Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Medicina (Buenos Aires)
versión impresa ISSN 0025-7680versión On-line ISSN 1669-9106
Medicina (B. Aires) v.63 n.4 Buenos Aires jul./ago. 2003
Effect of lactobacillus strains and saccharomyces boulardii on persistent diarrhea in children
Gaon D.1, Garcia H.2, Winter L.2, Rodríguez N.2, Quintas R.1, Gonzalez S. N.3, Oliver G.3
1Cátedra de Salud Pública, Facultad de Medicina, Universidad de Buenos Aires;
2Departamento de Pediatría, Hospital A. Posadas, Haedo, Provincia de Buenos Aires;
3Cerela (Centro de Referencia para Lactobacilos) y Departamento de Bioquímica, Facultad de Medicina, Universidad de Tucumán
Abstract
The efficacy of probiotics on persistent diarrhea remains uncertain. The purpose of this study was to evaluate the effect of Lactobacillus sp and Saccharomyces boulardii on persistent diarrhea in children. In a double-blind trial eighty-nine children, aged 6-24 months were randomly distributed to receive pasteurized cow milk containing 2 viable lyophilized strains Lactobacillus casei and Lactobacillus acidophillus strains CERELA, (1010-1012 colony-forming units per g) (n =30), or lyophilized S. boulardii, (1010-1012 colony forming units per g) (n =30) or pasteurized cow milk as placebo (n =29); on each diet 175 g was given twice a day for a 5 day period. Number of depositions, duration of illness and frequency of vomiting were considered. Enteric pathogens were isolated from stools in 40% of the patients, 27% had rotavirus. Lactobacillus and S.boulardii significantly reduced the number of depositions (p < 0.001) and diarrheal duration (p < 0.005). Similarly both significantly (p < 0.002) reduced vomiting as compared with placebo. There was no difference between treatments depending on rotavirus status. In conclusion, L. casei and L. acidophillus strains CERELA and S. boulardii are useful in the management of persistent diarrhea in children.
Key words: Persistent diarrhea; Probiotics; Lactobacillus sp;Saccharomyces boulardii; Rotavirus.
Resumen
La eficacia de los probióticos sobre la diarrea persistente en niños aún no ha sido comprobada. Este trabajo controlado doble ciego tuvo como propósito evaluar ese efecto usando Lactobacillus sp y Saccharomyces boulardii. Ochenta y nueve niños entre 6 meses y 2 años de edad fueron distribuidos al azar para recibir leche pasteurizada conteniendo cepas liofilizadas de Lactobacillus casei y Lactobacillus acidophillus desarrolladas por CERELA (Centro de Referencia para Lactobacilos (1010-1012 CFU por g), n=30, o cepas liofilizadas de S. boulardii (1010-1012 CFU por g), n = 30, o placebo, n =29. Cada niño recibió 175 g dos veces por día durante 5 días. Se evaluó el número de deposiciones/día, la duración de la diarrea y la duración de los síntomas. Se aislaron gérmenes patógenos en las heces en el 40% de los casos: 27% eran rotavirus. Lactobacillus sp y S. boulardii redujeron significativamente el número de deposiciones (p < 0.001), la duración de la diarrea y el número de vómitos (p< 0.005) y (p< 0.002) respectivamente, comparado con placebo. No hubo diferencias entre los tratamientos en pacientes con rotavirus-positivo o rotavirus-negativo. En conclusión, este trabajo demuestra que cepas de L. casei y L. acidophillus desarrolladas por CERELA y de S. boulardii son similarmente efectivas en el tratamiento de la diarrea persistente en niños.
Palabras clave: Diarrea persistente; Probióticos; Lactobacillus sp;Saccharomyces boulardii; Rotavirus.
The vast majority of young children with acute diarrhea and mild or no dehydration who are managed according to appropriate treatment protocols such as that promoted by World Health Organization (WHO)1 can be successfully treated with lactose-containing diets or with lactose- free ones2. Besides, available evidence indicates that undiluted non human milks and breast milk are well tolerated during diarrhea and may in fact reduce the severity and duration of the illness and prevent the nutritional status deterioration3, 4, Lactobacillus included in probiotics can also limit the course of acute diarrhea4,5 especially rotavirus diarrhea6, 7, although variable results can be found according to the use of diverse species and subspecies8 or to the number of colony forming units9(CFU).
Saccharomyces boulardii, a nonpathogenic yeast, has also been used empirically in the treatment of acute infectious diarrhea10, 11. S. boulardii adheres to human jejunal cells but it does not multiply in the gut and disappears from the stools once ingestion has stopped12 Both probiotics, Lactobacillus and Sacharomyces boulardii supposedly exert an important impact in either the small or large intestine but the exact nature by which they exert their protective effects is not known.
Persistent diarrhea in children is defined as the continuation of an acute diarrheal episode for at least two weeks13. The illness is associated with malnutrition, malabsortion and growth failure, and is widely prevalent in developing countries, where 8% of acute diarrheas turn into persistent type14, 15..The cause of persistent diarrhea is not known, pathogenic mechanisms are not well-understood and satisfactory treatment is not available15, so that many cases are refractory and death rates remain high, 45-70%14. A recent study16 showed that green banana and pectin mediated through their bacterial conversion into short-chain fatty acids (SCFA) are useful in the dietary management of persistent diarrhea. Similar beneficial effect with bifidobacteria and lactobacilli has also been recognized in the management of persistent diarrhea in several studies17,18 . Based on these data and others observed in controlled clinical trials, indicating that those microorganisms are effective in many intestinal disorders19, and since S. boulardii has never been tested in persistent diarrhea, we assessed by a well-controlled study the efficacy of Lactobacillus acidophillus subsp CRL 730 and Lactobacillus casei subsp CRL 431 from the Cerela Culture Collection (CRL) and S.boulardii in the management of children with persistent diarrhea.
Material and Methods
Patients. This, randomized, double-blind, placebo-controlled study was implemented in the Departamento de Maternidad e Infancia del Hospital A. Posadas, situated in Moron, a community on the Northwestern outskirts of Buenos Aires city, from January 1996 to April 1998. The children were selected from the outpatients department and admitted into a special study ward.
Children of both sexes between 6 to 24 months of age who presented the following characteristics were admitted: (1) a history of frequent loose stools (> 3 per day) for the last consecutive 14 days or more, (2) not being breast fed, (3) no history of allergy to cow´s milk, or no history of treatment with antimicrobial or antidiarrheal agents within the preceding 7 days, (4) an absence of concurrent systemic illness, a weight for age < 60% of the value for the 50 th percentile according to the tables of the American National Center for Health Statistics Standard, or dehydration of more than 10% of body weight (severe), and (5) ability to take oral food. For each child, an informed written consent was required, either from their parents or legal guardian, and the study protocol was approved by the Ethical Review Committee of Hospital A. Posadas.
Patients evaluation. Each patient remained in the hospital for at least 5 days. A complete history was recorded, and physical examination including assessment of dehydration was performed by a physician on admission to hospital (LW). Patients with mild or moderate dehydration were rehydrated ad libitum orally or by gastric tube for 4 to 6 hours with the standard WHO/UNICEF sodium- glucose-based oral rehydration salt (ORS) solution before starting the study. The fluid deficit was corrected within four to six hours of admission. After rehydration and until diarrhea stopped, ORS solution was offered according to WHO recommendations as a maintenance therapy.
The patient´s body weight, the amount of oral rehydration solution, the average energy intake, the mean volume of formula consumed, as well as the number and type of stools, (liquid, soft or normal) and clinical symptoms such as vomiting, abdominal distention (abdominal circumference) or colic, were daily recorded by specially trained nursing staff every eight-hours until discharge. Urine was collected separately in urine bags and weighed. At the end of the study, patients who had been discharged from the hospital without a complete clinical recovery were followed up daily either in the hospital or at home via a chart filled out by their parents for 12 days after starting the study.
Feeding was started as soon as children were considered to be fully rehydrated. Subjects were given soft foods from hospital according to their ages, including milk containing lactose, and milk products usually consumed by the child (fermented dairy products were excluded to avoid the effects of substances produced during fermentation process). Children under one year, received jelly, boiled mashed vegetables, potatoes with meat, as well as cooked cereal (rice, maize powder) and vegetable oil. The older children received an ordinary mixed diet. Diets and usual drink were freely given to the children by their mothers and under the supervision of nurses.
When an unformed stool was followed by 2 stools that retains its shape and does not stick to the container (formed stool), diarrhea was considered stopped.
An increase in fecal motion for 3 consecutive 8 hours periods; a recurrence of dehydration of more than 5% of body weight during maintenance oral rehydration therapy, or a recurrence or starting of gastrointestinal symptoms such as vomiting, abdominal distention and colic at any time during the treatment period, indicated a treatment failure.
Tolerability was assessed during each clinical evaluation, and all adverse events were recorded.
Study medication. After admission, children were allocated to receive one of three dietary treatments. Group 1 received pasteurized cow milk, as placebo, group 2 received lyophilized S. boulardii, and group 3 received pasteurized cow´s milk containing lyophilized Lactobacillus casei, and Lactobacillus acidophillus sp strains CERELA (1010-1012 CFU per g). The yeast was reconstituted in sterile distilled water at a concen-tration of 0.1 g/mL (1 g of powder per mL contained 1010 CFU of S. boulardii) in order to be suspended in pasteurized cow´s milk.
Treatments were given twice daily, at a dose of 175 g during 5 days. Each formula with the same consistence and appearence was supplied in opaque bottles and were given to the children by their mothers under the direct supervision of nursing staff. All study formula were given to children for 5 days. None of the staff had access to the randomization codes.
Laboratory determination. On admission, a fresh stool sample or occasionally a rectal swab was obtained from each of the patients to identify rotavirus and adenovirus by genus-specific enzyme-linked immunosorbent assay (ELISA kit, Abbott laboratories) and a standard microbiological culturing was performed for Escherichia coli, Salmonella, Shigella, Campylobacter and Yersinia 20. Specimens were transported in Cary-Blair medium to the microbiology laboratory (Hospital A. Posadas). After reception, they were inmediatly frozen and stored at –20° C until they were assayed (within 10 days). Stools specimens were examined microscopically for the presence of ova and parasites, and a special test for Giardia lamblia measured by enzyme-linked immunosorbent assay was performed. The presence of reducing substances in the stools were tested by Clinitest (Ames) on admission and on every alternate stool. A pH measurement of every stool was done with pH paper. The osmotic gap was calculated from electrolyte concentration on every stool water during the first 48 hours of study, using the following formula: 290-2([Na+]+[K+]), and stool fat was evaluated by means of a Sudan stain.
Anal swabing was cultivated in a LAPT soft agar medium20 in order to examine the implantation of lactobacilli in the intestine on admission, after stopping treatment, and even seven days after discontinuing each formula (Cerela).
Venous blood was obtained for determination of electrolytes ( Na+, K+, Cl-, HCO3-), glucose, proteins, hematocrit and total and differencial white cell count.
Statistical analysis. Data of the 3 treatment groups were analyzed by means of the non-parametrical U tests of Mann-Whitney and Kruskal- Wallis. To compare the differences between the curves, the Log Rank test was calculated. The sample size was estimated with reference to previous studies, with an assumption of a 50% reduction in stool frequency, a power of 80% and a P value of less than 0.05. (R.Q.).
Results
Patients. Of the 93 children enrolled, 4 were excluded before the final analysis: 2 due to vomiting (both receiving placebo) and 2 due to urinary tract infection (1 receiving S.boulardii and 1 receiving Lactobacillus). Five to 8 children were recruited each month.
A total of 89 patients were evaluated: 29 received placebo (group 1), 30 received S. boulardii (group 2) and 30 received Lactobacillus. The three study groups were comparable in their demographic and clinical characteristics at admission. There were no significant differences among the groups in the distribution of the enteropathogens at admission.
An etiologic agent was isolated in 35 of the 89 (40%) patients evaluated, with rotavirus, with rotavirus in 24 patients (Table 1).

Clinical Results
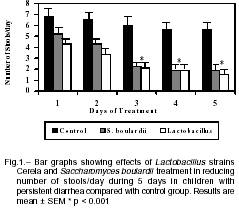
Number of depositions. Children receiving S. boulardii or lactobacilli had a gradual reduction in the number of daily stools, more noticeable after the first day of treatment, compared to those in placebo group. On day 5, the number of daily stools decreased to 2.0 ± 2.0 in the S. boulardii and 1.5 ± 0.9 in the lactobacilli group, respectively, in comparison with placebo (5.2 ± 3.0), (p< 0.001) (Fig 1).
Duration of diarrhea. In a similar way the duration of diarrhea (days mean ± SD) did not differ significantly between children receiving S. boulardi (3.8± 1.5) orlactobacilli (3.7 ± 1.3) compared with placebo (8.5 ± 4.2); p< 0.005 respectively.
The analysis of patients whose diarrhea did not stop within the 5-day study period were 17% in S. boulardii (5/30), 10% in lactobacilli (3/30) and 90% (26/29) in placebo, respectively. In the placebo group most children continued manifesting diarrhea up to 12 days.
With regard tothe duration of symptomsthe results showedthat patients treated with S. boulardii or Lacto-bacillus had a significant faster recovery compared with those receiving placebo (days, mean ± SD: 2.4 ± 1.6, 1.5 ± 1.4, 3.2 ± 2.0, p< 0.025 and p< 0.002 respectively) without a significant difference between both groups.
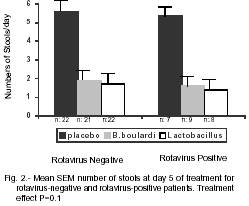
There was no difference between treatments depending on rotavirus status concerning recovery rates. Saccharomyces boulardii and Lactobacillus versus placebo were found to be similarly effective (p= 0.1) in both rotavirus-positive and rotavirus-negative (Fig 2).
The presence ofLactobacillus casei was investigated in the feces of 30 patients fromgroup 3 on day-5 and wasdetected in 28 patients with amean fecal count of 107 CFU/g. Nineteen of those patients (68%) still showed persistence of lactobacilli in the feces (105-106 CFU/g), seven days after discharge.
The presence ofreducing substances in stoolswas measured in all patients and there was no significant association between the presence of reducing substances and lack of efficacy of treatments. Stool pH mostly showed values of > 5.6, and the fecal osmotic gap was of 60.6 mOsm/kg (mean SD), suggesting that electrolytes account for most of stools osmolarity (secretory diarrhea) without substantial variation by feeding effects. The presence of excessively large and numerous fat globules by stain were only observed in a few cases (15%).There were no treatment failures neither appearance of symptoms possibly related to treatment.
Discussion
Childhood diarrhea accounts for substantial morbidity and mortality worldwide especially among children from developing countries who are more susceptible as a result of poor nutrition, impaired immune status or frequent exposure to infectious agents1.
The results of our studyestablished the efficacy of L. casei and L. acidophillus strains Cerela and S. boulardii in the management of persistent diarrhea in children. Both therapies significantly reduced the number of daily stools, shortened the duration of illness and produced a significant clinical recovery compared with those who received placebo. Within 5 days of starting Lactobacillus and S. boulardii, most children recovered completely from their illness and were discharged from the hospital. Only a few patients paid a return visit to hospital within 3 months after hospital discharge, probably indicating a low recurrence of diarrhea at home.
Lactobacillus and S. boulardii were found to be similarly effective to decrease the duration of diarrhea inboth rotavirus-positive and rotavirus-negative patients although statistical significance for reduction of symptoms may be doubtful probably due to the small number of children with rotavirus being studied. Further studies should be done to ascertain the precise role of these organisms on the gastroenteritis caused by rotavirus and other pathogens.
Rotavirus is a common cause of non-bloody diarrhea in children, accounting for 50-75% of episodes of acute diarrhea in children below3 years referred to the hospital21, but it is unknownif this percentage is always present in persistent diarrhea. In our study only 27% of patients had rotavirus. Rotavirus were observed continually on the time course of study.
Exactly how Lactobacillus and S. boulardii exert their protective effects has not yet been established.
Lactobacillus have traditionally been thought to work by competitive exclusion inhibiting the attachment and growth of pathogenic organisms, restoring the microbial balance in the gastrointestinal tract22. Lactobacillus may also enhance the immune-modulating effect on the host through Ig A response, or modifying mucosal IL-10 and Th1,Th2 lymphocytes thereby altering the cytokine profile23. Moreover, produce a proteinaceous factor(s) that alters epithelial permeability inhibiting bacterial translocation24 and may also influence the levels of gut mucin glycoproteins25.
Although any of the postulated mechanisms may lead to a shorter duration of acute or chronic diarrhea, several issues should be kept in mind when Lactobacillus have to be used in controlled trials. Those unresolved issues are: 1) strain selection, because not every strain shows efficacy and not every strain has efficacy in each disease8, 2) dosage9, 3) frequency of administration, 4) optimal delivery vehicle6, 5) time passed to develop formed stool and 6) single strain or combinations26. Thus it is important to select a well-characterized strain from a well known supplier.
The effect of Lactobacillus on diarrhea duration is not modifiable by country of study neither is modifiable when comparing preparations containing living or killed microorganism9.
Taken orally it does not persist in the gut, so continuous dosing is required27, 28.Respect Lactobacillus combinations, although the properties and activities of the individual bacterial constituents should be well established, the choice of proved combinations with presumed synergistic activities, used in controlled studies, may offer superior efficacy26.
In several reports Lactobacillus casei and L. acidophillus strains CERELA in a fixed combination, have proved to have better efficacy than placebo in randomized, double-blind, clinical trials of patients with various intestinal disorders, and this efficacy has been combined with a similar profile regarding the number of vomiting compared to placebo20, 28-31.
The protective effect of S. boulardii on acute diarrheahas been proposed, based mainly on results from animal studies32,33, but based on the data of Potholaulakis et al11 the effect of it on diarrhea appears to be manifested by a secreted product of the yeast, possibly a protease able to remove toxin receptors or brush border glycoproteins involved in adhesion of pathogens to the mucosa.
Green bananas and pectin also seem to be potential therapeutic agents for persistent diarrhea16. The underlying mechanism of actions of both, banana and pectin are postulated to be mediated by its high content of amylase-resistant starch which on reaching the colon is fermented by resident bacteria into SCFA. In the colon, SCFA provide energy and induce a trophic effect on the colonic as well as on small bowel mucosa. Similar observations consistent with this hypothesis have been reported by other investigators using other dietary fibers34.
Nutrition of colonic epithelial cells (colonocytes) is maintained in health by luminal SCFA chiefly by n-butyrate35 and its oxidation by colonocytes is involved in the regulation of sodium and water absorption from colon36. Hence, the malnutrition of colonocytes has been linked mainly with a diminished oxidation of SCFA or its luminal absence, supporting the concept that malnutrition of colonocytes plays a part in the pathogenesis of different forms of diarrhea37. Lactobacillus are also involved in the production of essential mucosal nutrients such as SCFA and amino acids such as arginine, cysteine and glutamine so that they may also participate in the regulation of intestinal functions38. Thus, the high luminal concentration of Lactobacillus casei in stool recovered from our patients on the seventh day of the discharge could be an important determining factor for the overall clinical course of diarrhea disease. These quality of Lactobacillus in addition to well-known trophic effects of S. boulardi on intestinal mucosa mediated by the release of polyamine33 are strongly giving support to further new investigation on these agents in diarrhea.
In conclusion our data show that L. casei and L. acidophillus strains CERELA and S. boulardii significantly reduce the number of depositions, shorten the duration of diarrhea and duration of symptoms in children with persistent diarrhea.
Acknowledgements: The study was partially supported by a grant from Sancor Cooperativas Unidas Ltda., Sunchales, Argentina and CONICET (National Research Council of Argentina)
1. The treatment and prevention of acute diarrhea: practical guidelines. 2nd ed. Geneva: World Health Organization (WHO),1989. [ Links ]
2. Brown KH, Peerson JM, Fontaine O. Use of nonhuman milks in the dietary management of young children with acute diarrhea: a meta-analysis of clinical trials. Pediatrics 1994; 93: 17-27. [ Links ]
3. Chew F, Penna FJ, Peret Philo LA, et al. Is dilution of cows´milk formula necessary for dietary management of acute diarrhea in infants aged less than 6 months? Lancet 1993; 341: 194-7. [ Links ]
4. Hove H, Norgaard H, Brobech Mortensen P. Lactic acid bacteria and the human gastrointestinal tract. Eur J Clin Nutr 1999; 53: 339-50. [ Links ]
5. Heyman M. Effect of lactic bacteria on diarrheal disease. J Amer Coll Nutr 2000; 19: 137S-46S. [ Links ]
6. Isolauri E, Juntunen M, Rautanen T, Sillanaukee P, Koivula T. A human Lactobacillus strain(Lactobacillus casei sp strain GG) promotes recovery from acute diarrhea in children. Pediatrics 1991; 88: 90-7. [ Links ]
7. Shornikova AV, Isolauri E, Burkanova L, Lukovnikova S, Vesikan T. A trial in the Karelian Republic of oral rehydration and Lactobacillus GG for treatment of acute diarrhea. Acta Paediatr 1997; 86: 460-5. [ Links ]
8. Fuller R. Probiotics in human medicine. Gut 1991; 32: 439-42. [ Links ]
9. Van Niel CW, Feudtner Chris, Garrison MM, Christakis DA. Review article. Lactobacillus therapy for acute infectious diarrhea in children:A meta-analysis. Pediatrics 2002; 109: 3-8. [ Links ]
10. Czerucka D, Roux I, Rampal P. Saccharomyces boulardii inhibits secretagogue-mediated adenosine 3´,5´-cyclic monophosphate induction in intestinal cells. Gastroenterology 1994; 106: 65-72. [ Links ]
11. Pothoulakis CH, Kelly CP, Joshi MA, et al. Saccharomyces boulardii inhibits Clostridium difficile toxin A binding and enterotoxicity in rat ileum. Gastroenterology 1993; 104: 1108-15. [ Links ]
12. Chapoy P. À propos du mode daction intestinal Saccharomyces boulardii. Gastroenterol Clin Biol 1986; 2: 860-1. [ Links ]
13. World Health Organization (WHO). Persistent diarrhea in children in developing countries. Memorandum of a WHO meeting. Bull World Health Organ 1988; 66: 709-13. [ Links ]
14. Black RE. Persistent diarrhea in children of developing countries. J Infect Dis 1993; 12: 751-76. [ Links ]
15. Walker-Smith JA. Diagnosis and dietary and pharmacological management of chronic diarrhea In: Lifschitz Ch, Nichols BL (eds) Malnutrition in chronic diet-associated infantile diarrhea: diagnosis and management. San Diego, CA: Academic Press, 1990: 281-90. [ Links ]
16. Rabbani GH, Teka T, Zaman B, Majid N, Khatun M. Clinical studies in persistent diarrhea: dietary management with green banana or pectin in Bangladeshi children. Gastroenterology 2001; 121: 554-60. [ Links ]
17. Boudraa G, Touhami M, Pochart P, Soltana R, Mary JY, Desjeux JF. Effect of feeding yogurt versus milk in children with persistent diarrhea. J Pediatr Gastroenterol Nutr 1990; 11: 509-12. [ Links ]
18. Roggero P, Volpe C, Ceccatelli MP, et al. Crystalline lactulose and oral preparations of micro-organisms for the treatment of chronic specific diarrhea in children. A controlled clinical study. Minerva Pediatr 1990; 42: 147-50. [ Links ]
19. Gurr MI. Nutritional aspects of fermented milk products. FEMS Microbiol Rev 1989; 46: 337-42. [ Links ]
20. Gonzalez SN, Cardozo R, Apella MC, Oliver G. Biotherapeutic role of fermented milk. Biotherapy 1995; 8: 129-34. [ Links ]
21. Yolken RH, Eiden JJ. Rotavirus, enteric adenoviruses, Norwalk viruses, caliciviruses, astroviruses and other viruses causing gastroenteritis.In: Belshe RB, ed. Texbook of human virology, 2nd. St Louis: Mosby Year Book, 1991: 804-21. [ Links ]
22. Alvarez-Olmos MI, Oberhelman RA, Probiotic agents and infectious disease: a modern perspective on a traditional therapy. Clin Infect Dis 2001; 32: 1567-76. [ Links ]
23. Perdigon G, Fuller R, Raya R. Lactic acid bacteria and their effect on the immune system. Curr Issues Intest Microbiol 2001; 2: 27-42. [ Links ]
24. Isolauri E, Majamaa H, Arvola T, Rantala I, Virtanen E, Arvilommi H. Lactobacillus casei strain GG reverses increased intestinal permeability induced by cow milk in suckling rats. Gastroenterology 1993; 105: 1643-50. [ Links ]
25. Depalncke B, Gaskins HR. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am Clin Nutr, 2001; 73 (suppl): 1131S-1141S. [ Links ]
26. Gionchetti P, Rizello F, Venturi A, et al. Oral bacterio-therapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology 2000; 119: 305-9. [ Links ]
27. Goldin BR, Gorbach SL, Saxelin M et al: Survival of a Lactobacillus (strain GG) in human gastrointestinal tract. Dig Dis Sci 1992; 37: 121-8. [ Links ]
28. Gaón D, Garmendia C, Murrielo NO,et al. Effect of Lactobacillus strains (L. casei and L. acidophillus strains CERELA) on bacterial overgrowth-related chronic diarrhea. Medicina (Buenos Aires) 2002; 62: 159-63. [ Links ]
29. Gonzalez S, Albarracin G, Locascio M, et al. Prevention of infantile diarrhea by fermented milk. Microbiologie Aliments Nutrit 1990; 8: 349-54. [ Links ]
30. Perdigon G, Alvarez S, Rachid M, Agüero C,Gobbato N. Symposium: probiotic bacteria for humans:clinical systems for evaluation of effectiveness. J Dairy Sci 1995; 78: 1597-606 [ Links ]
31. Perdigon G, Alvarez S. Probiotic and the immune state. pp 146-176. In: R. Fuller (ed) Probiotic: the scientific basis. Chapman and Hall. London. [ Links ]
32. Buts JP, Bernasconi P, Vaerman JP. Stimulation of secretory IgA and secretory component of immunoglobulin in small intestine of rats treated with Saccharomyces boulardii. Dig Dis Sci 1990; 35: 251-6. [ Links ]
33. Buts JP, De Keyser N, Marandi S, et al. Saccharomyces boulardii upgrades cellular adaptation after proximal enterectomy in rats. Gut 1999; 45: 89-96. [ Links ]
34. Alam NH, Meier R, Sarkar SA, et al. Efficacy of soluble fiber supplemented oral rehydration solution in the treatment of acute non-cholera diarrhea in children. Gastroenterology 1997; 112: A2. [ Links ]
35. Firmansyah A, Penn D, Lebenthal E. Metabolism of glucose, glutamine, n –butyrate, and B – hydroxybutyrate in isolated rat colonocytes following acute fasting and chronic malnutrition. Gastroenterology 1989; 97: 622-9. [ Links ]
36. Binder HJ, Mehta P. Short-chain fatty acids stimulate active sodium and chloride absorption in vitro in the rat distal colon. Gastroenterology 1989; 96: 986-9. [ Links ]
37. Roediger WEW, Duncan A, Kapaniris O, Millard S. Reducing sulfur compounds of the colon impair colonocyte nutrition: implications for ulcerative colitis. Gastroenterology 1993;104: 802-9. [ Links ]
38. Bengmark S. Colonic food: pre and probiotics. Am J Gastroentroent 2000; 95 (suppl): 5-7. [ Links ]
Recibido: 23 de diciembre de 2002
Aceptado: 19 de mayo de 2003














