Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Medicina (Buenos Aires)
versión impresa ISSN 0025-7680versión On-line ISSN 1669-9106
Medicina (B. Aires) v.63 n.2 Buenos Aires mar./abr. 2003
Von willebrand factor cleaving protease activity in the physiopathology of microangiopathic disorders
M. M. Amaral, A. C. Kempfer*, C. E. Farias*, G. A. Carballo, M. R. Silaf, M. A. Lazzari*
Instituto de Investigaciones Hematológicas, Academia Nacional de Medicina, Buenos Aires
*Miembro de la Carrera de Investigador del CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas)
Dirección postal: Lic. María Marta Amaral, Instituto de Investigaciones Hematológicas, Academia Nacional de Medicina, Pacheco de Melo 3081, 1425 Buenos Aires, Argentina.
Fax: (54-11) 4805-0712. E-mail: mmarta@hematologia.anm.edu.ar
Abstract
The von Willebrand factor cleaving protease (VWFCP) modulates the von Willebrand factor (VWF) multimeric size in normal plasma. VWFCP activity levels are decreased in different physiological and pathologic situations. Different techniques have been developed to unfold the purified VWF (perfusion at high shear rate, dialysis against urea in nitrocellulose filters), to detect the VWFCP activity on it (multimeric analysis of VWF, collagen binding to VWF assay) and to use the patient plasma both as the source of the enzyme and substrate. In this paper we compared the above mentioned methods with new ones: normal plasma dialyzed on membranes instead of purified VWF, dialysis of the samples against urea in tubing instead of nitrocellulose filters, and sonicated plasma to remove the endogenous VWF. The perfusion assay and detection by multimeric analysis showed a limit of detection (25%) of VWFCP activity. Dialysis against urea in both supports and detection by multimeric analysis, showed a better limit of detection (3%), but the recovery of the samples was not as efficient in nitrocellulose filters as it was in tubing. The detection by collagen binding to VWF has more advantages because it allows to analyze more samples than the multimeric analysis does in the same assay. The dialysis of plasma by membranes to obtain the source of exogenous VWF requires no complex equipment. The method, which uses patient plasma as the source of the enzyme and substrate, was inapplicable in our experience because the values could not be interpolated in the reference curve.
Key words: Von Willebrand factor; Cleaving protease; Thrombotic thrombocytopenic purpura.
Resumen
Detección de la actividad de la proteasa que cliva al factor von Willebrand en la patofisiología de las microangiopatías. La proteasa que cliva al factor von Willebrand (VWFCP) controla el tamaño multimérico del factor von Willebrand (VWF) en plasma normal. Sus niveles se encuentran disminuidos en diferentes situaciones fisiológicas y patológicas. Diferentes técnicas se desarrollaron para desplegar al VWF purificado (perfusión a alta fuerza de cizallamiento, diálisis contra urea en filtros de nitrocelulosa), para detectar la actividad de la VWFCP (análisis multimérico del VWF, enlace del VWF al colágeno) y para usar el plasma del paciente como fuente de enzima (VWFCP) y de sustrato (VWF). Este trabajo compara los métodos descritos con métodos nuevos: plasma dializado en membranas en lugar de VWF purificado, diálisis de la muestra contra urea en tubos, en lugar de filtros de nitrocelulosa y plasma sonicado para remover el VWF endógeno. El ensayo de perfusión y la detección por análisis mutimérico mostró un límite de detección de la actividad de la VWFCP del 25%. La diálisis contra urea en ambos soportes y la detección por análisis multimérico arrojó un límite mejor (3%), pero la recuperación de las muestras no fue tan eficiente en filtros de nitrocelulosa como en tubos. La detección por enlace del VWF al colágeno posee mayores ventajas al analizar más muestras en el mismo ensayo. La diálisis de plasma en membranas, para obtener VWF exógeno, no requiere equipos complejos. El método que usa el plasma del paciente como fuente de enzima y sustrato fue inaplicable en nuestras manos ya que los valores no pudieron ser interpolados en la curva de referencia.
Palabras clave: Factor von Willebrand; Proteasa; Púrpura trombocitopénica trombótica.
VWF is a glycoprotein circulating in plasma as a series of multimers ranging from 500 to 20.000 kD1. VWF precursor is synthesized as a very large protein in endothelial cells and megakaryocytes2,3. An important mechanism for depolymerization of the large multimers is the limited proteolysis by a VWFCP present in plasma. The apparent molecular weight of the enzyme is approximately 300 kD as estimated by gel filtration and sodium dodecyl sulfate-polyacrylamide gel electrophoresis1. The enzyme is found to specifically cleave the Y (842)-M (843) peptide bond in the A2 domain4. The shear stress may be a modulator of VWF size in the circulation because it increases the VWF susceptibility to proteolysis5. Proteolytic activity has a pH optimum at 8 to 9. The protease is activated by preincubation with divalent ions in the following order: Ba2+>Sr2+>Ca2+>Mg2+. It is inhibited by peptidyl diazomethyl ketones as Z-Phe-Phe-CHN2 and chelating agents as EDTA, EGTA1,5.
The active VWFCP is detected in human plasma, serum, cryoprecipitate supernatant, defibrinated plasma, and commercial plasma preparations including solvent-detergent-treated and methylene-blue-treated plasma1,6. When the protease is deficient in the plasma, ultralarge forms of VWF are released from damaged vascular endothelium7,8 and may cause thrombus formation because they aggregate platelets under high shear stress conditions9.
Two primary mechanisms for deficiency of VWFCP activity were identified, constitutive deficiency and presence of an acquired immunoglobulin inhibitor6,10. Recent studies showed complete constitutional deficiency of VWFCP activity in patients with familial thrombotic thrombocytopenic purpura (TTP)11,12 and the presence of autoantibodies inhibiting VWFCP in patients with nonfamilial TTP6, 10, 13. At the beginning, some authors as Furlan6, 11 and Tsai10 proposed that VWFCP levels could discriminate between TTP and hemolytic uremic syndrome (HUS). They demonstrated the deficiency of VWFCP in the plasma of patients with acute, sporadic, chronic relapsing, and familial TTP and they found that it was measurable in normal amounts in acute sporadic HUS. Nevertheless, in recent studies low levels of VWFCP activity were also found in patients with HUS14. Demonstration of its activity requires to subject substrate VWF to mechanical15, 5 or chemical denaturation1,16 to unfold VWF and to allow better access to the cleavage site. In vitro, the protease cleaves VWF under non-physiological conditions, such as low ionic strength in the presence of 1 to 1.5 M urea1, 6 or guanidine HCl16.
Till now, the methods developed to test the VWFCP effect on the VWF do not join all the necessary conditions: high sensibility, infallibility, and availability of the necessary substructure, quick and low cost. In the present report, we used the published techniques to unfold the purified VWF (perfusion at high shear rate, dialysis against urea in nitrocellulose filters), to detect the VWFCP activity on it (multimeric analysis of VWF, collagen binding to VWF assay) and to use the patient plasma both as the source of the enzyme and substrate. In addition we introduced the following modifications: filtered normal plasma instead of purified VWF, dialysis against urea in tubing of the samples instead of nitrocellulose filters and plasma sonicated to remove the endogenous VWF.
Materials and Methods
Materials
Type I agarose, type III collagen (from calf skin), 4-Chloro-1-naphtol, Tween 20, dialysis tubing D-9.277 with diameter size of 6mm (retain most proteins of mol. wt. 12.000 D or grater) were from SIGMA. Polyclonal antibodies (rabbit anti-human VWF, FITC rabbit anti-human VWF, biotinylated goat anti-rabbit IgG), and streptavidin-biotin-horse radish peroxidase complex (ABComplex/HRP) were from DAKO. Polystyrene beads with diameter size of 2.837m were from Polysciences, Inc. (Warrington, PA). Sephacryl S-1000 and Protein-G Sepharose 4B were from Pharmacia. Medicell membranes with diameter size of 20mm (retain most proteins of mol. wt. 1.000.000 D or grater) were from Spectrum Medical. Nitrocellulose filters with pore size of 0.025 and with size diameter of 25mm were from MILLIPORE.
All other reagents were of analytical grade.
Plasma preparation
Venous blood was collected from healthy volunteers donors, into 1:10 volume trisodium citrate to achieve a final concentration of 129mM. Platelet-poor plasma was obtained by centrifugation at 2.500g for 60 minutes at 4 °C, after which the plasma was transferred to another tube, it was centrifuged at 10.000 for 30 minutes and stored at -80 °C. Samples should contained less than 1000 platelets/mL. Pooled normal plasma (PNP) (n=15) was used as reference for VWF:CB.
Preparation of exogenous VWF
Purified VWF. VWF was isolated from fresh frozen plasma of normal volunteers following the procedures described by Thorell and Blombäck17. The eluted fractions containing VWF were pooled and concentrated using 20 % PEG 20.000 and dialyzed against 0.15M NaCl, pH 7.2 overnight.
Dialyzed plasma by membranes. Protease-depleted plasma by Medicell membranes, was used to replace the purified VWF. About 1mL of citrated plasma was depleted of VWFCP by dialysis against 0.15M NaCl, pH 7.2 for 19 hours. This method was developed at IIHEMA (not published yet).
Assay of VWFCP activity
The assay of VWFCP activity was performed in diluted plasma samples after activation by barium chloride and incubation with VWF substrate as previously described1 with some modifications. Curves with normal plasmas (n=10) were constructed undiluted and diluted from 1:2 to 1:32 with 0.15M NaCl, 10 mM Tris-HCl solution, pH 7.4. When the variations between the normals were estimated, a PNP was used to estimate the VWFCP %. BaCl2 (final concentration 0.01M) was added to each diluted plasma sample and then it was incubated at 37 °C for 30 minutes.
In a set of experiments, curves with normal plasmas (n=3) were constructed diluted from 1:2 to 1:32 with 0.15M NaCl, 10 mM Tris-HCl solution, pH 7.4 and Pefabloc SC 1mM.
Degradation of VWF from plasma
The degradation of VWF was performed by sonication using the method described by Casillas et al18.
Preparation of samples to be tested with exogenous VWF
Aliquots (148mL) of diluted plasma samples, after activation, by barium chloride were incubated with 36mL of exogenous VWF at a final concentration of 1U/mL to evaluate VWFCP activity.
Preparation of samples without exogenous VWF
500mL of the test plasma were placed in dialysis casing and so they were used as a source of the enzyme and substrate, negating the need for purified VWF or other preparation of normal plasma VWF19. The degree of VWF cleavage by the protease is then quantitated by exploiting the property of larger multimers to bind preferentially to human collagen type III20,21.
Conformational change of VWF
Perfusion at high shear rate
The perfusion assay was developed using the method previously described15.
Urea treatment
i) Dialysis on hydrophilic filter
100 mL of the test samples were dialyzed on the surface of a hydrophilic filter for 19 hours at 37 °C against 1.5M urea and 5mM TRIS (pH 8). The reaction was stopped by the addition of EDTA (final concentration 20mM, pH 7.4)6.
ii) Dialysis in tubing
Aliquots (184mL) of the test samples were dialyzed in tubing, which were immersed in 1.5 M urea 5mM Tris-HCl, pH 8, for 19 hours at 37 °C. Then, the samples were dialyzed against 1.5M NaCl, (pH 7.4). The reaction was stopped by the addition of EDTA (final concentration 20mM, pH 7.4.
Laboratory test to detect the effect of VWFCP on VWF
Multimeric analysis of VWF
A multimeric analysis of normal VWF, subject to proteolytic degradation, was performed on SDS-1% agarose gel electrophoresis and it was visualized by immunoenzymatic stain, like it was previously described22. VWF multimers were measured by densitometrical scanning of the stained gels with a Sharp Scanner (JX 330, Hamburg, Germany) using the software ImageMaster (Pharmacia, Newcastle, England). The used software gave the RF value automatically. The control in each running was the purified VWF (100% of present multimers) that was considered as RF=0.
Binding of VWF to collagen (VWF:CB)
Collagen has been shown to bind VWF, with the preferred binding with high molecular weight forms of VWF23,20. This assay is based on modifications of the VWF:CB assay previously described24. 7mL of buffer Horm (isotonic glucose solution, pH 2.7-2.9) were added to 1mL of a collagen solution (100mg/mL in isotonic glucose solution, pH 2.7-2.9), the mix was incubated 5 minutes at 37 °C. Finally 1000mL of polystyrene beads were washed and resuspended in 5mL of this last solution. The beads were coated by re-suspending them in 10 % BSA diluted in an ELISA buffer and they were incubated for 30 minutes.
A calibration curve was set up always using PNP (1:20 to 1:640). Aliquots of 20mL of beads were added to the treated samples and to PNP, and they were incubated gently rocking at room temperature for 1 hour in Eppendorf tubes. After the beads were washed (2 times), they were incubated with anti-VWF-FITC diluted 1:100 in ELISA buffer containing 0.1% BSA, in the darkness for 30 minutes at 37 °C. Finally the beads were centrifuged, washed with ELISA buffer with 0.1% BSA, re-suspended in 500mL of Isoflow and transferred to polyethylene tubes. All samples were measured by flow cytometry (FACSCAN, Becton Dickinson) and analyzed with the Cell Quest software. The results were expressed as the median of fluorescence.
The 1-U/mL VWF:CB represents the loss of large VWF multimers, which is directly proportional to VWFCP activity, so 1-U/mL VWF:CB = 0 corresponds to 100% of multimers.
Assay for VWF:Ag
This assay is based in the method previously described24. The solutions and materials used were identical to the VWF: CB assay previously described.
Statistical Analysis
The values were expressed in terms of media ±SD, and the significant differences were examined by the paired t test. P values below 0.001 were considered to be statistically significant. Inter-assay variation, expressed as between-run coefficient of variation, was determined by using normal and patients samples, they were frozen in aliquots and then subsequently thawed and tested repeatedly on different days. Intra-assay variation, expressed as within-run coefficient of variation, was determined using those samples, which were tested repeatedly on the same day.
Results
Detection of VWFCP activity after perfusion at high shear rate by multimeric analysis of VWF
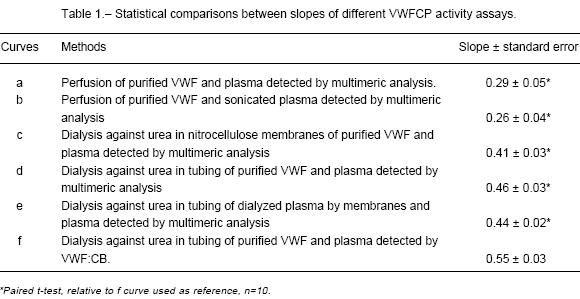
As previously described, in each curve the control RF=0 was the purified VWF. In all the assays, undiluted normal plasma was considered as 100% VWFCP. A quantification curve (n=10) was made with normal plasma (undiluted to 1:16) + purified VWF (Figure 1, curve a). The curve exhibits a good correlation (coefficient=0.96). No statistical differences (P=0.278) were found between the media ± SD of purified VWF and purified VWF + normal plasma (diluted in 1:8), suggesting that the limit of detection (P<0.001) was 25% of proteasic activity according to purified VWF + normal plasma (diluted 1:4). Figure 1, curve b, represents the quantification curve of sonicated samples and it presented a good correlation (coefficient=0.96). No statistical differences were found, for all the dilutions of quantification curve, between RF of sonicated plasma and non-sonicated plasma. No statistical differences were found, between RF of normal plasma dilutions with and without Pefabloc (data not shown).
No statistical differences of VWF:Ag were found between sonicated and non sonicated samples (data not shown).

Detection of VWFCP activity after dialysis against urea in nitrocellulose filters by multimeric analysis of VWF
A quantification curve (n=10) was made with normal plasma (undiluted to 1:32) + purified VWF (Figure 1, curve c). The correlation coefficient of the curve was 0.99. The obtained RF values by dialysis against urea in nitrocellulose filters method were significantly different (P<0.001) from all the curve dilutions regarding purified VWF. The limit of detection was 3% of proteasic activity according to purified VWF + normal plasma (diluted 1:32). No statistical differences of VWF:Ag were found between treated and untreated samples (data not shown).
Comparison of the RF values obtained by perfusion at high shear rate and dialysis against urea in nitrocellulose filters. Detection by multimeric analysis of VWF
The comparison of the RF values of plasma samples (n=10), which were exposed to both methods to get the conformational change of VWF, showed that dialysis against urea in nitrocellulose filters method presented statistically significant differences (P<0.001) regarding perfusion at high shear rate.
No statistical differences of VWF:Ag were found between treated and untreated samples (data not shown).
Detection of VWFCP activity after dialysis against urea in tubing by multimeric analysis of VWF
A quantification curve (n=10) was made with normal plasma (undiluted to 1:32) + purified VWF (Figure 1, curve d). It presented a correlation coefficient of 0.99. The obtained RF values by dialysis against urea in tubing method were significantly different (P<0.05) regarding purified VWF. The limit of detection was 3% of proteasic activity according to purified VWF + normal plasma (diluted 1:32). No statistical differences of VWF: Ag were found between treated and untreated samples (data not shown).
The comparison of RF values derived from the dialysis against urea in tubing method with two different exogenous VWF sources; purified (Figure 1, curve d) and dialyzed plasma by membranes (Figure 1, curve e), showed that no statistical differences were found between the dilutions of both quantification curve (purified VWF or dialyzed plasma by membranes) except at 1:32 plasma dilution (P<0.001) which corresponds to the limit of detection (3 % of proteasic activity). The Figure 1, curve e, shows a correlation coefficient of 0.99.
No statistical differences of VWF:Ag were found between samples with purified and dialyzed plasma by membranes (data not shown).
Detection of VWFCP activity after dialysis against urea in tubing by VWF:CB
Results suggested that all the 1-U /mL VWF (VWF:CB) values obtained from the quantification curve dilutions, were significantly different (P<0.001) from the purified VWF (Figure 1, curve f). The limit of detection (3% of proteasic activity) corresponds to purified VWF + plasma 1:32. No statistical differences of VWF: Ag were found between treated and untreated samples (data not shown).
When patients plasma was submitted to dialysis against urea in tubing method, within-run (n=15) coefficient of variation was 13.6%, and between-run (n=15) coefficient of variation was 18 %. For normal plasma within-run (n=17) coefficient of variation was 5.4 %, and between-run (n=17) coefficient of variation was 3.5%.
Comparison of quantification curves obtained by different methods to get the conformational change of VWF and to detect the VWFCP effect on VWF
The slope of the quantification curve that was obtained by dialysis against urea in tubing and detection by VWF:CB was significantly different (P<0.001) from the slopes of quantification curves given by the others methods above described (Table 1).
Dialysis against urea in tubing applied to plasma samples without exogenous VWF. Detection by VWF:CB
Undiluted and diluted (1:2) normal plasma samples were submitted to the conformational change of VWF by dialysis against urea in tubing. A PNP reference curve was used to interpolate these samples and extrapolate the 1-U/mL (VWF:CB) results. The fluorescence medians of the samples were under the 1:640 PNP dilution (data not shown).
No statistical differences of VWF:Ag were found between treated and untreated samples (data not shown).
Discussion
A VWFCP activity has been identified in normal plasma, which degrades VWF at position 842-843 of the subunit4. In vivo this protease appears to play a critical role by regulating the size of VWF multimers for optimal efficiency in platelet adhesion to the subendothelium under high shear conditions25. Deficiency of VWFCP (ADAMTS13) is the molecular mechanism responsible for TTP26. Recently, Mannucci27 measured the VWFCP in a population of healthy controls from newborns to elderly. The protease levels were low in newborns but became normal within 6 months. In healthy people the VWFCP was decreased since 65 years old, and in pregnancy it was lower in the last 2 trimesters than in the first. In cirrhosis, uremia, acute inflammation and postoperative period the VWFCP activity was also found decreased. Liver and renal diseases and inflammatory states are not unusual in thrombotic microangiopathies, but low protease values in these states do not mean TTP. In addition, the protease may be low in patients with HUS and renal or liver failure, therefore this observation seems to be opposed to the idea that normal protease levels accompany HUS6, 10,11. Remuzzi et al28 found that deficient ADAMTS13 activity does not distinguish TTP from HUS, at least in the recurrent and familial forms.
As VWFCP activity levels are decreased in different physiological and pathologic situations, we think that is very important to find the most effective method to test the VWFCP activity. Therefore, these protease levels could be related with clinics features in the diagnostic of patients.
The purpose of the present study was to find a sensitive method that does not require high technology to detect VWFCP activity.
Kempfer and Tsai5,15 measured the protease in plasma samples by perfusion at high shear rate. Since their observations, we attempted to use this method for assay the VWFCP quantification in normal plasma dilutions in which purified VWF was added. Nevertheless, we found that the limit of detection was low (25%).
Plasma sonication allows to obtain plasma samples free of VWF activity without modification of proteasic activity. We removed the endogenous VWF18 because at plasma dilutions lower than 1:20, endogenous VWF interferes in the VWFCP measurement, especially if it is decreased or if the cleavage site is disturbed. The obtained results suggested that the VWFCP activity was not affected by sonication. Remuzzi et al28 confirms their results by evaluating the cleavage of recombinant VWF A1-A2-A3 domains, which overcomes possible artifacts of the VWF:CB due to the presence of endogenous undegraded VWF in test samples.
In our experience, the method described by Furlan1 showed a lower limit of detection of VWFCP activity (3%) than perfusion at high shear rate (25%). Nevertheless, this method practically fails due to evaporation or hydratation of the samples during dialysis against urea buffer. Obert25 placed the nitrocellulose filters in Petri boxes, but we employed this alternative and did not improve the recovery of the samples. A similar limit of detection of VWFCP activity was obtained replacing nitrocellulose filters by dialysis tubing and it did not fail.
Since the preparation of purified VWF needs a complex substructure, we removed the plasma VWFCP by dialysis in membranes to obtain the source of exogenous VWF.
For the VWFCP activity detection we used the VWF:CB assay. It has a limit of detection similar to the obtained limit by multimeric analysis. In addition, this method allows to include much more samples than the multimeric VWF assay does and it is faster too (7 hours instead of 48 hours). We have observed that between-run and within-run precision data of the method were suitable.
Rick19 developed a VWF: CB that allows a quantitative test that can be performed in less than a day. In our experience, this technique was inapplicable because the values of the treated plasma were under the limit of detection of the reference curve of VWF:CB.
In conclusion, to quantify the VWFCP activity, we advise using dialysis against urea in tubing to unfold VWF and VWF:CB to detect the remanent VWF. VWF:CB technique by flow citometry is not available in all laboratories and it can be replaced by an ELISA method in micro wells29. To the best of our knowledge, our data provide a demonstration that dialyzed plasma in membranes could be used instead of purified VWF.
Acknowledgements: This work was supported by grants from the Alberto J. Roemmers Foundation, René Barón Foundation and CNICT, National Research Council, Argentina.
1. Furlan M, Robles R, Lämmle B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood 1996; 87: 4223-34. [ Links ]
2. Jaffe EA, Hoyer LW, Nachman RL. Synthesis of von Wille-brand factor by cultured human endothelial cells. Proc Natl Acad Sci U S A. 1974; 71: 1906-09. [ Links ]
3. Nachman R, Levine R, Jaffe EA. Synthesis of factor VIII antigen by cultured guinea pig megakaryiocytes. J Clin Invest. 1977; 60: 914-21. [ Links ]
4. Dent J, Berkowitz S, Ware J, Kasper C, Ruggeri Z. Identi-fication of cleavage site directing the immunochemical detection of molecular abnormalities in type IIA.Von Willebrand factor. Proc Natl Acad Sci USA 1990; 87: 6306-10. [ Links ]
5. Tsai H, Sussman I, Nagel R. Shear stress enhances the proteolysis of Von Willebrand factor in normal plasma. Blood 1994; 83: 2171-79. [ Links ]
6. Furlan M, Robies R, Galbusera M, et al. Von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic uremic syndrome. N Engl J Med 1998; 339: 1578-84. [ Links ]
7. Sporn LA, Marder VJ, Wagner DD. Inducible secretion of large, biologically potent von Willebrand factor multimers. Cell 1986; 46: 185-190. [ Links ]
8. Tsai HM, Nagel RL, Hatcher VB, Sussman II. Multimeric composition of endothelial cell-derived von Willebrand factor. Blood 1989; 73: 2074-76. [ Links ]
9. Moake JL, Turner NA, Stathopoulos NA, Nolasco LH, Hellmus JD. Involvement of large plasma von Willebrand factor (VWF) multimers and unusually large VWF forms derived from endothelial cells in shear stress-induced platelet aggregation. J Clin Invest 1986; 78: 1456-61. [ Links ]
10. Tsai H, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med 1998; 339: 1585-94. [ Links ]
11. Furlan M, Robles R, Solenthaler M, Wassmer M, Sandoz P, Lämmle B. Deficient activity of von Willebrand factor-cleaving protease in chronic relapsing thrombotic trombo-cytopenic purpura. Blood 1997; 89: 3097-103. [ Links ]
12. Furlan M, Lämmle B. Aetiology and pathogenesis of thrombotic trombocytopenic purpura and hemolytic uremic syndrome: the role of von Willebrand factor cleaving protease. Baillières Clin Haematol 2001; 14: 437-54. [ Links ]
13. Furlan M, Robles R, Solenthaler M, Lämmle B. Acquired deficiency of von Willebrand factor-cleaving protease in a patient with thrombotic trombocytopenic purpura. Blood 1998; 91: 2839-46. [ Links ]
14. Veyradier A, Obert B, Houllier A, Meyer D, Girma JP. Specific von Willebrand factor-cleaving protease in thrombotic microangiopathies: a study of 111 cases. Blood 2001; 98: 1765-72. [ Links ]
15. Kempfer AC, Farias CE, Schattner MA, Lazzari MA. Requirement of a plasma fraction for the loss of large VWF multimers induced by high wall shear rate. Medicina (Buenos Aires) 1997; 57: 409-16. [ Links ]
16. Tsai H. Physiologic cleavage of von Willebrand factor by plasma protease is dependent on its conformation and requires calcium ion. Blood 1996; 87: 4235-44. [ Links ]
17. Thorell L, Blomback B. Purification of the FVIII complex. Thromb Res 1984; 35: 431-49. [ Links ]
18. Casillas G, Simonetti C, Lazzari MA, Kempfer AC. Effects of sonication of factor VIII. Thromb Haemost 1980; 43: 29. [ Links ]
19. Rick M. E. A. Rapid Assay for the Protease. Thromb Hae-most 2001; 185: 184-5. [ Links ]
20. Santoro SA. Preferential binding of high molecular weight forms of von Willebrand factor to fibrillar collagen. Biochim Biophys Acta 1983; 756: 123-6. [ Links ]
21. Brown JE, Bosak JO. An ELISA test for the binding of von Willebrand antigen collagen. Thromb Res 1986; 43: 303-11. [ Links ]
22. Aihara M, Sawada Y, Ueno K, et al. Visualization of von Willebrand factor multimers by immunoenzymatic stain using avidin-biotin peroxidase complex. Thromb Haemost 1986; 55: 263-7. [ Links ]
23. Scott CM, Griffin B, Pepper DS, Barnes MJ. The binding of purified factor VIII von Willebrand factor to collagens of different type and forms. Thromb Res 1981; 24: 467-72. [ Links ]
24. Kempfer AC, Silaf MR, Farias CE, Carballo GA, Woods AI, Lazzari MA. Binding of von Willebrand factor to collagen by flow cytometry. Am J Clin Pathol 1999; 111: 418-23. [ Links ]
25. Obert B, Tout H, Veyradier A, Fressinaud E, Meyer D, Girma JP. Estimation of the von Willebrand factor-cleaving protease in plasma using monoclonal antibodies to VWF. Throm Haemost 1999; 82: 1382-5. [ Links ]
26. Levy GG, Nichols WC, Lian EC, et al. Mutations in a mem-ber of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature 2001; 6855: 488-94. [ Links ]
27. Mannucci PM, Canciani MT, Forza I, Lussana F, Lattuada A, Rossi E. Changes in health and disease of the metallo-protease that cleaves von Willebrand factor. Blood 2001; 98: 2730-35. [ Links ]
28. Remuzzi G, Galbusera M, Noris M, et al. von Willebrand factor cleaving protease (ADAMTS13) is deficient in recu-rrent and familial thrombotic trombocytopenic purpura and hemolytic uremic syndrome. Blood 2002; 100: 778-85. [ Links ]
29. Gerritsen H, Turecek P, Schuarz H, LämmLe B. Assay of Von Willebrand Factor (VWF)-cleaving protease based on decreased collagen binding affinity of degraded VWF. Thromb Haemost 1999; 82: 1386-9. [ Links ]
Received: 27de Julio 2002
Accepted: 3 de marzo 2003














