Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Medicina (Buenos Aires)
versión impresa ISSN 0025-7680versión On-line ISSN 1669-9106
Medicina (B. Aires) v.66 n.2 Buenos Aires mar./abr. 2006
Regional differences in astrocyte activation in HIV-associated dementia
María C. Vanzani, Rubén F. Iacono2, Roberto L. Caccuri1, Alcides R. Troncoso3, María I. Berria1
1Departamento de Microbiología, Facultad de Medicina; 2Cátedra de Inmunología, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires; 3Sala 21, Hospital de Enfermedades Infecciosas Francisco J. Muñiz, Buenos Aires
Postal address: Dra. María I. Berría, Departamento de Microbiología, Facultad de Medicina-UBA, Paraguay 2155, 1121 Buenos Aires, Argentina. Fax: (54-11) 4508-3705. E-mail: neurovir@fmed.uba.ar
Abstract
Since astrogliosis is a histological marker usually observed in HIV-associated dementia (HIV-D), we decided to investigate the potential relationship between the expression of glial fibrillary acidic protein (GFAP) and the regional distribution of cells positive (+) for this specific marker of astrocyte activation. Histological sections of brain tissues obtained at necropsy from 5 HIV-D patients and 5 age-matched controls without history of neuropsychiatric illness were immunostained with peroxidase. Mean numbers of GFAP(+) astrocytes were significantly increased in entorhinal cortex, hippocampus and subcortical white matter of patients, but values in frontal cortex and basal ganglia were similar to those of controls. In contrast, surface density of immunoreactive GFAP was significantly increased in all tested brain areas from all patients, including unusually affected regions such as entorhinal cortex and hippocampus. Therefore, such consistent finding of hypertrophic astrocytes, ranging from highest cell percentajes in subcortical white matter to lowest in basal ganglia indicates that quantification of surface density in GFAP (+) cells appears to be a more reliable approach to score gliosis than the counting of their cell nuclei. Because astrocyte activation involves both protective and detrimental effects on adjacent neuronal subsets, the evidence of regional differences in this reactive potential highlights the importance of accurately defining their contribution to the neuropathogenesis not only of HIV-D, but of a wide range of neurodegenerative disorders.
Key words: AIDS, astrocyte, GFAP, immunocytochemistry, cytomorphometry
Resumen
Diferencias regionales en la activación astrocitaria en demencia asociada a HIV. Siendo la astrogliosis un signo histológico habitualmente presente en demencia asociada a HIV, se investigó la eventual relación entre expresión de proteína gliofibrilar ácida (GFAP) y localización regional de células positivas para ese marcador específico de la activación astrocitaria. Por inmunoperoxidasa, se procesaron cortes histológicos de tejidos cerebrales obtenidos por necropsia de 5 pacientes y 5 controles de edades similares pero sin antecedentes neuropsiquiátricos. Según los valores de las medias registrados por conteo de astrocitos GFAP(+) en pacientes, el número fue significativamente mayor en corteza entorrinal, hipocampo y sustancia blanca subcortical, mientras que en corteza frontal y ganglios basales no se encontraron diferencias con controles. En cambio, la densidad de superficie del material GFAP inmunorreactivo en pacientes estuvo significativamente aumentada en todas las áreas cerebrales analizadas, incluso en regiones inusualmente afectadas, como corteza entorrinal e hipocampo. Entre esos astrocitos hipertróficos, el mayor porcentaje correspondió a sustancia blanca subcortical, y el menor a ganglios basales. Cabe concluir que el constante hallazgo de agrandamiento astrocitario señala a la medida de la superficie inmuno-reactiva como mejor índice de activación celular que el conteo de núcleos de las células marcadas. Dados los reconocidos efectos de la astrogliosis sobre las subpoblaciones neuronales vecinas, la comprobada regionalización de ese potencial reactivo destaca el interés de precisar su contribución en la neuropatogenia, tanto de demencia asociada a HIV como de otras enfermedades neurodegenerativas.
Palabras clave: sida, astrocito, GFAP, inmunocitoquímica, citomorfometría
Infection of the nervous system by HIV commonly causes a broad range of cognitive, behavioral and motor abnormalities known, in its most severe form, as HIV-associated dementia (HIV-D). Although the introduction of highly active antiretroviral therapy (HAART) in the 1990s has attenuated the incidence and severity of HIV-D, it remains as a common and disabling problem for those living with AIDS1, 2. Since HAART is effective in reducing the viral burden in the brain compartment3, increased prevalence rate of HIV-D4, 5 and concomitant neuropathological findings6 suggests that irreversible damage may already have occurred to parenchyma, or else that immune reconstitution has resulted in an upregulation of the inflammatory infiltrate. Therefore, a complementary pharmacological approach is required7, 8envisaging the use of neuro-protective agents or those with disease-modifying potential. However, in order to achieve such a goal, a deeper understanding of HIV-D pathophysiology is necessary. Taking into account that HIV-D appears as a metabolic encephalopathy that must be sustained by inflammatory neurotoxic responses9, neuronal injury would be indirectly mediated by a variety of postinflammatory cytokines released into the parenchyma by HIV-infected macrophages and microglia10, as well as by immune-activated macrophages and astrocytes11. However, direct injury of neurons by viral proteins cannot be ruled out. In this connection, detection of HIV-1 RNA or proviral DNA in astrocytes indicates that they are cell hosts of a restricted infection and thus able to synthesize potentially neurotoxic regulatory viral proteins12. Besides this deleterious potential, astrocyte activation per se would participate in dementia pathogenesis through dual effects, whether neurotoxic by releasing cytokines, free radicals, nitric oxide, and arachidonic acid, among others, as well as acting as neuroprotectors by reducing neurotoxic activities of macrophages, synthesizing anti-inflammatory cytokines, and taking up excitatory amino acids13.
Bearing in mind the secondary though relevant role played by astrocyte activation in the development of HIV-D14, it seemed of interest to characterize its topographical presence in brain areas and evaluate its regional intensity degree. In this connection, in vitro studies15 have disclosed a dissimilar reactivity potential in cultured types/subtypes of astrocytes according to their cell lineage. It was in order to discriminate an eventual differential expression of in vivo astrocyte activation, that we resorted to immunoperoxidase labeling of glial fibrillary acidic protein (GFAP), the specific marker of the reactive astrocyte, in tissue samples obtained from necropsied HIV-D patients. Since GFAP immunoreactivity is known to increase throughout adult life span16, 17, postmortem brain samples from age-matched subjects were used as controls.
Materials and Methods
Samples. Formalin-fixed archival brain tissues corresponding to 5 patients from the Hospital of Infectious Diseases "Francisco J Muñiz", Buenos Aires, Argentina, were employed. All subjects died presenting cognitive and motor disorders, following a clinical and laboratory diagnosis of AIDS, and all autopsies were performed within 24 h of death. Median age of such patients was 35.8 years (range 21 to 50), while controls were represented by 5 deceased individuals lacking neurological disease with an median age of 52.0 years (range 49 to 58).
Single immunohistochemistry. Following paraffin embedding, 5-µm histological sections were obtained and pretreated with 96% formic acid (Sigma, USA) for 10 min, and incubated first with 1/1.600 dilution of rabbit anti-cow GFAP (Dako A/S, Denmark) in Tris buffer, and then with 1/200 dilution of bio-tinylated goat anti-rabbit immunoglobulins (Dako A/S). Peroxidase-conjugated streptavidin (Dako A/S) at 1/200 dilution was finally employed. Reaction development was achieved by 10-min exposure to 0.03% DAB (Fluka AG, Switzerland) plus 0.02% H202. In all immunolabeled sections, light counterstaining with Mayer´s hematoxylin was performed.
Double immunohistochemistry. The peroxidase activity of GFAP-labeled sections was blocked by 30 min-treatment with methanol containing 3% of H2O2, and samples were subsequently incubated with 1/800 dilution of anti-proliferating cell nuclear antigen (PCNA) antibody (BioGenex, USA), followed by incubation with 1/200 dilution of biotinylated rabbit anti-mouse antiserurm (Dako A/S). Peroxidase conjugated streptavidin was finally added and the reaction developed with the AEC Chromogen Kit (Immunotech, France).
Quantitative evaluation. Immunolabeled sections were observed at 400x magnification through a Zeiss microscope. With the aid of a test square grid delimiting 0.01 mm2 in the section, the number of cell nuclei corresponding to GFAP(+) astrocytes was counted in each selected brain area, including entorhinal cortex, hippocampus, subcortical white matter, frontal cortex and basal ganglia. For such cell counting, 8 microscopic fields were randomly chosen by progressive displacement of the test square grid. Only process-bearing cells showing their nucleus in the plane of the section were recorded.
Resorting to a computer-assisted approach already described18, morphometric analysis of GFAP(+) cells was carried out by means of a stereological grid and following the point-counting method19 as applied to brain tissue20. To this end, 8 microscopic fields in each histological section were randomly analyzed for measurement of surface density of GFAP immunoreactive material, by selecting isolated labeled cells sharply demarcated from the negative background.
Counting and morphometric analysis of GFAP(+) astrocytes were carried out by at least two independent observers.
All data were analyzed using Students t-test for comparison of the means, taking p<0.05 and p<0.001 as significance levels.
Results
Demographic, clinical and laboratory data of the HIV-D cases are summarized in Table 1. Out of the 4 males, one was homosexual, and the remainder heterosexual, one of whom was intravenous drug user (IDU). Patient 4 (female) reported a regular HIV(+) and IDU male partner. All cases were severely immunosuppressed with CD4 cell counts <100 x 106 /l, out of whom 3/5 presented values lower than 25 x 106 /l. As up to 10 years had elapsed since their death, occurring before the HAART era, all cases had been limited to AZT treatment, which became highly irregular due to loss of patient compliance. Given these shortcomings, detection of viral persistence in plasma was not unexpected. All patients showed signs of neuropsychiatric and neurological disorders, as evidenced by progressive motor abnormalities, cognitive impairments and behavioral disorders, whose degree ranged from mild (patient 1) and moderate (patient 2) to severe (patients 3 to 5).
TABLE 1.- Demographic, clinical and laboratory data from HIV-D patients (n=5)
According to hematoxylin-eosin staining, perivascular infiltration by mononuclear cells was noticeable in white matter areas from all five patients samples, although vascular dilation accompanied by inflammatory exudate was more intense in cases 1 and 2.
As regards GFAP immunolabeling, preliminary cursory inspection of histological sections from patients disclosed signs of astrocyte activation, as evidenced by the ready detection of stained astrocytes (Fig. 1, A and B) and the enlargement exhibited by many of such cells (Fig. 1, C and D). Subsequent statistical analysis of cell count revealed that mean values were significantly greater in entorhinal cortex, hippocampus and subcortical white matter than those recorded in controls, while differences in frontal cortex and basal ganglia proved negligible (Table 2).
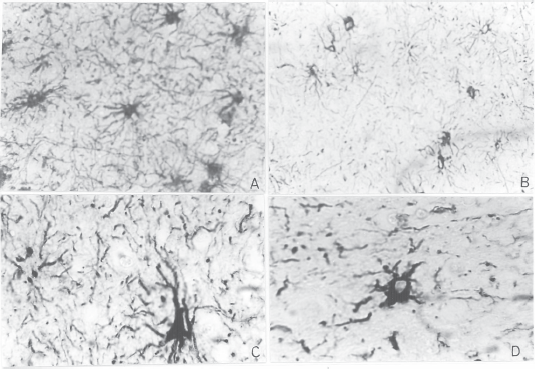
Fig 1.- Immunoperoxidase labeling of GFAP in subcortical white matter tissues from HIV-D patient 1 (A) versus age-matched control (B). Greater number of GFAP(+) astrocytes is observed in A when compared with B. Magnification: 400 X. According to enlarged cell body and increased number and length of cell processes, astrocyte hypertrophy is disclosed in basal ganglia tissues from the same patient (C), but not in age-matched control (D). Magnification: 1000 X.
TABLE 2.- Number of GFAP-immunoreactive cells and surface density of GFAP-immunoreactive material in selected brain areas from HIV-D patients (n=5) and controls (n=5)
Concomitantly, SvGFAP was significantly enhanced in all tested brain areas from all patients. By assuming that astrocyte hypertrophy was present when SvGFAP mean value was greater than the control mean + 1 SD in each selected brain area, cell enlargement was found in 72% of astrocytes located in entorhinal cortex, 85% in hippocampus, 97% in subcortical white matter, 75% in frontal cortex, and 75% in basal ganglia.
When GFAP(+) cell number for each of the patients was individually compared with the respective control mean (Fig. 2, a), cases 1 and 5 displayed significantly greater values in entorhinal cortex, hippocampus and subcortical white matter, but not in frontal cortex and basal ganglia. In all tested brain areas of remaining patients, GFAP(+) astrocyte number failed to differ from controls. In turn, SvGFAP appeared significantly enhanced in all brain samples from all patients, reaching highest values in subcortical white matter and lowest in basal ganglia (Fig. 2, b).

Fig 2.- GFAP immunoreactivity as evaluated by number of positive cells (a) and surface density (b) in all tested brain areas (Ent: entorhinal cortex; Hip: hippocampus; SWM: subcortical white matter; FC: frontal cortex; and BC: basal ganglia of HIV-D patients (P 1-5). Controls are represented by mean values of 5 subjects lacking a history of neuropsychiatric disease. Asterisks indicate significant differences (*p<0.05; **p<0.01)
In some strongly GFAP-immunoreactive samples from patients, attempts to disclose astrocyte mitotic activity were carried out according to an immunocytochemical approach formerly employed for cultured astrocytes18. Despite sequential staining with monoclonal anti-PCNA antibody, no positive labeling (reddish nuclei) was achieved.
Discussion
Although at cursory inspection the number of GFAP(+) astrocytes seemed to be increased in all tested brain areas, significant differences versus controls were only found in entorhinal cortex, hippocampus and subcortical white matter, but not in frontal cortex or basal ganglia. Therefore, statistical significance of mean values in entorhinal cortex, hippocampus and subcortical white matter was mainly provided by count data from cases 1 and 5 alone, since GFAP(+) astrocyte numbers in remaining patients failed to differ from those recorded in controls. In contrast, astrocyte hypertrophy as evaluated by surface density of GFAP immunoreactive material was a statistically significant finding in all patients and in all tested brain areas. Since our attempts to disclose PCNA staining in GFAP(+) astrocytes proved negative, GFAP reactivity was attributed to activation of former quiescent cells rather than from proliferation, thus confirming the absence of proliferation markers in AIDS brains21.
In fact, astrocyte enlargement was the only constant histological feature we found throughout, since perivascular monocytic infiltration was mainly limited to subcortical white matter, and neither multinucleated giant cells nor microglial nodules were observed. In this connection, it is known that such neuropathological changes, though specific of HIV-brain infection, may be lacking in over half of HIV-D cases22, 23.
Although astrogliosis has been classically defined as hypertrophy, hyperplasia and gliofibril hyperproduction, currently there is a trend to consider the presence of hypertrophic/hyperimmunoreactive cells as the most important feature, despite the existence of hyperplasia24. As highlighted by our results, quantification of surface density in GFAP (+) cells appears to be a more reliable approach to score astrocyte activation than the count of their cell nuclei. In addition to the expected detection of enlarged astrocytes in subcortical white matter and frontal cortex, we found they were also present in entorhinal cortex, hippocampus and basal ganglia. Hypertrophy was coincident with hyperplasia in entorhinal cortex, hippocampus and subcortical white matter, but not in frontal cortex or basal ganglia, two brain areas that failed to exhibit increased number of GFAP(+) cells. Such lack of correlation between astrocyte size and density had been already described in aging rats, which exhibited more pronounced hyperplasia in hippocampus, while hypertrophy was more accentuated in the frontal cortex25. In turn, aging rhesus monkeys showed an increase in GFAP(+) cell size but not in density in all subcortical white matter areas of the frontal, temporal and parietal cortices26.
It may be concluded that regional differences in the astrocyte activation deserve to be evaluated, because they may affect dementia course depending on the relation of such cell reaction, whether local, proximal or remote, with pathways to neuronal injury. In this connection, a process of macrophage/microglia and astrocyte activation leading to neurotoxicity has been described as sharing similarities between HIV-D and Alzheimer disease27 and even multiple sclerosis28. Therefore, accurate assessment of hyper-reactive astrocyte distribution, given its connections with neuronal subpopulations, will likely provide a deeper insight into the pathophysiology and treatment not only of HIV-D, but of other neurodegenerative diseases.
Acknowledgements: This work was partially supported by grants from the CONICET (National Research Council, Argentina) and the University of Buenos Aires.
References
1. Brew BJ. Evidence for a change in AIDS dementia complex in the era of highly active antiretroviral therapy and the possibility of new forms of AIDS dementia complex. AIDS2004; 18 (Suppl 1): 875-8. [ Links ]
2. McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol 2004; 157: 3-10. [ Links ]
3. Kandanearatchi AK, Williams B, Everall IP. Assessing the efficacy of highly active antiretroviral therapy in the brain. Brain Pathol 2003; 13: 104-10. [ Links ]
4. Gray F, Adle-Biassette H, Chretien F, et al. Neuropathology and neurodegeneration in human immunodeficiency virus infection. Pathogenesis of HIV-induced lesions of the brain, correlations with HIV-associated disorders and modifications according to treatments. Clin Neuropathol 2001; 20: 146-55. [ Links ]
5. Neuenburg JK, Brodt HR, Herndier BG, et al.HIV-related neuropathology, 1985 to 1999: rising prevalence of HIV encephalopathy in the era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2002; 31: 171-7. [ Links ]
6. Bell JE. An update on the neuropathology of HIV in the HAART era. Histopathology 2004; 45: 549-59. [ Links ]
7. McGuire, Marder K. Pharmacological frontiers in the treatment of AIDS dementia. J Psychopharmacol 2000; 4: 251-7. [ Links ]
8. Dou H, Kingsley JD, Mosley RL, et al. Neuroprotective strategies for HIV-1 associated dementia. Neurotox Res 2004; 6: 503-21. [ Links ]
9. Anderson E, Zink W, Xiong H, et al. HIV-1-associated dementia: a metabolic encephalopathy perpetrated by virus-infected and immune-competent mononuclear phagocytes. J Acquir Immune Defic Syndr 2002; 31 (Suppl 2): S43-54. [ Links ]
10. Kramer-Hammerle S, Rothenaigner I, Wolff H, et al. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res 2005; 11: 194-213. [ Links ]
11. Wesselingh SL, Thompson KA. Immunopathogenesis of HIV-associated dementia. Curr Opin Neurol 2001; 14: 375-9. [ Links ]
12. Gorry PR, Ong C, Thorpe J, et al. Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1 associated dementia. Curr HIV Res 2003; 1: 463-73. [ Links ]
13. Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS 1999; 3: 1-22. [ Links ]
14. Sabri F, Titanji K, De Milito A, et al. Astrocyte activation and apoptosis: their roles in the neuropathology of HIV infection. Brain Pathol 2003; 13: 84-94. [ Links ]
15. Wu VW, Schwartz JP. Cell culture models for reactive gliosis: new perspectives. J Neurosci Res 1998; 51: 675-81. [ Links ]
16. Hansen LA, Armstrong DM, Terry RD. An immunohistochemical quantification of fibrous astrocytes in the aging human cerebral cortex. Neurobiol Aging 1987; 8: 1-6. [ Links ]
17. Miguel-Hidalgo JJ, Baucom C, Dilley G, et al. Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biol Psychiatry 2000; 48: 861-73. [ Links ]
18. Vanzani MC, Iacono RF. Alonso A, et al. Immunochemical expression of proliferative cell nuclear antigen in aging cultured astrocytes. Medicina (Buenos Aires) 2003; 63: 303-6. [ Links ]
19. Weibel ER. Stereological methods. Vol 1. London: Academic Press, 1979. [ Links ]
20. Tranque PA, Suarez I, Olmos G, et al. Estradiol-indiced redistribution of glial fibrillary acidic protein immunoreactivity in the rat brain. Brain Res 1987; 406: 348-51. [ Links ]
21. Torres-Munoz JE, Redondo M, Czeisler C, et al. Upregulation of glial clusterin in brains of patients with AIDS. Brain Res 2001; 888: 297-301. [ Links ]
22. Glass JD, Wesselingh SL, Selnes OA, et al. Clinical-neuropathologic correlation in HIV-associated dementia. Neurology 1993; 43: 2230-7. [ Links ]
23. Seilhean D, Dzia-Lepfoundzou A, Sazdovitch V, et al. Astrocytic adhesion molecules are increased in HIV-1-associated cognitive/motor complex. Neuropathol Appl Neurobiol 1997; 23: 83-92. [ Links ]
24. Monzón-Mayor M, Álvarez MI, Arbelo Galván JF, et al. Long-term evolution of local, proximal and remote astrocyte responses after diverse nucleus basalis lesioning (an experimental Alzheimer model): GFAP immunocytochemical study. Brain Res 2000; 865: 245-58. [ Links ]
25. Amenta F, Bronzetti E, Sabbatini M, et al. Astrocyte changes in aging cerebral cortex and hippocampus: a quantitative immunohistochemical study. Microsc Res 1998; 43: 29-33. [ Links ]
26. Sloane JA, Hollander W , Rosene DL, et al. Astrocyte hypertrophy and altered GFAP degradation with age in subcortical white matter of the rhesus monkey. Brain Res 2000; 862: 1-10. [ Links ]
27. Smits HA, Boven LA, Pereira CF, et al. Role of macrophage activation in the pathogenesis of Alzheimer´s disease and human immunodeficiency virus type 1-associated dementia. Eur J Clin Invest 2000; 30: 526-35. [ Links ]
28. Minagar A, Shaoshak P, Fujumura R, et al. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurological disorders: HIV-associated dementia, Alzheimer disease and multiple sclerosis. J Neurol Sci2002; 202: 13-23. [ Links ]
Received: 12-12-2005
Accepted: 14-02-2006














