Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Medicina (Buenos Aires)
versión impresa ISSN 0025-7680versión On-line ISSN 1669-9106
Medicina (B. Aires) v.65 n.3 Buenos Aires mayo/jun. 2005
Immunochemical and morphometric features of astrocyte reactivity vs. plaque location in Alzheimer´s disease
María C. Vanzani1, Rubén F. Iacono2, Roberto L. Caccuri1, María I. Berria1
1Departamento de Microbiología, Facultad de Medicina; 2Cátedra de Inmunología, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires
Postal address: Dr. María I. Berría, Departamento de Microbiología, Facultad de Medicina-UBA, Paraguay 2155, 1121 Buenos Aires, Argentina, Fax: (54-11) 4508-3705, e-mail: neurovir@fmed.uba.ar
Abstract
The quantitative relationship between glial fibrillary acidic protein (GFAP) hyper-reactivity and b-amy-loid protein (bAP) deposition was investigated by double immunoperoxidase labeling of hippocampal and entorhinal cortex sections from five Alzheimer´s disease (AD) cases and five age-matched controls. bAP plaques, which were absent in controls, were found in all AD samples, without significant differences in number or perimeter according to their location among the regions studied. In contrast, the mean number of GFAP (+) cells was significantly greater in the hippocampus than in the entorhinal cortex from AD cases (49 vs.39). Although at lower values (30 vs. 20), predominance of astrocyte hyperplasia in hippocampus as compared with entorhinal cortex was also found in control samples. Concomitant astrocyte hypertrophy, as defined by surface density (Sv) values of GFAP-immunoreactive material exceeding those of control means, affected a similar proportion of cells in the hippocampus (73%) and the entorhinal cortex (74%) from AD cases. Since an increased number of GFAP (+) cells in the hippocampus was not accompanied by an increased number and/or perimeter of neighbouring plaques, such differential hyper-reactivity in samples from AD patients, as well as in those with normal aging, seems to depend partially on the regional location of the involved astrocyte.
Key words: Hippocampus, Entorhinal cortex, GFAP, bAP, Quantitative analysis
Resumen
Características inmunoquímicas y morfométricas de reactividad astrocitaria vs. localización de placas en enfermedad de Alzheimer. La relación cuantitativa entre la hiperreactividad de la proteína gliofibrilar ácida (GFAP) y los depósitos de proteína b-amiloide (bAP) fue investigada mediante doble marcación por inmunoperoxidasa en cortes histológicos de hipocampo y corteza entorrinal correspondientes a cinco casos de enfermedad de Alzheimer (AD) y cinco controles de edades similares. Las placas bAP, ausentes en controles, se encontraron en cambio en todas las muestras AD, donde no se observaron diferencias significativas en número o perímetro según su localización en las regiones estudiadas. En cambio, el número de células GFAP (+) fue significativamente mayor en hipocampo que en corteza entorrinal (49 vs. 39). Aunque a menores valores (30 vs. 20), el predominio de hiperplasia astrocitaria en hipocampo con respecto a corteza entorrinal, también se observó en controles. En AD, una concomitante hipertrofia astrocitaria, definida por valores de densidad de superficie (Sv) del material inmunorreactivo para GFAP, afectó a un número similar de células en hipocampo (73%) y corteza entorrinal (74%). Dado que el aumentado número de células GFAP(+) no se acompañó de mayor número y/o perímetro de placas vecinas, la hiperreactividad regional exhibida tanto por AD como por envejecimiento normal, parecería depender de la localización del subtipo astrocitario involucrado.
Palabras clave: Hipocampo, Corteza entorrinal, GFAP, bAP, Análisis cuantitativo
Astrocyte activation, known as the earliest CNS response to injury and initially described by enhanced number and size of such cells, is currently evidenced by the overimmunoreactivity of glial fibrillary acidic protein (GFAP), the main 8-9 nm intermediate filament of reactive astrocyte1. In this connection, a significant increase in GFAP expression has been observed in many neurodegenerative disorders, including Alzheimer´s disease (AD), the most common dementia in the elderly. Though the abundance of diffuse and dense-core amyloid plaques in specific brain areas is a neuropathological hallmark of AD, the concomitant presence of astrocyte activation is also a salient feature. Since the original description of astrocyte association with senile plaques2 has been repeatedly confirmed3-5, the pathogenic relevance of astrocyte response, either as a primary or early secondary reaction to amyloid deposition, has deserved growing attention6-8.
In order to determine whether increased GFAP immunoreactivity, as evidenced by a greater number and size of astrocytes, is spatially related to the number and the surface density (Sv) of amyloid plaques, we compared the distribution of activated astrocytes with that of bAP (+) deposits in brain tissues. In these astrocytes, signs of mitotic activity were searched through the detection of a specific marker such as proliferating cell nuclear antigen (PCNA).
Taking into account that entorhinal cortex and hippocampus are representative brain areas for the histopathological diagnosis of AD, they were chosen for the present study. Since GFAP immunoreactivity is known to increase throughout adult life span9-12, postmorten brain samples from age-matched subjects were used as controls.
Material and Methods
Samples. Postmorten hippocampus and entorhinal cortex from five patients (mean 69 years, range 60-75) with a clinical diagnosis of primary degenerative dementia (DSM IV-CERAD) as well as from controls represented by five age-matched deceased individuals lacking neurological disease (mean 67.2 years, range 60-77) were obtained from the Division of Pathology, Francisco Santojanni Foundation Hospital, Buenos Aires, Argentina. For initial screening, 5-µm thick sections from paraffin-embedded tissues were stained with hematoxylin-eosin.
Single immunohistochemistry. Serially obtained histological sections were microwaved before overnight incubation at 4 °C with a 1/1600 dilution of a rabbit serum against bovine GFAP (Dako A/S, Denmark), or a 1/100 dilution of a mono-clonal antibody against beta-amyloid Clone 6F/3D (Dako), or a 1/800 dilution of a monoclonal anti-PCNA antibody (BioGenex, USA), followed by incubation with a 1/200 dilution of biotinylated goat antibodies against rabbit immunoglobulins (Dako) or biotiny-lated rabbit antibodies to mouse immunoglobulins (Dako), depending on the species of the first antibody. Peroxidase-conjugated streptavidin (Dako) at 1/200 dilution was finally added. Second and third antibody incubations were performed at room temperature during 30 min, while reaction development was achieved by 10-min exposure to 0.03% diaminobenzidine tetrahydrochloride (DAB, Fluka AG, Switzerland) plus 0.02% hydrogen peroxide.
Double immunohistochemistry. The peroxidase activity of GFAP-labeled sections was blocked by treatment with methanol-hydrogen peroxide 3% for 30 min, and samples were subsequently incubated with anti-beta amyloid or anti-PCNA antibodies, followed by biotinylated rabbit anti-mouse antiserum in both cases. Samples were incubated with peroxidase-conjugated streptavidin and the reaction was developed with the AEC Chromogen Kit (Immunotech, France).
Quantitative evaluation of immunolabeled sections. Each double immunolabed section was observed at 400x magni-fication through a Zeiss microscope. The number of GFAP (+) cells and bAP (+) plaques was recorded in 8 areas of hippocampus (CA1 to CA4) and entorhinal cortex, which were determinated by sequential displacement of a test square grid delimiting 0.01 mm2 in the section. Only process-bearing astrocytes with their nuclei in the plane of the section were considered. For counting, bAP (+) plaques were recorded separately as diffuse (i.e., lacking-morphologically identified substructures) or dense core (i.e., exhibiting a compact central mass surrounded by an outer sphere).
Resorting to a computer-assisted approach already described13, morphometric analysis of both GFAP (+) cells and bAP (+) plaques was carried out by means of a stereological grid, following the point-counting method14 as applied in studies of rat brain tissue15. To this end, 8 microscopic fields in each histological section, in which the number of GFAP (+) cells and bAP (+) plaques had been recorded, were chosen for measurement of surface density of their respective immunoreactivity.
Counting and morphometric analysis were carried out by at least two independent observers.
All data were analyzed using Student´s t-test for comparison of the means, taking p<0.05 as significance level.
Results
Consistent labeling clearly distinguishable from the immunonegative background was achieved for bAP as well as for GFAP, thus allowing accurate quantification of the number and perimeter of plaques and astrocytes, respectively.
bAP diffuse and dense-core plaques were found in hippocampus (Fig. 1-a) and entorhinal cortex (Fig. 1-b) from all five AD cases, while such deposits were completely absent in equivalent samples from aged-matched controls. Plaques were mostly observed in CA1 to CA2 areas in the hippocampus, while their distribution was more homogeneous in entorhinal cortex. As shown in Table 1, bAP deposits did not differ significantly (p<0.05) in number or size according to their brain location among the regions studied. Nevertheless, a significant predominance of diffuse over dense-core plaques was recorded in both hippocampus and entorhinal cortex.

Fig. 1.– Double immunohistochemical labeling on 5-µm sections of hippocampus (a) and entorrhinal cortex (b) samples from an AD case. DAB-immunolabeled astrocytes (arrowheads) and EAC-immunolabeled plaques (arrows) using polyclonal anti-GFAP and anti-beta amyloid antibodies, respectively. 1000x
TABLE 1.– Mean values (± SD; p<0.05) of number and surface density (Sv) of βAP(+) plaques and GFAP(+) astrocytes in hippocampus and entorhinal cortex from five Alzheimer disease cases and five age-matched controls
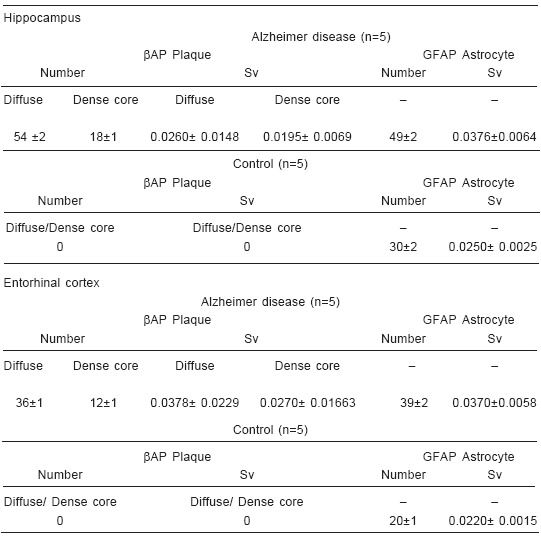
Differences in the number of GFAP (+) cells were found when the two areas selected for study were compared (Table 1). In histological sections corresponding to the five AD cases, GFAP-immunoreactive astrocytes were more numerous in hippocampus than in entorhinal cortex (49 ± 2 vs. 39 ± 2). Such regional increase was also observed in brains from age-matched controls, but at values significantly lower than in AD (30 ±2 vs. 20 ± 1).
Evident cell hypertrophy suggested by cursory inspection of AD samples as compared with those of controls (Fig. 2) was later confirmed by astrocyte morphometry, since significantly increased SvGFAP values were found in comparison with those of controls. On the basis of a cell size estimated by the number of intersections of the superimposed stereological grid with immunolabeled body perimeter and emerging processes (SvGFAP), 73% of labeled astrocytes in AD hippocampus and 74% in AD entorhinal cortex exceeded the mean values +SD in controls (Fig 3). A further peculiarity of GFAP-immunoreactive profile in AD was a greater number, width and branching of astrocyte processes as compared to those of controls.
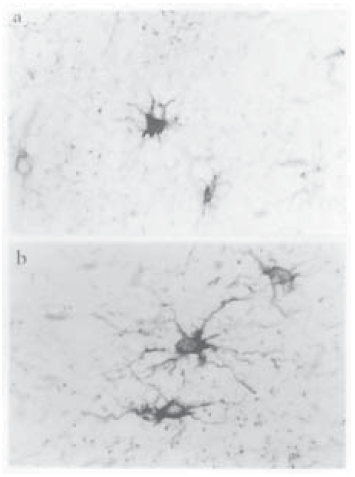
Fig. 2.– Single immunohistochemical labeling on 5-µm sections of entorhinal cortex samples. DABimmunolabeling of astrocytes using anti-GFAP. As compared with age-matched control (a), astrocyte hypertrophy in Alzheimer´s disease (b) is evidenced by enlargement of cell body and increased growth of cell processes. 1000x

Fig. 3.– Surface density (Sv) of GFAP-immunoreactive material (cell bodies and processes) in hippocampus (CA1 to CA4) and entorhinal cortex from five Alzheimer´s disease (AD) patients. Cell enlargement was assumed on the basis of SvGFAP values in hippocampus and entorhinal cortex that exceeded means +SD of five age-matching controls (0.0250+0.0025 and 0.0220+0.0015, respectively). Note the similar AD pattern in both brain areas.
PCNA labeling, as evidenced by reddish nuclei, was not found in GFAP (+) astrocytes, either in AD or in controls.
Discussion
As expected, astrocyte hyperplasia was significantly higher in hippocampal and entorhinal samples from AD patients than in those from controls. Since we did not find signs of mitotic activity in GFAP(+) cells, whether in AD or control samples, increased GFAP (+) cell counts appear to result from activation of former quiescent astrocytes rather than from proliferation. Nevertheless, attempts to disclose such sign of mitotic activity had been proved positive in experimental studies related to astrocyte aging, both in vivo16 and in vitro17, but in which harvest and fixation of samples had been experimentally controlled. It is known that mitosis is a relatively short event that may progress to completion even after bodily death, as long as individual cells survive.
In spite of similar distribution of bAP (+) plaques in the two evaluated brain areas, GFAP (+) astrocyte counts were higher in the hippocampus than in the entorhinal cortex of AD patients. Although at lower values than in AD, controls also exhibited greater GFAP-immunoreactive cells in the hippocampus. It may be assumed that in both AD and normal aging such differential GFAP reactivity is partly attributable to the regional location of astrocytes in brain. On the basis of GFAP quantitation by immunoblotting in diverse brain areas in the course of aging18, it has been demonstrated that astrogliosis starts in hippocampus, since it has been never observed in the entorhinal cortex alone and, once manifested in this brain area, it is systematically accompanied by a higher reaction in the hippocampus.
According to SvGFAP values, astrocyte hypertrophy was significantly greater in AD, involving almost identical percentages of GFAP (+) cells in hippocampal and entorhinal samples. Therefore, the recorded differences in the number of reactive astrocytes in hippocampus vs. entorhinal cortex were not found for SvGFAP values, which were similarly increased in both brain areas. Such lack of correlation between astrocyte density and cell size has been found in aging rats exhibiting pronounced hyperplasia in the hippocampus and accentuated hypertrophy in the frontal cortex19. In turn, aging rhesus monkeys show an increase in GFAP (+) cell size but not in cell density in all subcortical white matter areas of the frontal, temporal, and parietal cortices20. Furthermore, in frontal cortices and subcortical white matter of individuals displaying a variety of other diseases, ranging from AIDS to infarction, the extent of gliosis is reflected by an increase in cell size but not in the density or intensity of the GFAP staining of astrocytes21.
Although at cursory inspection bAP deposits appeared as more pronounced in hippocampus, no significant differences in plaque number and size were found according to their localization, either in hippocampus or in entorhinal cortex. On the basis of the proposed sequence of bAP deposition in AD22, entorhinal cortex and CA1 hippocampal sectors were involved subsequently to neocortex. Such temporo-spatial progression suggests that hippocampus and entorhinal cortex exhibit similar susceptibility to become involved, once they have received afferent input from previously affected neocortex. In this connection, topographical studies including entorhinal region, perirhinal cortex and hippocampal formation, indicate that neuritic plaques gradually develop in the projection areas of tangle-bearing neurons23. As Muramori et al24 suggested, attribution of AD changes to dementia should be neglected when confined to the entorhinal cortex, but appreciated when they spread to the hippocampal subiculum and/or cornus ammonis.
Since our characterization of plaques was carried out exclusively by bAP immunolabeling, thus overlooking detection of other plaque components identifiable by specific markers of glial cells25, 26 and dystrophic neurites27, 28, the predominance of diffuse over dense-core plaques herein observed can hardly be attributed to a prevailing earlier stage of plaque maturation alone, as the presence of late burnt-out plaques cannot be ruled out. Taking into account that amyloid plaque arrangement varies markedly among non-demented elderly individuals29 and is even absent in numerous cases30, 31, the lack of bAP reactivity in our five controls (mean age 67.2 years) is not unexpected.
To sum up, quantitation of astrocyte activation performed herein has allowed a more accurate characterization of astrocyte changes, including number and size, that take place in hippocampus and entorhinal cortex which, together with neocortex, are the first brain areas to evidence AD histopathological alterations. However, no quantitative correlation could be shown between astrocyte activation and bAP deposition in either hippocampus or entorhinal cortex. A better knowledge of the role played by glial cells, whether microglial or astroglial, may contribute to the development of therapeutic strategies designed to modulate the inflammatory processes recognized as contributory factors in the progression of CNS neurodegeneration32. Likewise, the unraveling of mechanisms responsible for the abundance of reactive astrocytes and activated microglia, may provide a deeper insight into the pathophysiology of AD33. To this end, all histocytomorphometric approaches enabling more objective characterization of activated glia, as well as its regional location, may help to validate the relevance of such cell response in the course of AD, as well as in normal aging.
Acknowledgments: To Dr. Angel O. Fernández, Division of Pathology, Francisco Santojanni Foundation Hospital, Buenos Aires, Argentina, who kindly provided the formalin-fixed brain tissues of both AD patients and controls. This work was partly supported by grants from National Council of Scientific and Technological Research (CONICET) and the University of Buenos Aires.
References
1. Eng LF, Guirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969-2000). Neurochem Res 2000; 25: 1439-51. [ Links ]
2. Duffy PE, Rapport M, Graf L. Glial fibrillary acidic protein and Alzheimer-type senile dementia. Neurology 1980; 30: 778-82. [ Links ]
3. Schechter R, Yen S-H C, Terry RD. Fibrous astrocytes in Alzheimer´s disease in senile dementia of the Alzheimer´s type. J Neuropathol Exper Neurol 1981; 40: 95-101. [ Links ]
4. Mancardi GL, Liwnicz BH, Mandybur TI. Fibrous astrocytes in Alzheimer´s disease and senile dementia of Alzheimer´s type. An immunohistochemical and ultrastructural study. Acta Neuropathol (Berl) 1983; 61: 76-80. [ Links ]
5. Vijayan V, Geddes JW, Anderson KJ, Chang-Chui H, Ellis WG, Cotman CW. Astrocyte hypertrophy in the Alzheimer´s disease hippocampal formation. Exp Neurol 1991; 112: 72-8. [ Links ]
6. Mandybur TI, Chiurazzi CC. Astrocytes and plaques of Alzheimer´s disease. Neurology 1990; 40: 635-9 [ Links ]
7. Pike CJ, Cummings BJ, Cotman CW. Early association of reactive astrocytes with senile plaques in Alzheimer´s disease. Exp Neurol 1995; 132: 172-9. [ Links ]
8. Cullen KM. Perivascular astrocytes within Alzheimer´s disease plaques. Neuroreport 1997; 8: 1961-6. [ Links ]
9. Amenta F, Bronzetti E, Sabbatini M, Vega JA. Astrocyte changes in aging cerebral cortex and hippocampus: a quantitative immunohistochemical study. Microsc Res Tech 1998; 43: 29-33. [ Links ]
10. Eriksdotter-Nilsson M, Dahl D, Rose G, Olson L. Image analysis of GFAP-positive astrocytes from adolescence to senescence. Exp Brain Res 1985; 58: 163-70. [ Links ]
11. Hansen LA, Armstrong DM, Terry RD. An immunohisto-chemical quantification of fibrous astrocytes in the aging human cerebral cortex. Neurobiol Aging 1987; 8: 1-6 [ Links ]
12. Beach TG, Walker R, McGeer EG. Patterns of gliosis in Alzheimer´s disease and aging cerebrum. Glia 1989; 2: 420-36. [ Links ]
13. Caccuri RL, Iacono RF, Weissenbacher MC, Avila MM, Berría MI. Long-lasting astrocyte reaction to persistent Junin virus infection of rat cortical neurons. J Neural Transm 2003; 110: 847-57. [ Links ]
14. Weibel ER. Practical methods for biological morpho-metry. In: Stereological methods, vol 1. London: Academic Press, 1979. [ Links ]
15. Tranque PA, Suarez I, Olmos G, Fernandez B, Garcia-Segura LM. Estradiol-induced redistribution of glial fibrillary acidic protein immunoreactivity in the rat brain. Brain Res 1987; 406: 348-51. [ Links ]
16. Vanzani MC, Iacono RF, Alonso A, Berría MI. Proli-ferative astrocyte potential during aging. Cell Mol Neurobiol 2003; 23: 273. [ Links ]
17. Vanzani MC, Iacono RF, Alonso A, Berría MI. Immunochemical expression of proliferative cell nuclear antigen in aging cultured astrocytes. Medicina (Buenos Aires) 2003; 63: 303-6. [ Links ]
18. David JP, Ghozali F, Fallet-Biando C, et al. Glial reaction in the hippocampal formation is highly correlated with aging in human brain. Neurosci Lett 1997; 235: 53-5 [ Links ]
19. Amenta F, Bronzetti E, Sabbattini M, Vega JA. Astrocyte changes in aging cerebral cortex and hippocampus: a quantitative immunohistochemical study. Microsc Res Tech 1998; 43: 29-33 [ Links ]
20. Sloane JA, Hollander W, Rosene DL, Moss MB, Kemper T, Abraham CR. Astrocyte hypertrophy and altered GFAP degradation with age in subcortical white matter of the rhesus monkey. Brain Res 2000; 862: 1-10. [ Links ]
21. Da Cunha A, Jefferson JJ, Tyor WR, Glass JD, Jannotta FS, Vitkovic L. Gliosis in human brain: relationship to size but not other properties of astrocytes. Brain Res 1993; 600: 161-5. [ Links ]
22. Thal DR, Rüb U, Orantes M, Braak H. Phases of Ab-deposition in the human brain and its relevance for the development of AD. Neurology 2002; 58: 1791-800. [ Links ]
23. Yilmazer-Hank DM, Hanke J. Progression of Alzheimer-related neuritic plaque pathology in the entorhinal region, perirhinal cortex and hippocampal formation. Dement Geriat Cogn Disord 1999; 10: 70-6. [ Links ]
24. Muramori F, Kobayashi K, Nakamura I. A quantitative study of neurofibrillary tangles, senile plaques and astrocytes in the hippocampal subdivisions and entorhinal cortex in Alzheimer´s disease, normal controls and non-Alzheimer neuropsychiatric diseases. Psychiatry Clin Neurosci 1998; 52: 593-9. [ Links ]
25. Styren SD, Kamboh, MY, Dekosky, ST. Expression of differential immune factors in temporal cortex and cerebellum: the role of a-1-antichymotrypsin, apolipo-protein E, and reactive glia in the progression of Alzheimer´s disease. J Comp Neurol 1998; 396: 511-20. [ Links ]
26. Dandrea MR, Reiser PA, Gumula NA, Hertzog BM, Andrade-Gordon P. Application of triple immunohistoche-mistry to characterize amyloid plaque-associated inflammation in brains with Alzheimer´s disease. Biotech Histochem 2001; 76: 97-106. [ Links ]
27. Dickson TC, Vickers JC. The morphological phenotype of beta-amyloid plaques and associated neuritic changes in Alzheimer´s disease. Neuroscience 2001; 105; 99: 107. [ Links ]
28. D´Andrea MR, Nagele RG. MAP-2 immunolabeling can distinguish diffuse from dense-core amyloid plaques in brains with Alzheimer´s disease. Biotech Histochem 2002; 77: 95-102 [ Links ]
29. Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol 2003; 60: 729-36. [ Links ]
30. Price JL, Morris JC. Tangles and plaques in nonde-mented aging and preclinical Alzheimer´s disease. Ann Neurol 1999; 45: 358-68. [ Links ]
31. Iseki E, Tsunoda S, Suzuki K, et al. Regional quantitative analysis of NFT in brains of non-demented elderly persons with findings in brains of late-onset Alzheimer´s disease and limbic NFT dementia. Neuropathology 2002; 22: 34-9. [ Links ]
32. Unger JW. Glial reaction in aging and Alzheimer´s disease. Microsc Res Tech 1998; 43: 24-48. [ Links ]
33. Meda L, Baron P, Scarlato G. Glial activation in Alzheimer´s disease: the role of Abeta and its associated proteins. Neurobiol Aging 2001; 22: 885-93. [ Links ]
Recibido: 29-09-2004
Aceptado: 4-02-2005














