Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Medicina (Buenos Aires)
versión impresa ISSN 0025-7680versión On-line ISSN 1669-9106
Medicina (B. Aires) v.66 n.4 Buenos Aires jul./ago. 2006
Medium and long term outcome of growth hormone therapy in growth hormone deficient adults
Hugo L. Fideleff, Hugo Boquete, Ana Giaccio, Patricia Sobrado, on behalf of KIMS1, Argentine Group2
Endocrinology Unit, Department of Medicine, Hospital T. Alvarez; Buenos Aires, Argentina
1Pfizer Internacional Database
2 KIMS Argentine Group: Darío Bruera (Hosp. de Clínicas, Córdoba), Alberto Chervin (Hosp. S. Lucía), Haraldo Claus Hermberg (Hosp. Alemán), Hugo L. Fideleff (Hosp. Alvarez), Oscar Levalle (Hosp. Durand), Mirta Miras (Hosp. de Niños, Córdoba), Isaac Sinay (Hosp. Francés), Graciela Stalldecker (Hosp. Pirovano).
Dirección Postal: Dr. Hugo L. Fideleff, Unidad de Endocrinología, Hospital T. Alvarez, Aranguren 2701, 1406 Buenos Aires, Argentina. Fax: (54-11) 4612-6563 E-mail: hugofideleff@arnet.com.ar
Abstract
We evaluated long-term replacement therapy outcomes in various subsets of patients with adult growth hormone (GH) deficiency (AGHD) as well as the patients' susceptibility to adverse events. Fifty-nine patients with AGHD were evaluated, 27 with childhood onset (CO) (18-44 years old, 12 females) and 32 with adult onset (AO) (27-70 years, 18 females). A significant improvement in HDL-cholesterol was observed in AGHD-AO males (basal: 41.3 ± 12.9 mg/dl, intratreatment: 47.5 ± 13.2 mg/dl, p= 0.009). However, individual analyses showed that total cholesterol decreased below 240 mg/dl in 33% of AGHD-CO patients and in 50% of AGHD-AO patients, and below 200 mg/dl in 67% of AGHD-CO patients and in 29% of AGHD-AO patients; in the AGHD-AO group, normalization of LDL-cholesterol (£ 160 mg/dl) and triglycerides (£ 200 mg/dl) was found in 100% and 50% of patients, respectively; the total cholesterol/HDL ratio decreased below 4.5 in 20% of AGHD-CO patients and in 25% of AGHD-AO patients. The cardiological evaluation showed a significant intra- and interindividual heterogeneity, but cardiac mass improved in patients with a baseline cardiac mass index below 60 g/m2. Markers of bone apposition increased significantly, while bone resorption markers were found to remain unchanged during treatment. A correlation was found between increased bone mineral content and lean body mass (p= 0.0009). Susceptibility to adverse events was not found to be dependent on gender or on the time of onset of the deficiency. Our findings would appear to confirm that a more severe metabolic impairment is correlated with a better therapeutic outcome.
Key words: Growth hormone, Adult growth hormone deficiency, Metabolic alterations, Growth hormone treatment
Resumen
Resultados en el mediano y largo plazo del tratamiento con hormona de crecimiento en adultos deficitarios de dicha hormona. Evaluamos resultados terapéuticos a largo plazo en subgrupos de pacientes con deficiencia de hormona de crecimiento (GH) del adulto (AGHD) y la susceptibilidad para desarrollar eventos adversos. Estudiamos 59 pacientes con AGHD, 27 de inicio en la infancia (CO) (18-44 años, 12 mujeres) y 32 de inicio en la adultez (AO) (27-70 años, 18 mujeres). El HDL-colesterol mejoró significativamente en varones AGHD-AO (basal: 41.3 ± 12.9 mg/dl, intratratamiento: 47.5 ± 13.2 mg/dl, p= 0.009). El análisis individual mostró que el colesterol total descendió por debajo de 240 mg/dl en el 33% de los AGHD-CO y en el 50% de los AGHD-AO, y por debajo de 200 mg/dl en el 67% de los pacientes AGHD-CO y en el 29% de los AGHD-AO; en el grupo AGHD-AO, se normalizó el LDL-colesterol (£ 160 mg/dl) y los triglicéridos (£ 200 mg/dl) en el 100% y 50% de los pacientes respectivamente; el índice colesterol total /HDL disminuyó por debajo de 4.5 en el 20% de los pacientes AGHD-CO y en el 25% de los AGHD-AO. La evaluación cardiológica mostró heterogeneidad intra e interindividual, con mejoría de la masa cardíaca en pacientes con valores menores a 60 g/m2 pretratamiento. Se encontró incremento significativo en los parámetros de aposición ósea, sin modificaciones en los de resorción durante el tratamiento. Se evidenció correlación entre el incremento del contenido mineral óseo y la masa magra (p=0.0009). No se hallaron subgrupos más susceptibles para el desarrollo de eventos adversos. Nuestros hallazgos parecerían corroborar que la mayor gravedad de afectación metabólica se correlacionaría con una mejor respuesta terapéutica.
Palabras clave: Hormona de crecimiento, Deficiencia de hormona de crecimiento del adulto, Alteraciones metabólicas, Tratamiento con hormona de crecimiento
Adult growth hormone (GH) deficiency (AGHD) has been recognized as a clinical entity since the 1980's, when it was described as a syndrome characterized by alterations in body composition, bone mineral density, lipid profile, decreased muscle strength and exercise performance, impaired heart function and structure with increased cardiovascular risk factors and impaired quality of life1, 2. In a previous study, we observed differences in impairment in AGHD patients, which are mainly dependent on gender and on the time of onset of the deficiency, and thus confirm the heterogeneity of this syndrome3.
Even if some of the beneficial effects of GH replacement therapy have been recognized, there are still some controversies over the medium- and long-term impact of GH therapy on the various areas involved2, 4-6.
The use of individualized GH dosages, adjusted according to insulin-like growth factor type I (IGF-I) levels, in contrast with earlier studies where fixed higher dosage schedules were used, resulted at present in a decreased incidence of adverse effects with an improved tolerance to treatment7, 8.
The aim of this study was to evaluate the potential differences in the outcomes of long-term GH replacement therapy in different subsets of patients and to verify potential differences in susceptibility to adverse effects.
Materials and Methods
Fifty-nine patients with AGHD were included: 27 with childhood onset (CO) with an age range between 18 and 44 years old (12 females and 15 males) and 32 with adult onset (AO) with an age range between 27 and 70 years old (18 females and 14 males). The etiologies of GHD for AGHD-CO were: idiopathic (17); craniopharyngioma (5); perinatal trauma or asphyxia, meningitis, oligodendroglioma, cholesteatoma, and pinealoma (1 each). GHD etiologies for AGHD-AO were: non-functioning pituitary tumor (8); prolactinoma (6); Sheehan's syndrome (5); craniopharyngioma (4); Cushing's disease (2); pituitary epidermoid cyst (2); pituitary granuloma, hypophysitis, empty sella, acromegalia and idiopathic (1 each).
The diagnosis of AGHD was made by the Insulin Tolerance Test (ITT)9. In patients in whom this test was contraindicated, an arginine test was performed10. In patients with isolated deficiency and in idiopathic patients, both tests were performed. Only patients with severe GH deficiency, defined by a peak GH response < 3 µg/l to any of the stimulation tests, were enrolled9.
In the AGHD-CO group, 19 patients had received GH therapy in childhood, but had discontinued such therapy at least 1 year before their enrollment in the study.
All patients received GH therapy at a starting dose of 0.1 mg/day, adjusted trying to maintain IGF-I levels between 0 and 2 SDS for gender and age. In the AGHD-CO group, the maintenance dose (mean and range) was 0.52 mg/day (0.17-1.07) in females and 0.33 mg/day (0.13-0.67) in males; in the AGHD-AO group, the maintenance dose was 0.38 mg/day (0.13-0.8) in females and 0.31 mg/day (0.13 -0.67) in males.
At baseline and during the fourth year of replacement therapy, the following evaluations were performed in 36 of the patients, 14 AGHD-CO (18-43 yr, mean 31.3 yr) and 22 AGHD-AO (31-66 yr, mean 48.2 yr): Anthropometric parameters: body mass index (BMI) and waist circumference; lipids: total cholesterol (TC), HDL-cholesterol, LDL-cholesterol, triglycerides (TG) and total cholesterol/ HDL-cholesterol ratio; cardiological evaluation: 2D echocardiogram with mitral Doppler (Esaote Model AU3). Diastolic function (A/E waves ratio), systolic function (ejection fraction) and cardiac mass index (CMI) were evaluated.
In 38 patients, 14 AGHD-CO (19-44 yr, mean 34 yr) and 24 AGHD-AO (31-66 yr, mean 48.5 yr), bone formation and resorption markers were measured at baseline and after 18 months of treatment: osteocalcin (RIA, Diagnostic Systems Laboratories, Inc., Webster, Texas, USA), bone-specific alkaline phosphatase (B-S ALP) and procollagen type I carboxyterminal propeptide (PICP), pyridinoline (Pyr) and deoxipyridinoline (DPyr) (Elisa, Metra Biosystems, Inc., Mountain View, CA, USA). In this group of patients, body composition was also evaluated at baseline and after 12-18 months of treatment by measuring the percentage of lean and fat mass with a Lunar DPX-L densitometer.
Two different assays were used to determine plasma levels of GH. First, an IRMA-Magnetic Solid Phase was used (Serono Maia Clone, Milan, Italy), calibrated against the 1st IRP 66/217. Then, a two-site chemiluminiscent enzyme immunometric assay (ICMA, Immulite, Diagnostic Products Corporation, Los Angeles, USA) was used, calibrated against the WHO IRP 80/505. The equation for the linear regression line comparing the two methods was log y = 0.9069 log x + 0.3172, where x was the IRMA and y was the ICMA (r: 0.9523). Serum IGF-I level was measured using IRMA after acid-ethanol extraction (Diagnostic Systems Laboratories, Inc., Webster, Texas, USA). Age -and sex-adjusted IGF-I values were obtained from a reference population obtained from blood donors: 384 serum samples from healthy adults (191 males and 193 females) with an age range between 18-70 years. The individual IGF-I standard deviation score (SDS) could then be calculated. SDS expresses the number of standard deviations away from the mean for the reference population.
Adverse events occurring during GH therapy were collected by means of a consistent questioning of subjects with regard to the occurrence of health-related events between visits, based on predesigned forms. Both GH-therapy possibly-related and possibly-unrelated events were recorded.
The statistical analysis was performed by the Wilcoxon test11 to assess treatment response (baseline vs. intra-treatment), separately in AGHD-CO and AGHD-AO. HDL, body composition and waist circumference were separately analyzed for each gender in each group. The Chi-square test and Fisher's test were used to compare the prevalence of adverse events in each group11. The correlation between bone mineral content and lean tissue percentage was analyzed by linear regression. These correlations were also compared to those from a control group (600 age-matched males and post-menopausal females) by means of an analysis of covariance (ANCOVA)12. Results are expressed as median and range (between brackets) for IGF-I values, bone markers and bone mineral content since these variables follow a non-parametric distribution, and as mean ± standard deviation (SD), for anthropometric parameters, lipids, cardiological evaluation and body composition.
Written informed consent was obtained from all patients, and the Ethics and the Education and Research Committees approved the study.
Results
During GH therapy, serum IGF-I levels in the AGHD-CO group were 210 ng/ml (44-280) and 165 ng/ml (110-240) in females and males, respectively; in the AGHD-AO group, serum IGF-I levels were 170 ng/ml (18-340) and 240 ng/ml (40-440) in females and males, respectively. These levels, expressed as SDS, correspond to: 0.22 (-3.30 to 1.35) and 1.40 (-0.80 to 2.30) in the AGHD-CO group, in females and males, respectively, and to 0.61 (-5.80 to 2.80) and 0.95 ( -3.60 to 5.90) in the AGHD-AO group, in females and males, respectively.
No difference was found in BMI between baseline and intra-treatment values (Table 1). In 20% of AGHD-CO patients and in 18% of AGHD-AO patients with elevated BMI at baseline (AGHD-CO: 26.1 ± 1.1; AGHD-AO: 26.9 ± 1.9), this index decreased to £ 25 kg/m2 (AGHD-CO: 24.3 ± 0.9; AGHD-AO: 24.5 ± 0.7).
TABLE 1.- Antropometric parameters and lipid profile in AGHD patients (childhood onset: CO, adult onset: AO): baseline and intra-treatment (fourth year of GH replacement therapy) data.
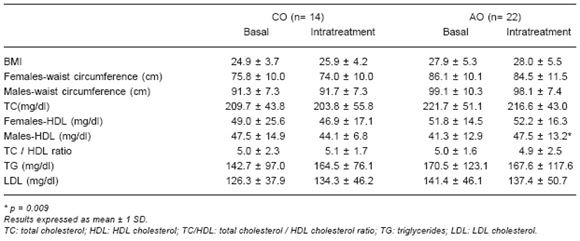
No differences were observed in waist circumference in any of the groups (Table 1). Normalization of waist circumference was observed (£ 84 cm in females and £ 92 cm in males) in 33% and 25% of AGHD-CO patients, females and males respectively (baseline females: 87 ± 2.5 and males: 96.2 ± 1.9; intra-treatment females: 79.1 ± 2.9 and males: 89.7 ± 2.1). In 30% of AGHD-AO females a similar normalization was observed (baseline: 85.3 ± 2.1; intra-treatment: 81.5 ± 2.4).
Results of total cholesterol, HDL-cholesterol, triglycerides, LDL-cholesterol, and the total cholesterol/HDL-cholesterol ratio are shown in Table 1. A statistically significant intra-treatment improvement was found in HDL-cholesterol in males of the AGHD-AO group (p = 0.009).
The individual analysis showed that, in patients with elevated basal levels, total cholesterol decreased below 240 mg/dl but above 200 mg/dl in 33% of AGHD-CO patients and in 50% of AGHD-AO patients, and, in those with basal levels above 200 mg/dl, it decreases below this value in 67% of AGHD-CO patients and in 29% of AGHD-AO patients. In the AGHD-AO group, normalization of LDL-cholesterol (£ 160 mg/dl) and triglycerides (£ 200 mg/dl) was found in 100% and 50% of patients with elevated levels at baseline, respectively. Thirty-three percent of AGHD-AO patients with normal cholesterol levels at baseline had increases above 160 mg/dl during treatment. The total cholesterol/HDL ratio decreased below 4.5 in 20% of AGHD-CO patients and in 25% of AGHD-AO patients.
None of the cardiological variables assessed showed significant changes after treatment (Table 2). The only finding was a consistent improvement in the CMI in patients (7) with lower baseline values (< 60 g/m2): 57 and 85 g/m2, 51 and 59 g/m2, 46 and 70 g/m2, 53 and 89 g/m2, 50 and 72 g/m2, 57 and 85 g/m2 and 57 and 72 g/m2 (basal and intra-treatment respectively).
TABLE 2.- Cardiological evaluation in AGHD patients (childhood onset: CO, adult onset: AO): baseline and intra-treatment (fourth year of GH replacement therapy) data.

The individual analysis showed heterogeneity in the echocardiographic response. Nevertheless, an improvement in diastolic function was observed in 50% of AGHD-CO patients and in 86% of AGHD-AO patients.
No differences were found between baseline and intra-treatment values in percentages of fat and lean tissues (Table 3).
TABLE 3.- Bone markers and body composition in AGHD patients (childhood onset: CO, adult onset: AO): baseline and intra-treatment (fourth year of GH replacement therapy) data.
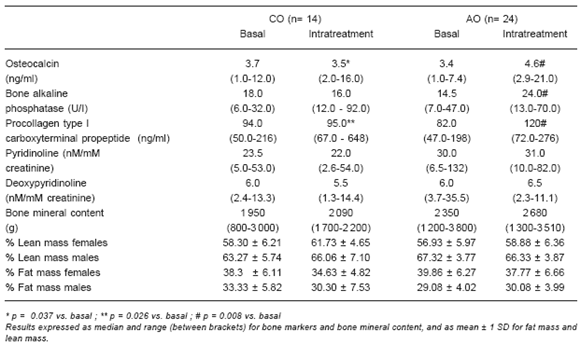
Results of osteocalcin, bone-specific alkaline phosphatase, procollagen type I carboxyterminal propeptide, pyridinoline, deoxypyridinoline and bone mineral content are shown in Table 3. A statistically significant intra-treatment increase was found in osteocalcin (p = 0.037) and procollagen type I carboxyterminal propeptide (p = 0.026) in the AGHD-CO group. In the AGHD-AO group, osteocalcin, bone alkaline phosphatase and procollagen type I carboxyterminal propeptide showed a statistically significant intra-treatment increase (p = 0.008). Correlations between bone mineral content and lean tissue percentage, both at baseline and during treatment, were significant (p= 0.0009) and did not differ significantly from the control group. Treatment resulted in a proportional increase in bone mineral content and lean mass in both genders; the curves intercepts remained unchanged. This effect was even more evident when only lower limbs measurements were considered (baseline r= 0.938, p= 0.0009; intra-treatment r= 0.949, p= 0.0008).
In the AGHD-CO group, 58% of females and 67% of males experienced adverse events during treatment. In the AGHD-AO group, adverse events were observed in 67% of females and in 50% of males.
Clinical adverse events reported in AGHD-CO females were: edema (4), myalgia, hypotension, depression, headache, arthralgia, abdominal pain (1 each); AGHD-CO males: myalgia (6), arthralgia (3), hypotension, edema, gastroenteritis, headache, trauma fracture, asthenia (2 each), diabetes (1); AGHD-AO females: arthralgia (9), edema (8), headache (4), gastroenteritis (3), tiredness, depression, trauma fracture, hepatitis, toothache (2 each), hypotension, breast adenoma, acute myocardial infarction, urinary infection, hypercalciuria, Sjogren's syndrome, epigastric distress, fever, weakness, myalgia, bronchitis, metrorrhagia, anemia, diabetes, death due to stroke (1 each); and AGHD- AO males: myalgia, bronchitis, hepatitis, facial palsy, anemia, intermittent claudication, enlargement of residual pituitary tumor, sleep apnea, multiple atheromatosis, edema, gynecomastia, blood hypertension (1 each). Biochemical adverse events observed in AGHD-CO females were: elevated liver transaminases (1); AGHD-CO males: increased levels of total cholesterol, increased levels of LDL cholesterol, increased triglycerides levels (4 each), elevated liver transaminases (2), increased glycosilated hemoglobin, increased total alkaline phosphatase (1 each); AGHD-AO females: increased levels of total cholesterol (5), increased tryglicerides levels (4).
No differences were found in the frequency of occurrence of adverse events between any of the subsets evaluated.
Discussion
Since the GH deficiency syndrome was first described in adults over 15 years ago, various studies have shown that patients suffering from this condition have experience of a number of alterations, as compared to age- and sex-matched healthy subjects. In patients with panhypopituitarism under standard hormone replacement other than GH, some authors reported an increased mortality rate related to an increased incidence of atherosclerosis13, 14. GH deficiency might play a role in this increase, possibly due to an increased abdominal adiposity and associated dyslipidemia. Various studies in which GH replacement therapy has been evaluated in GH deficient subjects have reported a marked improvement in the short-term in body composition4, 5, 15, lipid profile16, 17 and quality of life18, 19. However, data in the literature, as well as our data, show, in some aspects, controversial medium- and long-term outcomes. These discrepancies were first attributed to the use of inadequate replacement doses. On the one hand, excessively high doses were used (even with weight- and body surface area-adjusted dosages), which might account for many of the adverse effects observed. On the other hand, suboptimal doses have been associated with inadequate treatment responses20. Therefore, it has been suggested that the GH dosage should be adjusted to IGF-I concentrations. But IGF-I measurements expressed in absolute values showed some limitations. These limitations appear to be related to the wide range of IGF-I values in normal populations, with a resulting overlapping between healthy subjects and GH-deficient subjects, to circadian variations in plasma levels, and to the variability and reproducibility depending on the various measurement methods employed. For this reason, and to overcome some of these limitations, it has been suggested that values of IGF-I should be expressed in terms of SDS21 and should be maintained at approximately the 50th percentile, within 2 SD of the mean, in patients on GH therapy22. However, even the normalization of these parameters in the follow-up of patients has not been enough to solve all the controversies, and there is still a considerable heterogeneity in therapeutic outcomes. In our study, we succeeded in maintaining satisfactory IGF-I levels in most patients, but despite this, and in agreement with other authors' reports, outcomes have been clearly variable23. Some patients had IGF-I levels outside target values at some moment during follow-up. A clear example is the variability in the results reported in the literature with regard to the lipid profile24, 25. We have found a significant improvement in HDL-cholesterol levels only in the group of AGHD-AO males. However, the individual analysis showed that in a considerable number of patients, lipid parameters were clearly improved and even normalized in many of them. This could be partly attributed to a different response to treatment due to a different individual sensitivity, based on the degree of metabolic impairment, the severity of somatotropin deficiency, etc. This could be also explained by the fact that patients may often change their eating habits and physical activity lifestyle during follow-up, particularly in medium- and long-term studies such as this one. Some of these considerations might also account for the variability in the results obtained in body composition. Finally, we should highlight the limitations in the comparison between different studies, given the high percentage of biological and analytical variability observed in plasma lipid measurements. In this respect, coefficients of variation of up to 37% have been reported for triglycerides, 18.6% for LDL-cholesterol, 23.6% for HDL-cholesterol and 12.4% for total-cholesterol26.
The cardiological evaluation showed inter- and intra-individual heterogeneity, a finding that appears to be consistent in the interpretation of results27. In practice, this means that not all patients from a same group have a homogeneous behavior, but we have also observed that many patients did not show an identical response pattern in the medium and long term. Even if there is no clear explanation for this variability, it may be speculated that the difference in response is possibly related to variations in the concentration of GH receptors in the myocardium. It has been shown that by interacting with circulating GH, such receptors stimulate local biosynthesis of IGF-I, which acts in an autocrine or paracrine manner by binding to high-affinity sarcolemmatic receptors28. An exception to the heterogeneity reported above was the pretty consistent improvement in cardiac mass in patients with cardiac mass index values below 60 g/m2.
As regards bone mineral metabolism, we found a significant increase in apposition parameters, with no changes in resorption parameters during GH treatment. At least part of the bone anabolic effect of GH therapy is likely to be due to the increased muscle mass. In agreement with other authors, we were able to show a very good correlation between increased bone mineral content and increased lean mass29.
The high rate of adverse events is common to all pharmacovigilance studies, where both possibly-related and possibly-unrelated events are collected. Forty-five percent of adverse events could be considered to be possibly related to therapy. Most of them (94%) were mild and transient in nature, and generally dependent on fluid retention. The moderate prevalence of these events might be dependent on the initiation of treatment at low doses and on the dose adjustment according to IGF-I levels. Unlike other reports in the literature, we have not found susceptibility to adverse events to be dependent either on gender or on the time of onset of the deficiency30. However, because of the three possibly-related serious adverse events observed (two cases of diabetes and one of enlargement of residual pituitary tumor), high risk patients should be carefully monitored. As regards the patient who died due to a stroke, it is doubtful whether this should be considered as an adverse event since the patient had a history of vascular disease.
To sum up, from our findings and reports in the literature it can be inferred that the beneficial effects of GH replacement therapy in GH-deficient adults are mainly evidenced in subjects with a greater degree of impairment in each area (in our experience: cardiovascular parameters and lipid abnormalities). In the same way as in GH-deficient children a lower growth rate is one of the variables related to a better response to treatment, it is possible that in adults a more severe metabolic impairment may be correlated with a better outcome. This has already been shown in some areas such as quality of life (not assessed in this work)31, 32.
In conclusion, GH therapy is beneficial in deficient adults as replacement therapy, regardless of the time of onset of the deficiency (prepubertal period or adulthood). Other indications for GH therapy are being investigated and probably some of the ongoing studies may demonstrate further beneficial effects. Although replacement therapy started to be used in deficient adults over fifteen years ago, there are still unsettled issues regarding its long-term benefits and its influence on morbidity and mortality.
Acknowledgements: We wish to thank Carina Fideleff for her assistance in the correction of the English version. We thank Dr. Ricardo Barmat for his assistance in the interpretation of cardiological results. This study was partially supported by Pfizer Argentina SRL.
References
1. Cuneo RC, Salomon F, McGauley GA, Sönksen PH. The growth hormone deficiency syndrome in adults. Clin Endocrinol (Oxf) 1992; 37: 387-97.
2. Carroll PV, Christ ER, Bengtsson B-Å, et al. Growth hormone deficiency in adulthood and the effects of growth hormone replacement: a review. Growth Hormone Research Society Scientific Committee. J Clin Endocrinol Metab 1998; 83: 382-95.
3. Fideleff HL, Chervin A, Giaccio A, Sobrado P, Barmat R, Boquete H. Adult Growth Hormone Deficiency: Metabolic Alterations and Evaluation of Different Risk Groups. Medicina (Buenos Aires) 2004; 64: 13-9.
4. Salomon F, Cuneo RC, Hesp H, Sönksen PH. The effects of treatment with recombinant human growth hormone on body composition and metabolism in adults with growth hormone deficiency. N Engl J Med 1989; 321: 1797-803.
5. Jorgensen JOL, Pedersen SA, Thuesen L, et al. Beneficial effects of growth hormone treatment in GH-deficient adults. Lancet 1989; 1221-5.
6. Bengtsson B-Å, Edén S, Lünn I, et al. Treatment of adults with growth hormone (GH) deficiency with recombinant human GH. J Clin Endocrinol Metab 1993; 76: 309-17.
7. Drake WM, Coyte D, Camacho-Hubner C, et al. Optimizing growth hormone replacement therapy by dose titration in hypopituitary adults. J Clin Endocrinol Metab 1998; 83: 3813-9.
8. Murray RD, Skillicorn CJ, Howell SJ, Lissett CA, Rahim A, Shalet SM. Dose titration and patient selection increases the efficacy of GH replacement in severely GH deficient adults. Clin Endocrinol (Oxf) 1999; 50: 749-57.
9. Growth Hormone Research Society. Consensus guidelines for the diagnosis and treatment of adults with growth hormone deficiency: summary statement of the Growth Hormone Research Society workshop on adult growth hormone deficiency. J Clin Endocrinol Metab 1998; 83: 379-81.
10. Fideleff HL, Frigeri AE, Sobrado PG, Llano MN, Ruibal GF, Boquete HR. Reproducibility and variability of the arginine test in normal adults: Comparison between sexes. Medicina (Buenos Aires) 1999; 59: 249-53.
11. Siegel S. Non parametric statistics for the behavioral sciences, 5th ed. New York: Mc Graw, 1990.
12. Robert C Elston & William D Johnson. Principios de Bioestadística. Editorial: El Manual Moderno, S.A. de C.V. México, D.F.,1990, p 213-4.
13. Rosen T, Bengtsson B-Å. Premature mortality due to cardiovascular disease in hypopituitarism. Lancet 1990; 336: 285-8.
14. Bates AS, Van't Hoff W, Jones PJ, Clayton RN. The effect of hypopituitarism on life expectancy. J Clin Endocrinol Metab 1996; 81: 1169-72.
15. Binnerts A, Swart GR, Wilson JHP, et al. The effect of growth hormone administration in growth hormone deficient adults on bone, protein, carbohydrate and lipid homeostasis, as well as body composition. Clin Endocrinol (Oxf) 1992; 37: 79-87.
16. Russell-Jones DL, Watts GF, Weissberger A, et al. The effect of growth hormone replacement on serum lipids, lipoproteins, apolipoproteins and cholesterol precursors in adult growth hormone deficient patients. Clin Endocrinol (Oxf) 1994; 41: 356-63.
17. Beshyah SA, Henderson A, Niththyananthan R, et al. The effects of short and long-term growth hormone replacement therapy in hypopituitary adults on lipid metabolism and carbohydrate tolerance. J Clin Endocrinol Metab 1995; 80: 356-63.
18. Beshyah SA, Freemantle C, Shahi M. Replacement therapy with biosynthetic human growth hormone in growth hormone-deficient hypopituitary adults. Clin Endocrinol (Oxf) 1995; 42: 73-81.
19. Burman P, Broman JE, Hetta J, et al. Quality of life in adults with growth hormone (GH) deficiency: response to treatment with recombinant human GH in a placebo-controlled 21-month trial. J Clin Endocrinol Metab 1995; 80: 3585-90.
20. Underwood LE, Attie KM, Baptista J. Growth hormone (GH) dose-response in young adults with childhood-onset GH deficiency: A two-year, multicenter, multiple-dose, placebo-controlled study. J Clin Endocrinol Metab 2003; 88: 5273-80.
21. Boquete H, Sobrado P, Fideleff HL, et al. Evaluation of diagnostic accuracy of insulin-like growth factor (IGF)-I and IGF-binding protein-3 in growth hormone-deficient children and adults using Roc Plot analysis. J Clin Endocrinol Metab 2003, 88: 4702-8.
22. Vance ML, Mauras N. Growth hormone therapy in adults and children. N Engl J Med 1999; 341:1206-16.
23. Gibney J, Johannsson G. Long-term monitoring of insulin-like growth factor I in adult growth hormone deficiency: a critical appraisal. Horm Res 2004; 62 Suppl 1: 66-72.
24. Twickler T, Cramer M, Dallinga-Thie G, et al. Adult-onset growth hormone deficiency: Relation of postprandial dyslipidemia to premature atherosclerosis. J Clin Endocrinol Metab 2003; 88: 2479 - 88.
25. Gola M, Bonadonna S, Doga M, Giustina A. Growth hormone and cardiovascular risk factors. J Clin Endocrinol Metab 2005; 90: 1864 -70.
26. Cooper GR, Jay Smith S, Myers GL, Sampson EJ, Magid E. Estimating and minimizing effects of biologic sources of variation by relative range when measuring the mean of serum lipids and lipoproteins. Clin Chem 1994; 40: 227-32.
27. Fideleff HL, Boquete HR. Growth hormone (GH) deficiency and GH replacement therapy: effects on cardiovascular function. In: Abs R and Feldt Rasmussen U (eds). 10 Years of KIMS. London: Oxford PharmaGenesis: 2004, p 149-59.
28. Fazio S, Palmieri EA, Biondi B, Cittadini A, Sacca L. The role of the GH-IGF-I axis in the regulation of myocardial growth: from experimental models to human evidence. Eur J Endocrinol 2000; 142: 211-6.
29. Hermberg H, Fideleff H, Chervin A, et al. Effects of GH on the mineral, lean and fat masses in pan-hypopituitary men and women. J Bone Min Res 2001; 16 (Suppl 1): S 408.
30. Attanasio AF, Lamberts SWJ, Matranga AMC, et al. Adult growth hormone (GH) - deficient patients demonstrate heterogeneity between childhood onset and adult onset before and during human GH treatment. J Clin Endocrinol Metab 1997; 82: 82-8.
31. Shalet S. Growth hormone (GH) replacement is not justified for all adults with GH deficiency. J Clin Endocrinol Metab 2000; 85: 933-9.
32. Giaccio A, Boquete H, Sobrado P, Fideleff HL. Deficiencia de GH y calidad de vida: diferente respuesta al tratamiento sustitutivo prolongado según el momento de inicio del déficit y el sexo. Rev Arg Endocrinol Metab 2004; 41: 56 (resumen).
Received: 02-08-2005
Accepted: 10-05-2006














