Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Medicina (Buenos Aires)
versión impresa ISSN 0025-7680versión On-line ISSN 1669-9106
Medicina (B. Aires) v.66 n.6 Buenos Aires nov./dic. 2006
Rabbit IgG antibodies against Phospholipase A2 from Crotalus durissus terrificus neutralize the lethal activity of the venom
Juan P. Rodríguez1, Mauricio De Marzi2, Silvana Maruñak3, Emilio L. Malchiodi2, Laura C. Leiva1, Ofelia Acosta3
1 Cátedra de Química Biológica I, Facultad de Ciencias Exactas y Naturales y Agrimensura, Universidad Nacional del Nordeste;
2 Cátedra de Inmunología, Instituto de Estudios de la Inmunidad Humoral (IDEHU), CONICET-UBA. Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires;
3 Cátedra de Farmacología, Facultad de Ciencias Veterinarias, Universidad Nacional del Nordeste, Corrientes
Postal address: Dra. Ofelia Acosta, Facultad de Ciencias Veterinarias, UNNE, Sgto. Cabral 2139, 3400 Corrientes, Argentina, Fax: (54-03783) 425753 int. 113
E-mail: patmed@vet.unne.edu.ar
Abstract
Crotalus durissus terrificus (C.d.t.) (South American rattlesnake) venom possesses myotoxic and neurotoxic activities, both of which are also expressed by crotoxin, the principal toxin of this venom. Crotoxin contains a basic phospholipase A2 (PLA2) and a non toxic acidic protein, crotapotin. We have produced and investigated the ability of IgG antibodies raised in rabbits against PLA2 to neutralize the lethality of the whole venom. PLA2 was isolated by gel filtration chromatography (Sephadex G-75). Specific antibodies were obtained by subcutaneous and intramuscular inoculation of PLA2 (700 µg) with Freund adjuvant. Groups of six mice (20 + 2 g) were inoculated with 0.5 ml i.p. of C. d. t. venom (4 µg) or a mixture of venom that had been preincubated with the desired volume of IgG antibodies. Mortality, recorded 24 and 48 h after inoculation, showed that IgG anti-PLA2 were more effective than anticrotalic serum in neutralizing the lethal activity. These results demonstrate that it could be possible to obtain an anti-venom made by specific antibodies with a high level of protection against the lethal component of C.d.t. venom, and/or the inclusion of these antibodies as a supplement in heterologous anti-venoms.
Key words: Crotalus durissus terrificus; Phospholipase A2; Antibodies IgG; Neutralization
Resumen
Los anticuerpos IgG de conejos anti-fosfolipasa A2 de Crotalus durissus terrificus neutralizan la actividad letal del veneno. El veneno de Crotalus durissus terrificus (C.d.t.) (Cascabel de Sud América) posee actividad miotóxica y neurotóxica, actividades que también exhibe el complejo crotoxina, principal componente tóxico de este veneno. El complejo crotoxina está constituido por una fosfolipasa A2 básica (PLA2) y una proteína acídica no tóxica, el crotapotín. En este trabajo se estudió la capacidad neutralizante de anticuerpos IgG anti-PLA2 sobre la letalidad inducida por el veneno entero. El antígeno PLA2, fue aislado por cromatografía de filtración en gel (Sephadex G-75). Se inocularon conejos machos por vía subcutánea e intramuscular, con 700 µg de PLA2 y adyuvante para la obtención de anticuerpos específicos. La capacidad neutralizante del antisuero se analizó en ratones por inoculación con diluciones de veneno entero preincubado con un volumen adecuado de anticuerpos IgG anti-PLA2. Se inocularon ratones controles con 0.5 ml i.p. de veneno (4 µg.ml-1). El número de muertes fue contabilizado a las 24 y 48 h posteriores a la inoculación, demostrándose que la capacidad neutralizante de los anticuerpos IgG anti-PLA2 fue superior a la obtenida con el antiveneno crotálico. Los resultados obtenidos demuestran la potencial aplicación de antivenenos constituidos por anticuerpos específicos contra PLA2, y/o la inclusión de estos anticuerpos como suplementos en antivenenos polivalentes.
Palabras clave: Crotalus durissus terrificus; Fosfolipasa A2; Anticuerpos IgG; Neutralización
Crotoxin, the major toxin of South American rattlesnake (Crotalus durissus terrificus) (C.d.t.) venom1, is a potent phospholipase A2 neurotoxin that produces neuromuscular blockade2. Several additional biological activities, including myotoxicity3, hemolysis4 and platelet aggregating activity5, have also been attributed to crotoxin.
Crotoxin consists of a basic, weakly toxic phospholipase A2 (PLA2) subunit and an acidic, non-toxic subunit (crotapotin) devoid of enzymatic activity6, 7. Crotapotin acts as a chaperon in the complex by preventing non-specific binding of PLA2, thereby potentiating its toxicity8. Thus, the stability of the interaction between PLA2 and crotapotin plays a major role in the toxicity of crotoxin9.
Up to now, heterologous antibody administration has been the treatment of choice for snakebite victims. This practice requires the production, normally by horse immunization, of a high-titer anti-venom serum; semi purified globulin preparation or also fraction of antibodies, all of them against the whole venom10-13. Owing to the large amount of heterologous proteins administered, serum therapy may induce adverse reactions12, 13. In these circumstances specific antibodies against the lethal component of the venom would be more appropriate for immunotherapeutic applications.
In the present study, we examined the ability of specific IgG rabbit antibodies against PLA2 (IgG anti-PLA2) from C.d.t. venom to neutralize the lethal activity of this venom.
Materials and Methods
Venom and toxin (antigen)
Desiccated C.d.t. venom was obtained from Zoológico de la Ciudad de Corrientes. PLA2 was purified from C.d.t. venom by gel filtration chromatography. The procedure was carried out in one step using Sephadex G-75 (30x1 cm) pre-equilibrated with 20 mM glycin, 150 mM NaCl, pH 1.9, according to a modification of the method described by Landucci et al.5. Fractions of 0.75 ml/tube were collected at a flow rate of 12 ml/h.
Animals
Male Swiss white mice weighing 20-22 g were supplied by the animal facility of the Facultad de Ciencias Veterinarias, UNNE. The mice were housed at 25 °C on a 12 h light/dark cycle and had free access to food and water.
Male New Zealand white rabbits weighing 3 kg were housed individually with free access to food and water.
Anti-venom
The anti-crotalic venom used was a semi-purified immunoglobulin-rich solution produced by hyperimmunization of horses with C.d.t. venom provided by ANLIS (Instituto Nacional de Producción de Biológicos. Administracion Nacional de Laboratorios e Institutos de Salud "Dr. Carlos Malbrán, Buenos Aires, Argentina), and designated ACS (anti-crotalic serum).
Anti-sera raised in rabbits and IgG anti-PLA2
Rabbits were immunized by successive intramuscular and subcutaneous inoculations with 700 µg of PLA2 per rabbit. The first injections included Freund's complete adjuvant (Sigma) in a 1:1 ratio. The subsequent boosters were given in the same way, but using Freund´s incomplete adjuvant with the toxin dissolved in PBS.
The antibody levels in the sera were monitored by gel immunodiffusion14 and ELISA15. Blood samples were collected from a marginal ear vein and stored at 4 °C. The sera were subsequently separated by centrifugation and aliquots were stored at –70 °C.
IgG anti-PLA2 were obtained by ammonium sulfate precipitation, desionizated by exclusion molecular chromatography, Sephadex G-25 column (20 x 0.75 cm) equilibrated with 10 mM phosphate buffer pH 7.2. The desionizated IgG were then stored at –70 °C.
Antibody detection
Immunodiffusion assay
Double immunodiffusion was carried out on Petri plates with a base of 2% w/v agar coated with a shell of 1g of agar in 100 µl of 145 mM H3BO3, 50 mM NaOH pH 8.6. Pairs of wells were filled with 50 µl of 1 mg.ml-1 antigen (PLA2), and 50 µl of ACS, anti-sera raised in rabbits or IgG anti–PLA2. Immunodiffusion was allowed to proceed for 48 h at 4 °C until immunoprecipitin bands were observed.
Enzyme-linked immunosorbent assay (ELISA)
Microtiter plates (96 wells) were coated overnight at 4 °C with 100 µl of PLA2 (3 µg.ml-1) in phosphate-buffered saline (PBS). The plates were washed three times with PBS containing 0.5% Tween 20 (PBS/Tween) and unbound sites were blocked for 1 h at room temperature with 2% bovine casein in (PBS).
The plates were washed three times with PBS/Tween and used immediately for ELISA. To measure the serum titers, 100 ml of serial dilutions of serum (ACS or rabbit anti-sera; 80 mg.ml-1 of initial protein concentration which is used in antivenom therapy routine) were added to the plates and incubated for 1 h at 37 °C. The plates were then washed and incubated for 1 h with 100 µl of a goat anti-rabbit IgG–peroxidase conjugate (Sigma, 1:10.000 in PBS), followed by further washing. The substrate solution for the peroxidase assay (H2O2/OPD) was added and the enzymatic reaction allowed proceeding for 15 min at room temperature in the dark. The reaction was stopped with 50 µl of H2SO4 3N and the absorbance was read at 492 nm with a SpectraMax 340 multi-well plate reader.
Immunoblotting
Antigen (PLA2) and whole venom (1 mg.ml-1) were separated on 12.5% in SDS-PAGE at 200 V for 45 min and the proteins then transferred electrophoretically to nitrocellulose membranes (0.45 mm) in a transfer tank at 300 mA for 1 h. Subsequently, the membranes were blocked at room temperature for 2 h in a solution of 5% non-fat milk/0.05% Tween 20. After washing three times in Tris-buffered saline (TBS; 0.01 M Tris–HCl, 0.17 M NaCl, pH 7.6), the membranes were incubated overnight with rabbit anti-serum, IgG anti-PLA2 or ACS (diluted 1:1.000; 0,1 mg.ml-1; 1:2.000 in TBS, respectively). After washing again with TBS, bound antibodies were detected with a goat anti-rabbit IgG peroxidase conjugate (Sigma; 1:1.000 in TBS) for rabbit anti-serum or with a rabbit anti-horse IgG peroxidase conjugate (Sigma; 1:1.000 in TBS) for ACS for 1 h at room temperature with shaking. At the end of this incubation, the blots were washed, developed with 4-chloro-1-naphthol (Sigma; 0.03% in 0.05 M Tris-HCl, pH 7.6, containing 0.03% H2O2/OPD) and documented.Hemolytic activity neutralization
In order to evaluate the ability of the IgG anti-PLA2 to neutralize the phospholipase activity of the venom, an assay to determine the indirect hemolytic activity was carried out16. Twenty five milliliters of 1% (w/v) agar in PBS (pH 8.1) containing 0.3 ml of packed sheep erythrocytes, 0.3 ml of egg yolk in saline solution (1:3) and 0.25 ml of 0.01 M CaCl2, was applied to plastic plates (135 x 80 mm) and allowed to gel. Then, 3 mm diameter wells were filled with 15 µl of venom or venom preincubated with different amount of ACS or IgG anti-PLA2. One hemolytic dose (HD) was defined as the lowest amount of venom (µg) which induces a hemolytic halo around the well of 19 mm. The PLA2 neutralizing ability of the anti-sera was expressed as ED50 defined as the amount of anti-venom (mg of protein) needed to reduce by 50% the hemolytic activity of the whole venom.
Lethal activity neutralization
Neutralization of lethality was performed by incubating different mixtures of venom and antivenom at 37 °C for 30 min. Then 0.5 ml of the mixture was injected i.p. into mice (20 + 2g, 6 mice per group) and animals were observed for 48 h. Each mouse received 4 µg of venom, corresponding to 4 x LD50. Neutralization activity was expressed as ED50 defined as the amount of anti-venom (mg of protein) needed to reduce by 50% the lethal potency of the venom.
Results
Antigen purification
Sephadex G-75 chromatography column was an efficient step in the antigen purification. Results from a typical purification of PLA2 are shown in Fig. 1. The homogeneity of the purified PLA2 was examined by SDS-PAGE and by immunoprecipitation analysis. The antigen showed a single band of 16 kDa by SDS-PAGE method (Fig. 2) and gave a single precipitation line in the Ouchterlony method when the antigen was tested against ACS (Fig. 3).
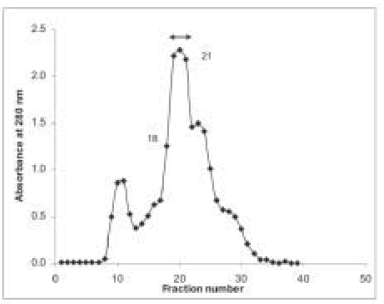
Fig. 1.– Elution profile of Crotalus durissus terrificus venom fractionated on Sephadex G-75. Venom (0.04 g.ml–1, 0.1 ml) was applied to a column (1 x 30 cm) of Sephadex G-75 pre-equilibrated and eluted with glycine buffer 20 mM, 150 mM NaCl, pH 1.9. Double arrow showed fractions with PLA2 activity.

Fig. 2.– SDS-PAGE of the purified PLA2. Electrophoresis was performed on 15% of polyacrylamide gel in SDS-PAGE at 200 V for 45 minutes. The gel was stained with 0.1% Coomasie blue R-250 (in 40% methanol and 12% Acetic acid) and distained with 10% acetic acid. (A) C.d.t. Venom (1 mg.ml-1). (B) PLA2 (2 mg.ml-1).

Fig. 3.– Inmunodifussion of PLA2 against ACS. 1) ACS, 2) Purified PLA2 (1 mg.ml-1), 3) whole venom (4 mg.ml-1) and 4) whole venom (1 mg.ml-1).
Antibody production
Antibody production during the immunization process was monitored by double immunodiffusion until strong immunoprecipitin bands were consistently obtained (Fig. 4). Rabbits were bled and antibody serum titers were determined by ELISA. Fig. 5 shows the reactivity between PLA2 and serial dilutions of the anti-sera raised in rabbits, and with ACS. The endpoint dilution of the anti-sera raised in rabbits was greater than for ACS, 1/218.700 and 1/8100, respectively.

Fig. 4.– Ouchterlony double diffusion test. Immunodifussion of PLA2 (1mg.ml-1) (3) against anti-sera raised in rabbits (2) and IgG anti-PLA2 (1).
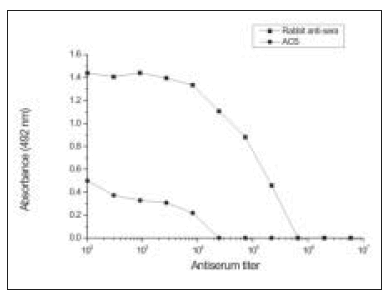
Fig. 5.– ELISA. Reactivity of anti-sera raised in rabbits and ACS with PLA2 from C.d.t.
Immunoblotting
Figure 6 shows immunoblots of PLA2 and whole venom detected with anti-sera raised in rabbit (diluted 1:1.000) IgG anti-PLA2 (0.1 mg.ml-1), and with ACS (diluted 1:2.000). All of the anti-sera recognized PLA2 from C.d.t. venom.
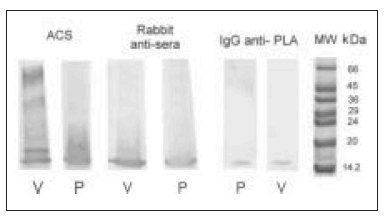
Fig. 6.– Immunoblotting. Blotting shows the reactivity of anti-sera raised in rabbits, ACS and IgG anti-PLA2 with whole venom (V) and PLA2 from C.d.t. (P).
Hemolytic activity neutralization
ED50 for ACS and IgG anti-PLA2 were calculated according to the amount of anti-venom (ACS or IgG anti-PLA2) that reduce 50% of hemolytic activity. The results showed in Table 1 demonstrated that minor amounts of IgG anti-PLA2 were needed to neutralize 1 HD (100 µg) of C.d.t. venom.
TABLE 1.– Comparison of the neutralization of the PLA2 activity and lethal potency in C.d.t. venom with ACS or IgG anti-PLA2
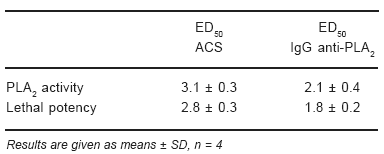
Lethal activity neutralization
The values obtained for ACS and IgG anti-PLA2 in protecting against lethality in mice are shown in Table 1. IgG anti-PLA2 proof to be better in neutralizing lethal activity.
Discussion
The methodology employed to purify PLA2 consisted in only one step, a gel filtration chromatography at extremely acid conditions thus obtaining the enzyme in a high purity grade. This resulted in a simple method compared to Landucci et al.5technique in which two steps were applied, a gel filtration chromatography at pH 8, followed by an ion exchange chromatography. The molecular weight of the isolated PLA2 proved to be in agreement with that reported by others17, 18. The SDS–PAGE analysis and the single precipitation line exhibited by immnunodiffusion analysis showed that the isolated PLA2 was not contaminated either by crotapotin or other proteins present in whole venom. Immunoblotting test confirmed the purity of the antigen when it was revealed with ACS.
The high titer obtained by ELISA confirmed the excellent reactivity of the anti-sera raised in rabbits to PLA2, whereas ACS was demonstrated to be about 16-fold less reactive to this protein.
In order to improve the neutralization capacity of the antibodies, per mg of protein, specific antibodies, IgG anti-PLA2 were purified. These antibodies neutralized the hemolytic activity and the lethal potency of the whole venom. ACS also efficiently neutralized the whole venom, but major quantities of proteins were required.
The greater neutralizing capacity of IgG anti-PLA2 compared to ACS could be due that ACS is often semipurified globulin-rich preparation containing non-IgG proteins and aggregates11, 19. However, the electrophoretical test of ACS demonstrated that it does not contain those proteins (data not shown).
These results demonstrate that it is possible to obtain anti-venom made by specific antibodies with a high level of protection against the lethal component of the C.d.t. venom, and/or the inclusion of these antibodies as a supplement in heterologous anti-venoms. Further physiological and histopathological studies will be conducted in order to evaluate possible sequels induced by this type of treatment.
Acknowledgements: This work was financially supported by SGCyT UNNE. PI 610. J.P. Rodríguez is the recipient of a student fellowship from SGCyT - UNNE Res. 436/03 C.S. Argentina. E.L.M. receives financial support from UBA, CONICET and ANPCyT.
References
1. Slotta KH, Fraenkel-Conrat HL. Schlangengifte, III: Mitteilung Reiningung und Krystallization des Klappers-changengiftes. Ber Dich Chem Ges 1938; 71: 1076-81.
2. Brazil OV. Pharmacology of crystaline crotoxin. II. Neuromuscular blocking action. Mem Inst Butantan 1966; 33: 981-92.
3. Azevedo-Marques MM, Cupo P, Coimbra TM, Hering SE, Rossi MA, Laure CJ. Myonecrosis, myoglobinuria and acute renal failure induced by South-American rattlesnake (Crotalus durissus terrificus) envenomation in Brazil. Toxicon 1982; 23: 631-6.
4. Rosenfeld G. Symptomatology, pathology and treatment of snake bites in South America. In: Bücherl W, Buckley EE (eds). Venomous Animals and their Venoms, vol. 2. New York: Academia Press, 1971, p 345-84.
5. Landucci, EC, Condino-Neto A, Perez AC, et al.Crotoxin induces aggregation of human washed platelets. Toxicon 1994; 32: 217-26.
6. Hendon RA, Fraenkel-Conrat H. Biological roles of the two components of crotoxin. Proc. Natl Acad Sci USA 1971; 68: 1560-3.
7. Breithaupt, H. Enzymatic characteristics of Crotalus phospholipase A2 and the crotoxin complex. Toxicon 1976; 14: 221-33.
8. Bon C, Changeux JP, Jeng TW, Fraenkel-Conrat H. Postsynaptic effects of crotoxin and of its isolated subunits. Eur J Biochem 1979; 99: 471-81.
9. Faure G, Harvey AL, Thomson E, Saliou B, Radvani F, Bon C. Comparison of crotoxin isoforms reveals that stability of the complex plays a major role in its pharmacological action. Eur J Biochem 1993; 214: 491-6.
10. Richardson WH, Tanen DA, Tong TC, et al. Crotalidae polyvalent immune Fab (Ovine) antivenom is effective in the neutralization of South American Viperidae venoms in a murine model. Ann Emerg Med 2005; 45: 595-602.
11. Otero R, Núñez V, Barona J, Díaz A, Saldarriaga, M. Características bioquímicas y capacidad neutralizante de cuatro antivenenos polivalentes frente a los efectos farmacológicos y enzimáticos de Bothrops asper y Porthidium nasutum de Antioquia y Chocó. Iatreia 2002; 15: 1-10.
12. Chipaux JP, Goyffon M. Venoms, antivenoms and inmunotherapy. Toxicon 1998; 36: 823-46.
13. De Roodt A, García S, Gómez C, et al. Antitoxinas y antivenenos para uso terapéutico. Acta Toxicol Argent 2004; 12: 29-41
14. Ouchterlony O. Antigen-antibody reactions in gels. Acta Path Microbiol Scand 1949; 26: 507-15.
15. Theakston RDG, Warrell DA, Griffiths E. Report of a WHO workshop on the standardization and control of antivenoms. Toxicon 2003; 41: 541-57.
16. Gutierrez JM, Avila C, Rojas E, Cerdas L. An alternative in vitro method for testing the potency of the polyvalent antivenom produced in Costa Rica. Toxicon 1988; 26: 411-3.
17. Habermann E, Rübsamen K. Biochemical and Pharmacological analysis of the so-called crotoxin. In: De Vries, A, Kochva, E. (eds). Toxin of Animal and Plants Origin, vol I. London: Gordon and Breach, 1971, p 333-41
18. Omori-Satoh T, Lang J, Breithhaupt H, Habermann E. Partial aminoacid sequence of the basic Crotalus phospholipase A. Toxicon 1975; 13: 69-71,
19. León G, Lomonte B, Gutiérrez JM. Anticomplementary activity of equine whole IgG antivenoms: comparison of three fractionation protocols. Toxicon 2005; 45: 123-8. [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ]
Received: 27-07-2005
Accepted: 31-05-2006














