Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Medicina (Buenos Aires)
versión impresa ISSN 0025-7680versión On-line ISSN 1669-9106
Medicina (B. Aires) v.67 n.1 Buenos Aires ene./feb. 2007
Ketoconazole therapy: an efficacious alternative to achieve eucortisolism in patients with Cushing's syndrome*
Daniel Moncet, Daniel J. Morando, Fabián Pitoia, Silvia B. Katz, María A. Rossi, Oscar D. Bruno
División Endocrinología, Hospital de Clínicas José de San Martín, Facultad de Medicina, Universidad de Buenos Aires
*Presented in part at The Endocrine Society 82nd Annual Meeting, Toronto, Canada, 24-26/6/2000.
Postal address: Dr. Oscar D. Bruno, División Endocrinología, Hospital de Clínicas, UBA, Avenida Córdoba 2351, 1120 Buenos Aires, Argentina Fax: (54-11) 4805-0631 e-mail: divendhcli@intramed.net
Abstract
Cushing's syndrome (CS) is a serious condition requiring drug management in diverse clinical settings. Fifty four patients (44 females, 10 males) with CS, aged 14-63, received ketoconazole (KTZ) prior to surgery (n= 27), as complementary therapy after surgery and/or radiotherapy (n= 16), or as primary treatment (n= 11). It was given at a 600 (500 - 600) mg/day (median - CI95) maintenance dose for periods ranging from 15 days to 13 years. Clinical signs, hepatic enzymes and urinary free cortisol (UFC) were evaluated before and during KTZ treatment. UFC normalised or decreased to subnormal values in 85% of the patients, in 5 to 150 days after starting treatment; although failing to normalise, UFC decreased to 12-48% of pre-treatment values in the remaining patients. Clinical signs improved throughout. Side effects were adrenal insufficiency (18.5%), reversible hepatic toxicity (11%), allergic skin rash (5.5%) and gastric intolerance (3.7%); in 11% of patients, an "escape phenomenon" was observed. Twenty-four out of the total (44.4%) were treated for prolonged periods, from one up to 13 years. In conclusion, this study confirms that KTZ is an effective and generally well tolerated treatment for CS particularly: a) shortly before surgery, b) because of persistent hypercortisolism after surgery or awaiting the results of radiotherapy, c) as a reasonable option in patients with CS of unknown aetiology and, d) as long-term therapy in any case of unsolved hypercortisolism after failure of current treatments.
Key words: Cushing's syndrome therapy; Hypercortisolism; Ketoconazole; Urinary free cortisol
Resumen
Tratamiento con ketoconazol. Una alternativa eficaz para lograr el eucortisolismo en pacientes con síndrome de Cushing. El síndrome de Cushing (SC) es un trastorno grave que requiere frecuentemente tratamiento medicamentoso. Cincuenta y cuatro pacientes (44 mujeres, 10 varones) de 14-63 años de edad con SC, recibieron ketoconazol (KTZ) previo a cirugía (n=27), como complemento luego de cirugía y/o radioterapia (n=16), o como tratamiento primario (n=11). La dosis de mantenimiento fue de 600 (500 - 600) mg/día (mediana-IC95) durante 15 días a 13 años. Los signos clínicos, hepatograma y cortisol libre urinario (CLU) fueron evaluados antes y durante tratamiento con KNZ. El CLU cayó a valores normales o subnormales en 85% de los pacientes, 5 a 150 días luego de iniciar el tratamiento; aún sin normalizar, el CLU disminuyó a 12-48% de los valores pre-tratamiento en el resto de los pacientes acompañándose de mejoría de los signos clínicos. Los efectos colaterales fueron: insuficiencia adrenal (18.5%), toxicidad hepática reversible (11%), "rash" cutáneo (5.5%) e intolerancia gástrica (3.7%); en 11% de los pacientes se observó un fenómeno de "escape". Veinticuatro pacientes (44.4%) fueron tratados por períodos prolongados, de uno a trece años. Este estudio confirma que el KTZ constituye un tratamiento eficaz y generalmente bien tolerado del SC, en particular: a) como preparación para cirugía b) en casos de hipercortisolismo residual luego de cirugía o en espera de resultados de radioterapia, c) como una alternativa razonable en pacientes con SC de origen desconocido y, d) como tratamiento crónico en casos de hipercortisolismo no resuelto luego de fracaso de las terapéuticas habituales.
Palabras clave:Síndrome de Cushing; Hipercortisolismo; Ketoconazol; Cortisol libre urinario
Endogenous Cushing's syndrome (CS) is a severe condition that results from chronic exposure to excess glucocorticoids produced by the adrenal cortex. It may be etiologically classified into ACTH dependent CS, which includes Cushing's disease (CD) and ectopic Cushing's syndrome (ECS), and ACTH independent CS mainly due to autonomous adrenal hypersecretion of cortisol by an adrenal tumour (ACS)1. Cushing's syndrome is often fatal owing to cardiovascular, thromboembolic or hypertensive complications or increased susceptibility to bacterial infections. Although surgery is the therapy of choice for most cases of CS, there are various clinical settings for which drug treatment may prove useful, e.g., prior to surgery, when surgical treatment cannot be performed, when hypercortisolism persists after surgery, or while awaiting for the results of radiation therapy. In these cases, there are several alternatives such as the use of steroidogenic blocking drugs, adrenolytic drugs, neuromodulators and steroid receptor antagonists2.
Ketoconazole (KTZ), an imidazol derivative, inhibits both adrenal and gonadal steroidogenesis by blocking P-450 enzymes including P-450 (scc), P-450 (17 alpha lyase) and P-450 (11 β/18)3. This drug has been mainly used to achieve eucortisolism shortly before to surgery, in any type of CS; however, few reports have dealt with its efficacy in long-term controlling of hypercortisolism3-6.
In this study, we evaluated the biochemical and clinical response, as well as secondary side effects of KTZ treatment, in 54 patients with endogenous CS. In a significant proportion of these patients, KTZ was used for extended periods of time as the only specific drug to control hypercortisolism.
Materials and Methods
We included in the study 54 patients with proven CS undergoing KTZ treatment (44 women and 10 men), with a mean age at diagnosis of 38 ± 13.1 (range 14-63) years. Diagnosis of hypercortisolism was confirmed by performing overnight low dose dexamethasone suppression test (Nugent's test)7 and free cortisol measurements (UFC) on 24-h and spot (22-23 h) urinary samples8, 9. Etiological diagnosis was made by performing: 1) overnight 8-mg dexamethasone suppression test10, 2) measurement of basal plasma ACTH levels, 3) imaging techniques including MRI of the sellar region and/or CT scan of lungs and adrenals. In two patients with doubtful results, a CRH-desmopressin test was done11.
We classified the whole group of patients into three subgroups, according to the goal of treatment: a) in 27 patients (# 1-27), KTZ was administered to achieve eucortisolism before surgery (pre-surgical subgroup, PS), b) eleven patients (# 28-38), who refused or were considered non-elective for surgery, had CS of non-confirmed aetiology (one with an image suggestive of microadenoma on MRI, eight with biochemical diagnosis of Cushing's disease but normal or empty sella on MRI and two with conflicting tests); in these patients, KTZ was indicated as primary or definitive treatment (first choice subgroup, FC), c) finally, in the remaining 16 patients (# 39-54), treatment was complementary to surgery and/or radiotherapy, due to persistence of hypercortisolism after such procedures (post-surgery/radiotherapy subgroup, SR).
Ketoconazole was administered as 200 mg pills in single or fractionated (every 8 or 12 hours) doses, ranging from 200 to 1200 mg daily. In all patients, the presence of arterial hypertension (blood pressure ≥ 140 mm Hg systolic and >90 mm Hg diastolic), myopathy (defined by subjective and objective weakness), overweight and/or obesity (body mass index >25 kg/m2), lower limb oedema, menstrual disturbances (oligomenorrhea or amenorrhea) and diabetes, were evaluated before and during KTZ treatment. Hepatic enzymes were measured in all patients before treatment, 20-30 days after beginning KTZ therapy and at regular intervals afterwards. Urinary free cortisol was measured by using a commercial kit (DPC, Los Angeles, CA, USA) after extraction with dichloromethane. Biochemical response to the drug was evaluated through 24-h UFC measurements (normal range 20-90 µg/24 h). Once 24-h UFC values attained normal range, controls were performed every 30-60 days. In this study, we defined as long-term treatments those which lasted for at least one year.
Statistical analysis: As data could not be normally distributed, it were expressed as median-range or median - confidence intervals 95% (CI95) and analyzed by nonparametric tests, i.e. Mann Whitney U test and Kruskal Wallis ANOVA. Statistical analysis was performed by Statistica 5.5 for Windows® (Stat Soft Inc., Tulsa, OK, USA). Significance was set at 5% level.
Results
Cushing's disease was the final diagnosis in 37 patients, ACS in five (four due to adrenal adenoma and one due to adrenal carcinoma), and one patient had ECS. The cause of hypercortisolism could not be confirmed by pathological and/or surgical results in eleven patients. Population features for each subgroup are shown in Table 1; there were non-significant differences between subgroups.
TABLE 1.- Population features, dose and treatment duration of each subgroup of CS patients
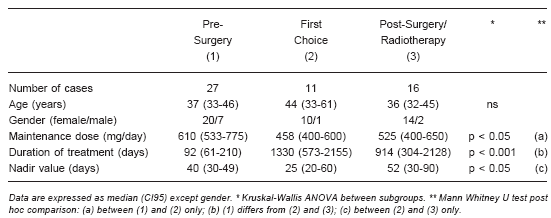
Duration of treatment: Mean duration of KTZ administration was 0.8 years (range: 15 days - 13 years) in the whole group of patients. The shortest period (15 days) corresponded to patient number 15, in whom the drug had to be withdrawn because of a rapid 6-fold increase in hepatic aminotransferase above normal range. Duration of treatment for each subgroup is shown in Table 1; the length of treatment in PS was significant lower than the others subgroups. Long-term therapy with KTZ was performed in 24 patients: 4/26 in PS, 10/21 in the FC and 10/16 in the SR subgroups (44% of the total cohort); for these patients, mean duration of KTZ administration was 3.6 years (range: 1-13 years).
KTZ dosage: For the whole group of CS patients, mean maintenance dose was 600 (500-600) mg/day. Dose values for each subgroup were: PS: 610 (533-775) mg; FC: 458 (400-600) mg and SR: 525 (400-650) mg (p<0.05 between PS and FC subgroups only).
Clinical response (Fig. 1): Forty-three patients (80%) had hypertension before initiating KTZ treatment; only 41 were considered for analysis, since KTZ was withdrawn before 30 days in two. Under KTZ therapy, 33 (80%) patients normalised their blood pressure, 18 with KTZ alone, while in the remaining 15 patients daily dosage of antihypertensive drugs was reduced. Myopathy was present in 27 (50%) patients, out of whom 20 (74%) clearly improved their subjective and objective muscular strength under treatment. Forty-five patients (83%) presented overweight or obesity, out of whom 23 (51%) reduced their body weight after three months' treatment, while 17 achieved weight-loss between one and 5 kg, five between 6 and 10 kg and one patient lost more than 10 kg of her initial body weight. Twenty patients (37%) had lower limb oedema; in ten (50%), it disappeared under KTZ treatment. Twenty-one out of 35 (60%) premenopausal women had menstrual disturbances, out of whom 16 (76%) normalised their cycles under treatment. Eleven out of the 54 patients (20%) had diabetes, eight of whom (73%) normalised their glucose levels by dieting and KTZ alone, while oral antidiabetic drugs were required in two and insulin in one to achieve normal glucose levels. An "escape phenomenon" (12) was observed in six patients (11%), five with proven CD and the remaining one with undetermined CS, appearing after 4 to 11 months of treatment under doses ranging from 400 to 800 mg/day; however, in three, urinary cortisol excretion was normalised by increasing the daily KTZ dosage.

Fig. 1.- Frequency of clinical manifestations of hypercortisolism and response after ketoconazole (KTZ) treatment in 54 patients with Cushing's syndrome.
Adverse side effects (Table 2): Side effects were seen in 18 (33.0%) out of the 54 treated patients, three of whom had more than one side effect. Treatment had to be definitively discontinued due to such reactions in only four out of the total. The main adverse effects are described below.
TABLE 2.- Adverse events of KTZ therapy registered in the whole cohort (n= 54). Data are shown as number and percentage

Transient subclinical hepatic injury developed in six patients (11%) after 15 days to 17 months of starting KTZ, in four of whom alterations in hepatic biochemical tests disappeared after switching to aminogluthetimide, while the other two were kept without enzyme blocking therapy until surgical procedures were performed.
Ten patients (18.5%) developed signs suggesting adrenal failure from 5 to 90 days after initiating KTZ (in four of them, before 10 days), with doses ranging from 400 to 800 mg/day. Failure was confirmed by finding subnormal 24-h UFC levels in six patients, whereas diagnosis was made on clinical grounds in the remaining 4. In six out of the ten, oral glucocorticoid doses were given while reducing KTZ dosage; in the remaining patients, signs of adrenal insufficiency disappeared by merely decreasing KTZ dosage.
Skin rash was observed in three (5.5%) patients, proving transient in two. However, in a single case (n° 19) KTZ had to be withdrawn due to severe cutaneous ampullaceous rash. Finally, two patients (3.7%) showed moderate digestive intolerance which improved after the first two months of KTZ treatment.
Biochemical response: Except for patient number 19, excluded because KTZ was definitively discontinued after 20 days' administration, the entire population (53 cases) was evaluated in order to analyse biochemical response. The whole cohort (WC) analysis showed that UFC excretion significantly decreased after 40 (30-45) days of KTZ treatment. The time of response (nadir value) for each subgroup was: PS: 40 (30-49) days, FC: 25 (20-60) days and SR: 52 (30-90) days (p<0.05 between FC and SR subgroups only). Urinary free cortisol figures dropped from 570 (170-1260) (median and range) to 80 (22-405), 314 (136-942) to 72 (10-409) and 230 (143-1276) to 66 (12-153) µg/24 hs in subgroups PS, FC and SR, respectively (Fig 2). At this time, normal or subnormal 24-h UFC levels were reached in 44 out of 52 (85%) patients evaluated (patient n° 29 was excluded, since no record of initial KTZ biochemical response could be found). In eight patients KTZ failed to normalise 24-h UFC levels: patient number 11 (with CD) had pituitary surgery 30 days after starting KTZ treatment, before normalising 24-h UFC levels; however, at that time UFC values had already dropped from 364 to 200 µg/day. Although failing to reach normal figures, the remaining seven patients (n° 8, 13, 17, 34, 36, 44 and 53) showed a fall in 24-h UFC to 23, 45, 12, 48, 20, 12 and 40 per cent of their basal levels, respectively (p<0.0001). Fig. 2 shows the change in urinary cortisol values of each subgroup, before and after KTZ treatment. Only pre-treatment UFC values in the PS subgroup were significantly higher than those obtained in the other subgroups. KTZ treatment significantly reduced UFC values in all subgroups, without significant differences between them.

Fig. 2.- Urinary free cortisol (UFC) levels before and after ketoconazole (KTZ) therapy in patients with Cushing's syndrome (CS): whole group (WG, n = 53); pre-surgical subgroup (PS, n = 27); first choice subgroup (FS, n = 11), and post-surgery/radiotherapy subgroup (SR, n = 16). Data expressed as median - CI95.
Discussion
Cushing's syndrome is a life-threatening clinical condition which can be correctly managed in most cases. In order to reduce intra and/or postoperative complications derived from myopathy, hypertension, and susceptibility to infection, it seems advisable to reduce hypercortisolism in the more affected, if not in all patients before surgery by drug administration. In addition, there is an increasing number of cases in which etiological diagnosis is uncertain, implying difficulties to select adequate therapy. Finally, some patients may reject surgery, or there may be medical contraindications. In such situations, cortisol blocking drug therapy could be useful to ameliorate and control the clinical manifestations of glucocorticoid excess. Many different therapeutic strategies have been proposed, adapted to the etiological diagnosis and/or to the severity of the clinical picture. Generally speaking, these can be divided into drugs acting on neurotrans-mitters, when dealing with CD, or those affecting cortisol biosynthesis used for any type of CS. Neurotransmitter modulation in CD has given inconstant results12 yet new compounds under evaluation may perhaps change this view in the future13. As a conclusion, cortisol synthesis blockage is used in most cases of CS. Although there are several options to achieve medical eucortisolism with steroid blocking drugs, we currently use KTZ, because of its efficacy and easy availability. Aminogluthetimide is employed as an alternative, when KTZ intolerance develops. Metyrapone and op'-DDD are expensive and not readily available in Argentina.
Pont et al. 14 first described the effect of ketoconazole on steroid synthesis in healthy humans and isolated adrenal cells from rats. They showed that cortisol response to ACTH was blunted after KTZ administration and suggested the possible use of this drug for the treatment of CS. In the present study, treatment with KTZ resulted in persistent suppression of cortisol secretion and dramatic improvement of clinical features. Eighty-five per cent of our patients achieved normal or subnormal 24-h UFC values, while the remaining 15% had significant decreases in 24-h UFC values, ranging from 52 to 88% of their initial levels. Pre-treatment basal UFC figures were significantly higher in PS than in the two other subgroups suggesting a more severe Cushing's syndrome in this subgroup; however, there was no difference in UFC values attained under treatment.
Although not employed in our cases, the association with other drugs could be useful for those who fail to achieve eucortisolism with KTZ alone, as shown in four patients with ECS simultaneously treated with octreotide15. In all our patients, the fall in cortisol excretion was regularly accompanied by clinical improvement. Clinical signs and symptoms of hypercortisolism showing amelioration were, in order of frequency: arterial hypertension in 80% of patients, menstrual dysfunction in 76%, diabetes mellitus in 73%, muscle weakness in 60%, obesity or overweight in 51% and oedema in 50%. Our results confirm and extend, on a greater series of cases, previously published observations on the beneficial effect of KTZ treatment on clinical manifestations of hypercortisolism3.
Although there are only scarce reports, KTZ has been employed successfully in pituitary-dependent CS during pregnancy16 and in patients older than 75 years of age17. In contrast, it has recently been reported that there was no recovery in decreased bone mineral content of a small group of Cushing's patients after KTZ, as against surgical treatment18. In the present work, no evaluation of bone mass was performed.
Although severe hepatic toxicity occurs in barely 1/15,000 treatments, the use of KTZ as antifungal agent has been associated with 10-15% incidence of increased transaminases or cholestasis, attributed in turn to idiosyncratic or immunoallergic mechanisms19. KTZ induced hepatitis is more frequent in the elderly and in women, and usually resolves within three months of therapy discontinuation20. However, fatal liver insufficiency induced by KTZ has also been described21 including a girl with CS22. In our series, there was a prevalence of 11% hepatotoxicity, related neither to duration of KTZ treatment nor to its dosage and self limited in all cases.
Adrenal insufficiency due to KTZ has been described in patients treated for mycosis and prostate cancer23, 24. In those reports, hypocortisolism was seen after doses ranging from 800 to 1200 mg/day. The hypothalamic-hypophyseal-adrenal axis generally recovers quickly after withdrawing the drug, though it took over two years in a case documented by Best et al.25. In our study, 18.5% of treated patients presented hypoadrenalism, a figure quite similar to that observed by Tabarin et al.26 From the present data, it must be underlined that in the four patients who developed signs of adrenal insufficiency shortly after starting treatment, the drop in UFC values was achieved with doses as low as 200 to 400 mg/day, which was also observed in the case reported by Best et al.25 as early as three days after giving 200 mg KTZ daily.
The escape phenomenom was described as a frequent event in a report of Diop et al.27 on 5 patients treated with KTZ for periods of 2 to 28 months. However, that phenomenon was observed in only 11% of our patients.
Twenty-four out of our 54 patients (44%) had long-term treatment with KTZ (mean 3.8 ± 2.7 years, range 1-12 years), ten of whom received KTZ as first and only treatment. Most of these patients had biochemical tests pointing to a pituitary CS but with a normal pituitary or an empty sella turcica on MRI28, or hypercortisolism was of undefined origin. At the time of writing this paper, six patients in this group continue under KTZ therapy (n° 29, 30, 32, 33, 35 and 38) after 1.5 to 9.2 years. Out of the remaining 18 with protracted treatment, seven patients with a troublesome diagnosis had received KTZ for periods ranging from 1.3 to 2.0 years until they were operated on, two were lost to follow up, one died, and eight still remain under long-term treatment (1-13 years) after failure of surgery and/or radiotherapy. In most previously reported series or individual cases, KTZ was administered for periods no longer than one year with the exception of three patients from Sonino et al. 3 who were treated for 2-3 years, three cases treated for 2.7, 6.9 and 7.1 years without complications by Chou et al.4, one patient of Spagnoli et al.5 who received KTZ for at least seven years and the case reported by Carral San Laureano et al6 who used KTZ during 2 years in a pediatric patient.
Some individual cases of our series better illustrate the usefulness of long-term treatment with KTZ. Patient n° 29 is a 23-year-old female put on KTZ at the age of 14 years when she presented with CS of indeterminate origin. She achieved eucortisolism, a normal height and had menarche two years after starting KTZ. To date, she is in her 9th year of treatment and remains symptom-free. The longest treatment was given to a 32-year-old female with CD (patient 49) who had undergone partial hypophysectomy; she then received pituitary radiation, but remained with hypercortisolism after one year and refused any other invasive treatment. Therefore, KTZ was begun and continued for more than 13 years, up to now. In both cases, interruption of KTZ for short periods was followed by a rebound of UFC to abnormally high values, so the drug had to be reinitiated.
The present study confirms that KTZ is an effective and generally well tolerated treatment for CS. It affords a useful tool for hypercortisolism control mainly in the following clinical settings: a) shortly before surgery, b) due to hypercortisolism persisting after surgery or while awaiting results of radiotherapy and, c) as a reasonable option in patients with CS of indeterminate origin. Finally, ketoconazole should be considered a valuable alternative as chronic long-term treatment of difficult cases when other therapeutic means are not available, or when there are definite contraindications for its use.
Acknowledgments: We would like to express our appreciation to Dr. Alejandra Schvartz and to Dr. Héctor Alejandro Sierra for most valuable help and criticism in the preparation of this manuscript.
References
1. Schteingart D. Cushing syndrome. In Principles and Practice of Endocrinology and Metabolism, 3th Ed, ch 75, pp723-738. Philadelphia: Lippincott Williams & Wilkins, 2001.
2. Morris D, Grossman A. The medical management of Cushing's syndrome. Ann N Y Acad Sci 2002; 970: 119-33.
3. Sonino N, Boscaro M, Paoletta A, Mantero F, Ziliotto D. Ketoconazole treatment in Cushing's syndrome: experience in 34 patients. Clin Endocrinol 1991; 35: 347-52.
4. Chou SC, Lin JD. Long-term effects of ketoconazole in the treatment of residual or recurrent Cushing's disease. Endocrinol J 2000; 47: 401-6.
5. Spagnolli W, Ramponi C, Davi MV, Francia G. Cushing's disease associated with empty sella: a clinical case treated for years with ketoconazole. Ann Ital Med Inter 1996; 11: 275-8.
6. Carral San Laureano F, Lechuga Campoy JL, Merino López J, Caro Contreras J, Aguilar Diosdado M. Eficacia terapéutica del ketoconazol en el síndrome de Cushing. Ann Pediatr 2000; 52: 381-4.
7. Nugent CA, Nichols T, Tyler FH. Diagnosis of Cushing's syndrome. Single dose dexamethasone suppression test. Arch Inter Med 1965; 116: 172-6.
8. Contreras LN, Hane S, Tyrrell JB. Urinary cortisol in the assessment of pituitary-adrenal function: utility of 24-hour and spot determination. J Clin Endocrinol Metab 1986; 62: 965-9.
9. Contreras LN, Masini AM, Rossi MA, Bruno OD. Spot urinary cortisol in assessing circadian variability in patients with Cushing's syndrome (CS), obesity and hypothyroidism. Journées Internationales Henri-Pierre Klotz d'Endocrinologie Clinique, París, 1988; p. 41.
10. Bruno OD, Rossi MA, Contreras LN, et al. Nocturnal highdose dexamethasone suppression test in the aetiological diagnosis of Cushing's syndrome. Acta Endocrinol 1985: 109: 158-62.
11. Newell-Price J, Perry L, Medbak S, et al. A combined test using desmopressin and corticotropin-releasing hormone in the differential diagnosis of Cushing's syndrome. J Clin Endocrinol Metabol 1997; 82: 176-80.
12. Nieman LK. Medical therapy of Cushing's disease. Pituitary 2004; 5: 77-82.
13. Van der Hoek J, Lamberts SW, Hofland LJ. The role of somatostatin analogs in Cushing's disease. Pituitary 2004; 7: 257-64.
14. Pont A, Williams PL, Loose DS, Feldman D, Reitz RE, Bochra C. Ketoconazole blocks adrenal steroid synthesis. Ann Int Med 1982; 97: 370-2.
15. Vignati F, Loli P. Additive effect of ketoconazole and octreotide in the treatment of severe adrenocorticotropin-dependent hypercortisolism. J Clin Endocrinol Metab 1996; 81: 2885-90.
16. Berwaerts J, Verhelst J, Mahler C, Abs R. Cushing's syndrome in pregnancy treated by ketoconazole: case report and review of the literature. Gynecol Endocrinol 1999; 13: 175-82.
17. Berwaerts J, Verhelst J, Verhaert GC, Verhaegen AA, Abs R. Corticotropin-dependent Cushing's syndrome in older people: presentation of five cases and therapeutical use of ketoconazole. J Amer Geriat Soc 1998; 46: 880-4.
18. Luisetto G, Zangari M, Camozzi V, Boscaro M, Sonino N, Fallo F. Recovery of bone mineral density after surgical cure, but not by ketoconazole treatment, in Cushing's syndrome. Osteoporosis Intl 2001; 12: 956-60.
19. Lewis JH, Zimmerman HJ, Benson GD, Ishak KG. Hepatic injury associated with ketoconazole therapy. Gastroenterology 1984; 86: 503-13.
20. Lake-Bakaar G, Scheuer PJ, Sherlock S. Hepatic reactions associated with ketoconazole in the United Kingdom. Brit J Med 1987; 294: 410-22.
21. Findor JA, Sorda JA, Igartúa EB, Avagnina A. Ketoconazole-induced liver damage. Medicina (Buenos Aires) 1998; 58: 277-81.
22. Zollner E, Delport S, Bonnici F. Fatal liver failure due to ketoconazole treatment of a girl with Cushing's syndrome. J Pediat Endocrinol Metab 2001; 14: 335-8.
23. Pillans PI, Cowan P, Whitelaw D. Hyponatremia and confusion in a patient taking ketoconazole. Lancet 1985; 1: 821-2.
24. Trachtenberg J, Pont A. Ketoconazole therapy for advanced prostate cancer. Lancet 1984; 2: 433-5.
25. Best TR, Jenkins JK, Murphy FY, Nicks SA, Bussell KL, Vesely DL. Persistent adrenal insufficiency secondary to low-dose ketoconazole therapy. Amer J Med 1987; 82: 676-80.
26. Tabarin A, Navarranne A, Guerin J, Corcuff JB, Parneix M, Roger P. Use of ketoconazole in the treatment of Cushing's disease and ectopic ACTH syndrome. Clin Endocrinol 1991; 34: 63-9.
27. Diop SN, Warnet A, Duet A, Firmin C, Mosse A, Lubetzki J. Traitement prolongé de la maladie de Cushing par le kétoconazole. Presse Méd 1989; 18: 1325-8.
28. Manavela MP, Goodall CM, Katz SB, Moncet D, Bruno OD. The association of Cushing's disease and primary empty sella turcica. Pituitary 2001; 4: 145-51. [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ]
Received: 13-03-2006
Accepted: 14-09-2006














