Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Medicina (Buenos Aires)
versión impresa ISSN 0025-7680versión On-line ISSN 1669-9106
Medicina (B. Aires) v.67 n.2 Buenos Aires mar./abr. 2007
Alanine-aminotransferase: an early marker for insulin resistance?
Martín R. Salazar1, Horacio A. Carbajal1, José O. Curciarello3, Marcelo Aizpurua2, Raúl E. Adrover3, Beatriz Riondet1
1 Centro de Referencia Provincial de Hipertensión Arterial, Ministerio de Salud, Provincia de Buenos Aires;
2 Hospital Municipal de Rauch,
3 Fundación para el Estudio de las Enfermedades Hepáticas de la Provincia de Buenos Aires
Postal address: Dr. Martín R. Salazar, 14 N° 320, 1900 La Plata, Argentina. Fax: (54-221) 483-3292
e-mail: salazarlandea@gmail.com
Abstract
In a population-based sample, after excluding alcohol consumption, hepatotoxic drugs and hepatitis Band C infected, we investigated if alanine-aminotransferase (ALT) was associated with metabolic syndrome and insulin resistance, and if this association was caused by non-alcoholic fatty liver disease (NAFLD). The sample (432 female and 119 male) was divided into two ALT thresholds corresponding to the 50th and 75th percentiles (P) (female ≤ 15 and ≤ 19 U/L; male ≤ 17 and ≤ 23 U/l, respectively). Blood pressure, body mass index, waist circumference, cholesterol, HDL cholesterol (HDLc), triglyceride (TG), TG/HDLc ratio, glycemia and homeostasis model assessment of insulin resistance (HOMA-IR) were compared between those above and below each ALT threshold. Female placed above the 50th P of ALT had higher levels of TG/HDLc ratio (p=0.029), glycemia (p=0.028), and homeostasis model assessment of insulin resistance, (p=0.045), and above the 75th P had higher SBP (p=0.036), DBP (p=0.018), TG (p=0.024), TG/HDLc ratio (p=0.028), glycemia (p=0.004) and HOMA-IR (p=0.0014). Male placed above the 50th P of ALT had higher BMI (p=0.017) and TG/HDLc ratio (p=0.048), and above the 75th P had lower values of HDLc (p=0.042). Only 16.5% of women and 14.5% of men, above the 75th P of ALT, showed an increase in liver brightness in the echography. This work shows in woman an early association of ALT with TG/HDLc ratio and HOMA-IR. Since the last two are independent predictors of cardiovascular risk, attention should be drawn to ALT values near the upper limit of the normal range even in the absence of NAFLD and obesity.
Key words: Alanine-aminotransferase; Insulin resistance; Metabolic syndrome
Resumen
Alanino-aminotransferasa: ¿un marcador temprano de resistencia a la insulina? En una muestra poblacional, luego de excluir a quienes consumían alcohol y drogas hepatotóxicas y a los infectados con virus B y C de la hepatitis, investigamos si la alanino-aminotransferasa (ALT), o transaminasa glutámico pirúvica (TGP), se asociaba con el síndrome metabólico y con resistencia a la insulina y si esta asociación se explicaba por enfermedad hepática grasa no alcohólica (NAFLD). La muestra (432 mujeres y 119 varones) se dividió por los percentilos (P) 50 y 75 de la distribución de ALT (mujeres ≤ 15 y ≤ 19 U/l; varones ≤ 17 y ≤ 23 U/l, respectivamente). Las mujeres a partir del P50 de ALT tuvieron valores más altos de índice triglicéridos (TG)/HDLc (p=0.029), glucemia (p=0.028) y de la valoración del modelo homeostático de insulino-resistencia (HOMA-IR) (p=0.045); a partir del P75 tuvieron valores más altos de presión arterial sistólica (PAS) (p=0.036), presión arterial diastólica (PAD) (p=0.018), TG (p=0.024), índice TG/HDLc (p=0.028), glucemia (p=0.004) y HOMA-IR (p=0.001). Los varones a partir del P50 de ALT tuvieron valores más altos del índice de masa corporal (p=0.017) y del índice (TG/HDLc (p=0.048); a partir del P75 mostraron valores más bajos de HDLc (p=0,042). Sólo 16.5% de las mujeres y 14.5% de los varones, a partir del P75 de ALT, mostraron aumento del brillo hepático en la ecografía. Este trabajo muestra, en mujeres, asociación temprana de ALT con el índice TG/HDLc y el HOMA-IR. Dado que estos dos últimos son predictores independientes del riesgo cardiovascular se debería prestar atención a los valores de ALT cercanos al límite superior aun en ausencia de NAFLD y de obesidad.
Palabras clave: Alanino-aminotransferasa; Resistencia a la insulina; Síndrome metabólico
Non-alcoholic fatty liver disease (NAFLD) is clearly associated with the metabolic syndrome and with insulin resistance1-3. However, the scope of the liver disease in metabolic syndrome could exceed these morphological alterations. In this sense it has been observed that g-gltamyltransferase (GGT) is associated with arterial hypertension and its incidence4, metabolic syndrome components and development of diabetes type 25-9. While GGT is a sensitive but unspecific marker of liver injury since it also increases by oxidative stress and alcohol intake10, 11, alanine aminotransferase (ALT) is the most specific marker of liver disease in clinical practice. Recently, high levels of ALT have been reported associated with obesity, hyperglycemia and the development of both diabetes and metabolic syndrome5, 9, 12-17.
The explanation for this relationship between hepatic enzymes and metabolic syndrome has been centered in the existence of non-alcoholic fatty liver disease (NAFLD), and insulin resistance has been proposed as a link between metabolic syndrome and liver steatosis18-21.
HOMA-IR (homeostasis model assessment insulin resistance) has been utilized and validated in populationbased samples as an indicator of insulin resistance22. Recently Reaven23 has also emphasized the usefulness of triglyceride (TG)/HDLc ratio as an insulin resistance indicator.
For the diagnosis of liver steatosis, echography is a non-invasive, reasonably sensitive and specific procedure2, 24, 25. It is based on the detection of an increase in liver brightness in comparison with that of spleen and kidney, and it has been estimated that 96% of the asymptomatic non-drinkers with altered hepatic enzymes and bright liver have NAFLD26.
The aim of the present work was to determine in a population sample if ALT was associated with components of the metabolic syndrome and insulin resistance and if this association is explained by NAFLD.
Materials and Methods
A cross-sectional study was performed in a population-based sample of the city of Rauch. The universe was the inhabitants of Rauch between 15 and 75 years of age. This city lies in the centre-Southeast region of the province of Buenos Aires, 36° 45' 00" south latitude and 59° 04' 00" west longitude. It is 270 km far from Buenos Aires city. According to the 1991 National Census, there were 13.909 inhabitants in Rauch, 8.246 between 15 and 75 years of age (4.166 men and 4.080 women). No abnormalities had been shown since 1991 to assume changes in the population. In 1997, randomly chosen blocks were considered as units for the sampling. 1.526 inhabitants constituted the initial sample; their composition was previously described27. The initial survey was performed on subjects living in the randomly chosen blocks. Since the socio-economic features and the number of inhabitants were similar, a proportional probability was not taken into consideration. In that opportunity, we found a high prevalence of hypertension (43.20% in men and 28.50% in women) and obesity-overweight (54.81% in men and 44.65% in women). Smoking was present in 34.61% of men (14.80 ± 0.74 cigarettes per day) and in 20.83% of women (12.63 ± 0.74 cigarettes per day). Average alcohol intake was 163.02 ± 10.24 and 25.32 ± 2.42 g per week for men and women, respectively. A 1.2% of the population was illiterate while the percentages with a level of education of incomplete primary, complete primary, incomplete secondary, complete secondary, tertiary or incomplete university and complete university level were 16.5%, 37.4%, 17.5%, 9.4%, 5.2% and 12.7%, respectively. Other characteristics were previously published27. After six years (2003) of the initial survey on prevalence, a cohort study was performed as a second step28. 855 women and 452 men were re-interviewed (n=1307) by previously trained nurses from Rauch Hospital. Some subjects were not re-interviewed: 71 had died (4.65%) and 148 (9.70%) were not found due to several causes (moved to another city, out of home). Systolic blood pressure (SBP) and diastolic blood pressure (DBP), weight, height and waist circumference (WC) were measured in the domicile of each re-interviewed subject; and personal information and alcohol and drugs intakes were recorded. Alcohol intake was registered by specifically asking about the consumption of wine, beer, distilled beverages, and aperitifs and other beverages. In order to calculate alcohol intake in grams per week (g/week), weekly intake reported in centilitres was multiplied by 0.12, 0.05, 0.40 and 0.18 in the case of wine, beer, distilled beverages, and aperitifs and other beverages, respectively.
Blood pressure was measured on the right arm relaxed and at the heart level using a mercury-scale sphygmomanometer after five minutes of rest. SBP was the reading coinciding with the first arterial sound and DBP with the last one. This procedure was repeated three times and SBP and DBP were defined as the means of the three measurements. Weight was registered with light clothes using a personal scale calibrated before each measurement. Height was determined barefoot and with a metal tape measure. WC was measured with a metal tape measure over the iliac crest parallel to the floor and with a relaxed abdomen. Blood extractions were performed, to whom attended voluntarily, with no less than eight hour fasting, in Rauch Hospital. Samples were processed to obtain serum for determinations of glycemia, ALT, cholesterol, HDL cholesterol (HDLc), TG and the remaining serum was put in the fridge and then frozen within twelve hours at -18 °C for determinations of insulinemia and serology for hepatitis B and C.
ALT (GPT UV AA, Wiener Lab) (normal rank: 6-40 U/l for men and 5-31 U/l for women) (laboratory variability < 10%), glycemia, cholesterolemia, HDLc and TG, were determined (colorimetric method) using an autoanalyzer Technicon RA 1000. Insulinemia was measured by radio-immunometric method. Antibody against hepatitis C virus (anti-HCV) and antibody anti-core of hepatitis B virus (anti-HBc) were determined by enzyme-immunoassay, while surface antigen of hepatitis B virus (anti HBS) was determined by ELISA. Body mass index (BMI) (weight/height2) and HOMA-IR (insulinemia (µUml) x [glycemia (mg/dl)/18]/22.5)19 were calculated. Any amount of alcohol intake (> 0.00 g/week), drug intake with acknowledged hepatotoxicity29, presence of anti-HCV and of anti-HBc with negative anti-HBs were the exclusion criteria.
A bidimensional liver echography was performed with equipment Sonoace 6.000 c digital color and multifrequence convex transductor, and liver fat was defined by two independent observers, comparing liver brightness with that of kidney and spleen. An interobserver variation of < 10% was accepted.
Two ALT thresholds were separately defined in men and women, corresponding to the 50th and 75th percentiles (P) of ALT distribution. BP, BMI, WC, cholesterol, HDLc, TG, TG/HDLc ratio, glycemia and HOMA-IR were compared between those above and below each ALT threshold. The percentage of subjects with increased liver brightness was compared among quartiles of ALT.
Statistics: continuous variables were expressed as mean ± SE and were compared with t test for independent samples. Discrete variables were expressed as percentage and compared using chi2 test. P value < 0.05 was considered significant. Data were processed with the statistical program SPSS 11.0.
Results
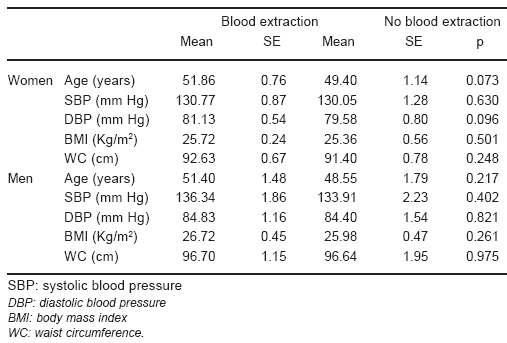
394 alcohol-consuming subjects, 13 subjects consuming drugs with acknowledged hepatotoxicity and 5 subjects with anti-HCV were excluded. Subjects with anti-HBc had anti-HBs positive and consequently were not excluded. The definitive sample was constituted by 895 subjects (690 women and 205 men); 551 attended voluntarily to have blood extractions (432 women and 119 men). There were no significant differences in age, BP, BMI and WC among those having blood extractions and those not having them (Table 1). The average value of ALT was 17.28 U/l (CI 95% 16.42-18.14) in women and 20.78 U/L (CI 95% 18.64-22.92) in men.
TABLE 1.- Features of subjects who had blood extractions and who had not
ALT thresholds for the 50th P and 75th P were ³ 15 and ³ 19 U/l in women and ³ 17 and ³ 23 U/l in men, respectively.
The age of women with ALT ³ 15 (n=245) and with ALT < 15 U/l (n=187) was 52.48 ± 0.98 and 51.05 ± 1.21 years, respectively (p=0.353); while for those with ALT ³ 19 (n=109) and < 19 U/l (n=323) it was 53.92 ± 1.38 and 51.17 ± 0.91 years, respectively (p=0.097). The age of men with ALT ³ 17 (n=67) and with ALT < 17 U/l (n=52) was 51.13 ± 1.94 and 51.75 ± 2.29 years, respectively (p=0.837); while for those with ALT ³ 23 (n=30) and < 23 U/l (n=89) it was 46.30 ± 2.73 and 53.12 ± 1.71 years, respectively (p=0.044).
Women above the 50th P of ALT (Table 2) had higher TG/HDLc ratio (p=0.029), glycemia (p=0.028), and HOMA-IR (p=0.045). Above the 75th P of ALT, they had higher SBP (p=0.036), DBP (p=0.018), TG (p=0.024), TG/HDLc ratio (p=0.028), glycemia (p=0.004) and HOMA-IR (p=0.001). Men above the 50th P of ALT (Table 3) had higher BMI (p=0.017) and TG/HDLc ratio (p=0.048). Above the 75th P of ALT, they had lower HDLc (p=0.042).
TABLE 2.- Indicators of metabolic syndrome and insulin resistance in women according to the 50th and 75th percentiles of ALT*

TABLE 3.- Indicators of metabolic syndrome and insulin resistance in men according to the 50th and 75th percentiles of ALT*

There were no significant differences in the percentage of subjects with an increase in liver brightness among ALT quartiles. Above the 75th P of ALT, 16.5% of women and 14.5% of men had an increase in liver brightness (Table 4).
TABLE 4.- Percentage of subjects with an increase in liver brightness in echography among ALT quartiles

Discussion
It has been observed that GGT is associated with arterial hypertension and its incidence4, metabolic syndrome components and development of diabetes type 25-9. While GGT is a sensitive but unspecific marker of liver injury since it also increases by oxidative stress and alcohol intake10, 11, ALT is the most specific marker of liver disease in clinical practice.
This work shows that women in upper levels of the ALT distribution, in which viral hepatitis and alcohol and hepatotoxic drugs intake have been excluded, have higher levels of components of metabolic syndrome and insulin resistance. This association was early evidenced (above the 50th P of ALT) with indicators of dislipidemia (TG/HDLc ratio) and disglycemia (glycemia, HOMA-IR) and the association with BP became evident only above the 75th P of ALT distribution. In men, the scarce number of non-drinkers and the misbalance in ages limit the analysis above the 75th P. However, above 50th P of ALT, TG/HDLc ratio and BMI were higher.
Likewise other epidemiological studies that evaluate the association of ALT with the development of diabetes and with the components of the metabolic syndrome, such as the ones carried out on the Pima Indians12, the West of Scotland Coronary Prevention Study13 and the Insulin Resistance Atherosclerosis Study15, 17, this work is based in only one determination of ALT.
The mechanisms of the association of ALT with metabolic syndrome and insulin resistance are unclear. NAFLD is clearly associated with metabolic syndrome1, 2 and insulin resistance evaluated by HOMA-IR21. However, only a minority above the 75th P of ALT showed an increase of liver brightness in the echography. This could be caused by the fact that ALT was more sensitive than the echography to detect early fat deposits in the liver or by the existence of other mechanisms to account for the association of ALT to metabolic syndrome and insulin resistance. It has been postulated that inflammation30 could be the mechanism for explaining the elevation of hepatic enzymes in subjects with metabolic syndrome, but there has not been a consistent association between ALT and inflammation markers in population studies9. It is very important to point out that ALT levels in our study are within normal limits or slightly elevated. It is possible that the top extreme of the population´ALT distribution expresses a functional disorder, with an increase of hepatocyte turnover, more than a hepatic cytolysis. In animal models the decrease in the production of "hepatic insulin sensitizing substance" (HISS) produces insulin resistance31 suggesting a bidirectional pathway between metabolic syndrome and hepatic injury.
Recently, in a consensus meeting of the International Diabetes Federation, it was proposed that central obesity is a necessary component for the diagnosis of metabolic syndrome32. However, Reaven23, 33 sustains that an elevated TG/HDLc ratio indicates early metabolic syndrome and insulin resistance in subjects without obesity. In the case of women, our study showed that TG/HDLc ratio and HOMA-IR were higher in those with higher ALT despite no differences in BMI or WC. This is consistent with the findings in elderly Caucasian men from the British Regional Heart Study, in which the association between hepatic enzymes with HOMA-IR, TG and HDLc remained significant after adjusting by BMI9. Similarly, in a multivariable regression analysis in the Mexico City Diabetes Study, ALT showed a significant correlation with TG and fasting insulinemia and no correlation with BMI or WC5. Likewise, in the Insulin Resistance Atherosclerosis Study, the upper quartile of ALT distribution predicted the development of diabetes mellitus and metabolic syndrome, independent of obesity markers15, 17.
Although there exist other causes of hepatic damage that were not investigated, their frequency is too low to modify the results of this study. Among them it is worth mentioning the Wilson's disease, with a prevalence in most populations of one in 30.00034, and homozygous genetic hemochromatosis wich prevalence ranges from 0.2% to 0.4% in general populations from Australia35, Europe36 and North America37.
As HOMA-IR38, 39 and TG/HDLc ratio40-42 are independent predictors of cardiovascular risk, attention should be paid to ALT levels near the upper limit of the normal range even when they are not associated with obesity or echographic data of NAFLD. On the other hand, it would be of unquestionable interest to identify subjects with insulin resistance through simple and widely available clinical data. A recent work43 indicates that a TG/HDLc ratio higher than 3 and a TG level higher than 130 mg/dL are adequate for that purpose and similar to the ATPIII definition in sensitivity and specificity. More research is necessary in order to elucidate whether ALT can contribute in this aspect.
Acknowledgements: This work was supported in part by the "Fundación para el Estudio de las Enfermedades Hepáticas de la Provincia de Buenos Aires" (FUNDEHBA). We are very grateful to Dr. Raúl Echeverría for his review of the manuscript, to Dr. Daniel Cocozzella for in-person interview and technical support, to Dr. Daniel Aizpurúa for performing the liver echographies and Graciela Caresia and Jorge González for performing the laboratory measurements.
References
1. Cause of cryptogenic cirrhosis. JAMA 2003; 289: 3000-4.
2. Adams LA, Angulo P, Lindor KD. Nonalcoholic fatty liver Disease. CMAJ 2005; 172: 899-905.
3. Bloomgarden ZT. Second World Congress on the Insulin Resistance Syndrome. Insulin resistance syndrome and nonalcoholic fatty liver disease. Diabetes Care 2005; 28: 1518-23.
4. Stranges S, Trevisan M, Dorn JM, Dmochowski J, Donahue RP. Body Fat distribution, liver enzymes, and Risk of hypertension. Evidence from the Western New York Study. Hypertension 2005; 46: 1186-93.
5. Nannipieri M, Gonzales C, Baldi S, et al. Liver enzymes, the metabolic syndrome, and incident diabetes. The Mexico City Diabetes Study. Diabetes Care 2005; 28: 1757-62.
6. Lee DH, Ha MH, Kim JH, et al. Gamma-glutamyltrans-ferase and diabetes: a 4 year follow-up study. Diabetologia 2003; 46: 359-64.
7. Nakanishi N, Suzuki K, Tatara K. Serum g-glutamyl-transferase and risk of metabolic syndrome and type 2 diabetes in middle-aged Japanese men. Diabetes Care 2004; 27: 1427-32.
8. Lee DH, Silventoinen K, Jacobs DR, Jousilahti P, Tuomileto J. Gamma glutamyltransferase, obesity, and the risk of type 2 diabetes: observational cohort study among 20,158 middle-aged men and women. J Clin Endocrinol Metab 2004; 89: 5410-4.
9. Wannamethee SG, Shaper AG, Lennon L, Whincup PH. Hepatic enzymes, the metabolic syndrome, and the risk of Type 2 diabetes in older men. Diabetes Care 2005; 28: 2913-8.
10. Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci 2001; 38: 263-355.
11. Sillanaukee P, Massot N, Jousilahti P, et al. Dose response of laboratory markers to alcohol consumption in a general population. Am J Epidemiol 2000; 152: 747-51.
12. Vozarova B, Stefan N, Lindsay RS, et al. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes 2002; 51: 1889-95.
13. Sattar N, Scherbakova O, Ford I, et al. The West of Scotland Coronary Prevention Study: Elevated alanine aminotransferase predicts new-onset type 2 diabetes independently of classical risk factors, metabolic syndrome, and C-reactive protein in the West of Scotland Coronary Prevention Study. Diabetes 2004; 53: 2855-60.
14. Erbey JR, Silberman C, Lydick E. Prevalence of abnormal serum alanine aminotransferase levels in obese patients and patients with type 2 diabetes. Am J Med 2000; 109: 588-90.
15. Hanley AJG, Williams K, Festa A, et al. Elevations in markers of liver injury and risk of type 2 diabetes: The Insulin Resistance Atherosclerosis Study. Diabetes 2004; 53: 2623-32.
16. Stranges S, Dorn JM, Muti P, et al. Body fat distribution, relative weight, and liver enzyme levels: a population-based study. Hepatology 2004; 39: 754-63.
17. Hanley AJG, Williams K, Festa A, Wagenknecht LA, D'Agostino RB Jr, Haffner SM. Liver markers and development of the metabolic syndrome: The Insulin Resistance Atherosclerosis Study. Diabetes 2005; 54: 3140-7.
18. Bugianesi E, Gastaldelli A, Vanni E, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia 2005; 48: 634-42.
19. Ardigo D, Numeroso F, Valtuena S, et al. Hyperinsulinemia predicts hepatic fat content in healthy individuals with normal transaminase concentrations. Metabolism 2005; 54: 1566-70.
20. Donati G, Stagni B, Piscaglia F, et al. Increased prevalence of fatty liver in arterial hypertensive patients with normal liver enzymes: role of insulin resistance. Gut 2004; 53: 1020-3.
21. Angelico F, Del Ben M, Conti R, et al. Insulin resistance, the metabolic syndrome, and nonalcoholic fatty liver Disease. J Clin Endocrinol Metab 2005; 90: 1578-82.
22. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA-IR modeling. Diabetes Care 2004; 27: 1487-95.
23. Reaven G. Metabolic Syndrome. Pathophysiology and implications for management of cardiovascular disease. Circulation 2002; 106: 286-8.
24. Joseph AE, Saverymuttu SH, al-Sam S, Cook MG, Maxwell JD. Comparison of liver histology with ultrasonography in assessing diffuse parenchymal liver disease. Clin Radiol 1991; 43: 26-31.
25. Saverymuttu S, Joseph A, Maxwell J. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J 1986; 292: 13-5.
26. Joy D, Thava VR, Scout BB. Diagnosis of fatty liver disease: is biopsy necessary? Eur J Gastroenterol Hepatol 2003; 15: 539-43.
27. Carbajal HA, Salazar MR, Riondet B, et al. Variables asociadas a hipertensión arterial en una región de la Argentina. Medicina (Buenos Aires) 2001; 61: 801-9.
28. Salazar MR, Carbajal HA, Aizpurúa M, et al. Decrease of blood pressure by community-based strategies. Medicina (Buenos Aires) 2005; 65: 507-12.
29. Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med 2006; 354: 731-9.
30. Kerner A, Avizohar O, Sella R, et al. Association between elevated liver enzymes and C-reactive protein: Possible hepatic contribution to systemic inflammation in the metabolic syndrome. Arterioscler Thromb Vasc Biol 2005; 25: 193-7.
31. Lautt WW. A new paradigm for diabetes and obesity: The Hepatic Insulin Sensitizing Substance (HISS) Hypothesis. J Pharmacol Sci 2004; 95: 9-17.
32. Alberti KG, Zimmet P, Shaw J; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome- a new worldwide definition. Lancet 2005; 366: 1059-62
33. Reaven GM. The metabolic syndrome: Requiescat in pace. Clin Chem 2005; 51: 931-8.
34. Sternlieb I. Perspectives on Wilson's disease. Hepatology 1990;12: 1234-9.
35. Leggett BA, Halliday JW, Brown NN, Bryant JJ, Duplock L. Prevalence of haemochromatosis amongst asymptomatic Australians. Br J Haematol 1990; 74: 525-30.
36. Simon M, Brissot P. The genetics of haemochromatosis. J Hepatol 1988; 6: 116-24.
37. Adams PC, Gregor JC, Kertesz AE, Valberg LS. Screening blood donors for hereditary haemochromatosis: decision analysis model based on a 30-year database. Gastroenterology 1995; 109: 177-88.
38. Reilly MP, Wolfe ML, Rhodes T, Girman C, Mehta N, Rader DJ. Measures of insulin resistance add incremental value to the clinical diagnosis of metabolic syndrome in association with coronary atherosclerosis. Circulation 2004; 110: 803-9.
39. Bonora E, Formentini G, Calcaterra F, et al. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: Prospective data from the Verona Diabetes Complications Study. Diabetes Care 2002; 25: 1135-41.
40. Gaziano JM, Hennekens CH, O'Donnell CJ, Breslow JL, Buring JE. Fasting triglycerides, high-density lipoprotein, and risk of myocardial infarction. Circulation 1997; 96: 2520-5.
41. Jeppesen J, Hein HO, Suadicani P, Gyntelberg F. High Triglycerides and low HDL cholesterol and blood pressure and risk of ischemic heart disease. Hypertension 2000; 36: 226-32.
42. Jeppesen J, Hein HO, Suadicani P, Gyntelberg F. Low triglycerides-high high-density lipoprotein cholesterol and risk of ischemic heart disease. Arch Intern Med 2001; 161: 361-6.
43. McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven GM. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med 2003; 139: 802-9.
Received: 17-VIII-2006
Accepted: 26-XII-2006














