC-ESI-MS/MS system. Ionization was performed at 4000 V assisted by nitrogen as a nebulizing gas at 4.0 bar and as a drying gas at 200 °C and a flow rate of 8.0 L.min-1. Positive ions were detected using full scan (m/z 80-1200) and normal resolution (scan speed 10300 m/z/s; peak with 0.6 FWHM/m/z). Trap parameters were set in ICC using manufacturer default parameters, and maximum accumulation time of 200 ms. CID was performed by collisions with a helium background gas present in the trap and automatically controlled through SmartFrag option. All extractions were analyzed using a Phenomenex, Luna C18(2); 250 × 4.6 mm maintained at 35 °C. The HPLC-MS analyses were performed using a linear gradient solvent system consisting of 0.5% formic acid in water (A) and 0.5% formic acid in methanol as follows: 80% to 50% A over 3 min, maintaining to 50% A at 8 min, 50 to 30 % A over 15 min maintaining to 30% A at 20 min changing to 20% A at 21 min, maintained at 30 min changing at 20% at 31 min, maintained from 45 min. The flow rate was 0.4 mL-min-1 and the volume injected was 40 μL. The quantification of anthocyanins was based on external calibration curves from available standards. Plots were built by comparison of area and concentration in the range of 1-500 ppm. Results are expressed as mg.g-1 samples.
RESULTS AND DISCUSSION
In this work, Z. punctata shrubs were sampled in four localities (Fig. 1). In each of them were identified, for the first time, two morphotypes of Z. punctata in wild populations, one with yellow fruits and other with red-brown fruits (Fig. 2). Both morphotypes grow in the same populations (Fig. 3) even in those found in different locations at different altitude levels, in more water-limited environments as well-drained piedmont slopes or ridges (“Ampimpa” or “Tío Punco”) or places with more moisture near of Santa María River (“Fuerte Quemado”). In all cases, both morphotypes are found together, being more abundant the plants with reddish brown fruits than plants with yellow fruits. Both morphotypes, have the same morphological aspect, glutinous and aromatic with pinnate resinous leaves with glandular dots on both surfaces of the leaflet blades in all places (Figs. 2 and 3). Yellow fruits have the same size (10 mm long. × 6 mm lat.) and have bristles and contained one seed as red-brown fruits (Fig. 2).
Cytogenetics studies
The sporophytic chromosome number of Z. punctata was determined for the first time as 2n = 24. Since the genera Caesalpinia Plum. ex L. and Hoffmannseggia Cav., more closely related to Zuccagnia, have a chromosome number 2n = 2x = 24, it is highly likely that it is a diploid (Zanin & Cangiano, 2001; Cangiano & Bernardello, 2005; Nores et al., 2012).
The karyotype of plants with yellow fruits showed 24 metacentric chromosomes (24 m). The somatic complement was composed of 12 pairs of chromosomes that gradually decreased in size and only three pairs exhibited a satellite (numbers 6, 7 and 10) (Table 2 and Fig. 4A, C). The total length of the haploid chromosome complement was 38.25 µm, with an average length of chromosomes 3.18 µm (length from 1.67 to 4.76 µm). According to average centromeric index values (42.23), chromosomes are classified as metacentric. The values of the intrachromosomal index (A1 = 0.24) suggest a symmetric karyotype, while the interchromosomal index (A2 = 0.28) showed chromosomes with uniform size.

Fig. 2 Zuccagnia punctata with fruits of two morphotypes. A, plant with yellow fruits. B, not mature yellow fruits. C, mature yellow fruits. D, plant with red-brown fruits. E, not mature red brown fruits. F, mature red-brown fruit. The pictures were obtained by the authors during samples collection from Valles Calchaquíes in Tucumán, Argentina. A-F scale = 1cm. Color version at http://www.ojs.darwin.edu.ar/index.php/darwiniana/article/view/1083/1292

Fig. 3 A, two morphotypes of Zuccagnia punctata in the same population of plants in Amaicha del Valle, Tucumán. Color version at http://www.ojs.darwin.edu.ar/index.php/darwiniana/article/view/1083/1292

Fig 3 B, Zuccagnia punctata with yellow fruits (YF) next to Z. punctata with red-brown fruits (RBF). Color version at http://www.ojs.darwin.edu.ar/index.php/darwiniana/article/view/1083/1292

Table 2 Quantitative parameters of chromosomes. Abbreviations: C, mean chromosome length; l, mean length of the long arm; S, mean length of the short arm; Ci, mean centromeric index; m, metacentric; sm, submetacentric; sat, satellite; SE, standard error.
The RBF morphotype, unlike YF morphotype, showed karyotype formula 18m + 6sm (Fig. 4B, D) (18 metacentric chromosomes + six submetacentric chromosomes). The chromosome complement was composed of 12 pairs of chromosomes that gradually decrease in size and also, three pairs of satellite (numbers 4, 6, and 7). The total length of the haploid chromosome complement was 38.8 µm, with an average length of chromosomes 3.17 µm (length from 1.67 to 4.96 µm). According to average centromeric index values (40.27), the chromosomes could be classified as metacentric. The values of the intrachromosomal index (A1 = 0.27) suggest a symmetric karyotype, while the interchromosomal index (A2 = 0.29) showed chromosomes with uniform size like yellow fruit morphotype (Table 2).

Fig. 4 Metaphase plate and idiograms of two Zuccagnia punctata morphotypes. A, metaphase plate of yellow fruits. B, metaphase plate of red-brown fruits. The satellites are indicated with arrows. C, idiograms of yellow fruits; 12 pairs of chromosomes metacentric, satellite in pairs chromosome 6, 7, 10. D, idiograms of red-brown fruits; 18 metacentric chromosomes and six submetacentric chromosomes, satellite in chromosome 4, 6, 7. Abbreviations: m, metacentric chromosome; sm, submetacentric chromosome; numbers indicate the chromosome sizes. Satellite indicated in grey colour. A-B scale = 10µm; C-D scale 2µm.
The total haploid complement length, centromeric index, and A1/A2 were very similar in both morphotypes. However, they have different karyotype formulas: the red-brown fruit plant has three submetacentric chromosomes (pairs 6, 7 and 8) as well as a different pair of chromosomes satellited (4 instead of 10). In vascular plants, different karyotypes between individuals from the same species were found, e. g. in Mikania Willd. (Ruas et al., 2000), Prospero Salisb. (Jang et al., 2013) and Carthamus L. (Uysal et al., 2018) and those authors concluded that distinct cytotypes could occur on individuals of the same species. Thus, both karyotypes could be at least two different cytotypes of the same species.
Chemical characterization of yellow and red-brown fruits and aerial vegetative parts of Z. punctata
The content of phenolic compounds in yellow fruits and red-brown fruits (62.31 and 73.40 mg GAE.g-1, respectively) were significantly lower than the content of phenolic compounds in aerial vegetative parts of both morphotypes of Z. punctata (132 and 179 mg GAE.g-1 for yellow and red-brown fruits morphotype, respectively) being greater the content in fruits and aerial vegetative parts of morphotype RBF. Fruits and aerial vegetative parts of the red-brown fruits morphotype showed also higher flavonoid content than the yellow fruits morphotype (Table 3).
Both morphotypes showed similar profiles of phenolic components (Fig. 5). Mass fragmentation patterns revealed the presence of 1-methyl-3(4′ hidroxyphenyl propil caffeic) ester, 7HF, 3,7 DHF, 3,7 DHMF, and two chalcones, DHdHC and DHC, previously described in leaves, flowers and fruits of Z. punctata (De la Rocha et al., 2003; Svetaz et al., 2004, 2007; Zampini et al., 2005, 2008, 2012; Morán-Vieyra et al., 2009; Agüero et al., 2010; Isla et al., 2016; Carabajal et al., 2017, 2019, 2020; Moreno et al., 2018; Gomez et al., 2020) (Table 4). The chalcones, DHdHC and DHC were considered the chemical and biological markers of Z. punctata preparations (Isla et al., 2016, 2021a, b). The content of DHC was higher in YF than RBF (Table 3), while its level in aerial vegetative parts of both morphotypes was twenty-fold higher than fruits and similar between them. Chalcone isomerase (CHI) catalyzes the stereospecific and intramolecular isomerization of chalcone into its corresponding flavanones which further derive into flavonoids, including flavones, isoflavones, flavonols, and anthocyanidins (Nabavi et al., 2020).
Table 3. Total phenolic, flavonoids, anthocyanins, 2′, 4′-dihydroxychalcone, of two morphotypes of Z. punctata (red-brown and yellow). Values are reported as mean ± standard deviation of triplicates. For all variables with different letters in the same line, there are significant differences among the means according to Tukey’s test (p ≤ 0.05). Degrees of freedom = 8. Abbreviations: SP, soluble principles; TPC, total phenolic compounds (mg GAE.g-1 plant material); F, flavonoids (mg QE. g-1 plant material); AC, anthocyanins (mg C3GE.g-1 plant material); DHC, 2′, 4′-dihydroxychalcone (mg.g-1 plant material); RBF, red-brown fruits; YF, yellow fruits; RBFAP, aerial vegetative part of red-brown fruits morphotype; YFAP, aerial vegetative part of yellow fruits morphotype; ND, non detected.

Fig. 5 HPLC-DAD profiles of the phenolic-enriched extracts of Zuccagnia punctata aerial vegetative parts and fruits of two morphotypes, red and yellow fruits. A, yellow fruits extract. B, extract of aerial vegetative part of morphotype-yellow (YAP). C, red fruits extract. D, extract of aerial vegetative part of morphotype-red (RAP). Principal components: 1, 7-HF; 2, 1-methyl-3(4′ hidroxyphenyl propil caffeic) ester; 3, 3,7 DHF; 4, 3,7 DHMF; 5, 2′, 4′ DHdHC; 6, 2′, 4′ DHC.
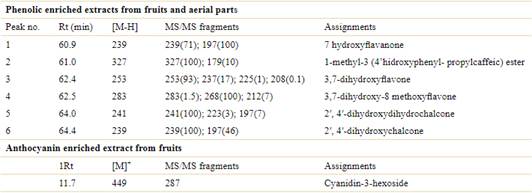
Table 4 Assignment of Z. punctata phenolics based on HPLC-ESI-MS/MS. Abbreviations: Rt, retention time (minutes); MS/MS, fragmentation pattern with the relative abundance in parenthesis for each proposed phenolic compound; [M-H], negative ion mode; [M]+, positive ion mode.
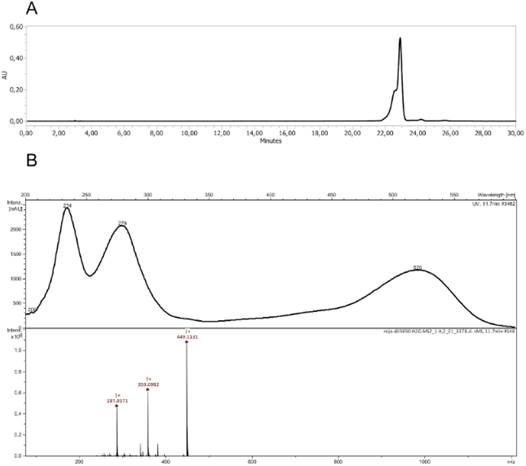
Anthocyanins were also detected in fruits of RBF morphotype but not in fruits of YF morphotype. The anthocyanin content in RBF was 4.75 mg.g-1. The anthocyanin level in Z. punctata fruits was similar to the other sources rich in this pigment, i.e. blueberries, dark grapes, black carrots, black currants and corn are 4.9, 6.0, 1.82, 4.75 and 4.9 mg.g-1 fresh weight, respectively (Somavat et al., 2018). Anthocyanins are one of the most commonly utilized water-soluble natural colorants because they exhibit bright colors that range from red to blue and have bioactive properties linked to certain health benefits, e.g., antioxidant, anti-diabetic, anti-inflammatory, and anti-cancerous effects (Chen et al., 2019).
HPLC analysis of the anthocyanins-enriched extract showed a main compound, with a maximum absorption of 520 nm and a [M]+ ion at m/z 449. MS/MS analyses indicated the neutral loss of 162 amu, in agreement with hexose (Fig. 6). The compound was assigned as cyanidin-3-hexoside, in agreement with the literature (Ha et al., 2010). Mass fragmentation patterns and identification of the anthocyanin are shown in Table 4. Consequently, the red pigmentation of fruits from RBF morphotype of Z. punctata could be due to the presence of anthocyanins. Genetic, biochemical, and cell biological evidence suggests that transcriptional activators and repressors function as part of a network to regulate anthocyanin biosynthesis linked to external factors, including temperature and light, excess metal stress and pathogen infection or developmental processes as well as hormone and sugar content (Jaakola, 2013; Chen et al., 2019; Dong et al., 2019; Chao et al., 2021). However, in this case the two chemotypes are present in the same population of Z. punctata at several altitudes and various environmental conditions. Further studies are needed to determine the factors that regulate the expression of anthocyanins in Z. punctata. Other plant species had similar patterns of fruit color variation; for example, Psidium cattleyanum Sabine and Solanum betaceum Cav. has two morphotypes: one with yellow-orange fruits and the other with red fruits, both with high content of anthocyanins (Isla et al., 2022; Montanari Beltrame et al., 2022).
CONCLUSIONS
Two morphotypes corresponding to two different cytotypes and two chemotypes of Z. punctata were described for the first time. The morphological character of diagnostic value to distinguish both morphotypes is the color of the fruits. The morphotypes analyzed are diploid with 2n = 24. The karyotypes were analyzed for the first time for the species. The YF morphotype showed a karyotypic formula of 24 m while RBF morphotype showed 18m + 6sm. The results obtained confirm that both morphotypes produce chalcones, the main compounds with pharmacological activity of Z. punctata and only the RBF morphotype produces anthocyanins, an antioxidant pigment, in similar concentrations to that of some commercial red fruits. This work constitutes an interesting discovery that contributes to the knowledge of this endemic plant from Argentina with wide potential for use in health care and that could be used to promote regional economies in the environments where it grows, taking into account that its management must be sustainable.












 uBio
uBio