Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Medicina (Buenos Aires)
versión impresa ISSN 0025-7680versión On-line ISSN 1669-9106
Medicina (B. Aires) v.67 n.3 Buenos Aires mayo/jun. 2007
Susceptibility tests to second line drugs and re-treatment of tuberculosis. Revisiting Early Experiences
Isabel N. De Kantor1, Lucía Barrera2
1 Panel de Consultores en Tuberculosis, OMS, Buenos Aires
2 Instituto Nacional de Enfermedades Infecciosas, ANLIS C. G. Malbrán, Buenos Aires
Postal address: Dr. Isabel N. de Kantor. Av. Libertador 7504, 1429 Buenos Aires, Argentina. Fax (54-11) 4701-7731 e-mail: ikantorp@fibertel.com.ar
Abstract
The value of susceptibility tests in guiding antituberculous therapy with second-line drugs remains controversial. We reanalyzed three reports regarding the relationship between in vitro susceptibility of Mycobacterium tuberculosis and the clinical outcome of in-patients treated with these drugs at the Muñiz Hospital, Buenos Aires, during the sixties. These patients had been irregularly treated with a standard regimen consisting of isoniazid, streptomycin and PAS; they developed resistance to at least the first two drugs and persisted culture-positive. Susceptibility testing to ethionamide, cycloserine and kanamycin were performed by the proportion method on Löwenstein Jensen medium. Some level of resistance was detected among isolates from patients not previously treated with these drugs, that could be due to cross resistance with previously administered first line structural analogs. However, the studies evidenced significant association between resistance to ethionamide and cycloserine and prior treatment with these drugs. Increased resistanceto all three drugs was detected within the first three months of treatment. In vitro resistance to ethionamide emerged earlier and was the most frequent followed by resistance to cycloserine and kanamycin. The low frequency of resistance to kanamycin could be related to the low dosage of this drug used at that time. Simultaneous resistance to the three agents, but not to two or one drug, appeared to be a marker of treatment failure. An apparent reversion of drug resistance was observed in near 6% of patients, for whom susceptibility tests were repeated on subsequent isolates, indicating this percentage of inconsistency in reproducibility of test results.
Key words: Tuberculosis; Drug susceptibility tests; Second line anti tuberculosis drugs; Multi drug resistance; Argentina
Resumen
Las pruebas de sensibilidad a drogas de segunda línea y el re-tratamiento de la tuberculosis. Visión retrospectiva de tres estudios. La correlación entre resultados de pruebas de sensibilidad a drogas antituberculosas de segunda línea y evolución de los pacientes en tratamiento, aún es discutida. Se reanalizan aquí tres estudios realizados en la década del 60, sobre la relación entre resultados de pruebas de sensibilidad y tratamiento con estas drogas, en pacientes crónicos, internados en el hospital Muñiz, Buenos Aires, que habían sido tratados con el entonces régimen estándar, integrado por isoniacida, estreptomicina y PAS; se habían hecho resistentes al menos a dos de estas drogas y continuaban con cultivo positivo. La prueba de sensibilidad a etionamida, cicloserina y kanamicina se efectuó por el método de las proporciones en medio Löwenstein Jensen. Entre 4 y 13% de los pacientes previamente no tratados con estas drogas presentó cierto nivel de resistencia, fenómeno atribuido a la administración previa de drogas de primera línea con moléculas análogas. Se halló asociación significativa entre resistencia a etionamida y cicloserina, y tratamiento previo con estas drogas. La resistencia a las tres drogas fue detectada en los primeros tres meses de tratamiento, siendo la resistencia a etionamida la más frecuente, y la primera en emerger, seguida por cicloserina y kanamicina, cuya baja frecuencia en alcanzar resistencia estaría relacionada con las bajas dosis administradas. La resistencia simultánea a las tres drogas, pero no a una o dos, resultó marcadora de fracaso terapéutico. Se observó en cerca del 6% de los pacientes aparente reversión de la resistencia, en pruebas hechas en aislamientos sucesivos, interpretada como falla en la reproducibilidad de resultados.
Palabras clave: Tuberculosis; Pruebas de sensibilidad; Drogas de segunda línea; Multirresistencia; Argentina
Multi drug resistant tuberculosis (MDR TB), i.e. simultaneously resistant to isoniazid (INH) and rifampicin (RFP), is a universally extended problem, even though its relevance varies in different regions of the world. In Argentina, 2.1 and 13.3% of new and previously treated patients included in a National survey (2005-2006) presented MDR (Not published preliminary data, ANLIS C. Malbrán, Reference Laboratory for the WHO/ International Union against Tuberculosis and Lung Disease [IUATLD] Global Project on anti-TB Drug Resistance Surveillance). MDR can entail resistance to new or second line antituberculous drugs (SLD) which is seen as an emerging public health threat1-3. The successful application of the DOTS-Plus Program for the management of MDR counts upon reliable and timely reported bacteriological results, including those of first and second-line antituberculous drug susceptibility testing (DST)4-6.
The tests that detect drug resistance (DR) must fulfill basic requisites to be applied under programmatic conditions. They should be accurate, standardized and quality assured7. To be feasible in low/medium income countries they should also be inexpensive, easy-to- perform and, as much as possible, independent of equipment and reagents importation. The proportion method (PM) developed by Canetti Rist and Grosset largely demonstrated to fit in unindustrialized scenarios to test first-line anti-TB drugs8-10. Most National Reference Laboratories in Latin American countries and most WHO/IUATLD Supranational Reference Laboratories in the world are still using the PM on egg media for DST.
While new DST methods are being developed and/or evaluated to test SLD, the PM either on semi synthetic Middlebrook agar or on egg-based media, is widely used as reference to standardize them11-19. However, the value of susceptibility tests to SLD as predictors of clinical evolution, and thus its appropriateness for guiding MDR treatment still is a controversial issue20.
In this situation, the analysis of the experience gained in different settings is of utmost interest. In Argentina, the medical assistance of drug-resistant TB has been concentrated at the Muñiz Hospital, an Infectious Diseases Referral Center located in Buenos Aires21-23. In the early sixties, RFP had not been introduced there yet. The main drugs then employed in the standard chemotherapy regimen were INH, streptomycin (SM), and eventually para-amino-salicylic acid (PAS) and thioacetazone. Simultaneous resistance to INH and SM occurring in those times could be equated to the current MDR. Resistance to PAS was rarely detected, and susceptibility to thioacetazone was not tested.
Several SLD were used for the therapy of TB resistant to INH and SM and, sometimes, also to PAS. In 1963-65 the second line or minor anti-TB drugs in use in this hospital were: ethionamide (ETH), kanamycin (KM), cycloserine (CS) and viomycin (VM). Capreomycin and ethambutol were barely available, and they were used as experimental drugs in clinical trials. Second line drugs were administered either as a last chance to cure these polyresistant chronic patients, or to prepare them for lung surgery.
Severe, cavitary pulmonary TB cases were hospitalized. Even though, treatment was not supervised, what poses a limitation in the re-analysis of the data presented in the reports produced in those years. Usual doses were 750 mg/day the first week, followed by 1g/d for ETH; 500 mg/d for CS, and 3 g/week or 500 mg/d for KM21. Doses recommended by WHO are 500-750 mg/d for ETH and CS, and 750 mg-1 g/d for KM, 5-6 days per week24. Then, in Buenos Aires during the 1960s, dosages were higher for ETH, similar for CS and lower for KM in relation to those currently prescribed.
HIV infection had not emerged yet, and the main causes of immune-depression were diabetes, malnutrition and alcoholism. Mortality among chronic immune-depressed TB in-patients, was high (6-10% during the first year follow-up)22.
In 1961-62 Abel Cetrángolo, then Director of the TB Laboratory at the Muñiz Hospital, and the Department Pneumo-Phtisiology, Buenos Aires University, visited the Pasteur Institute and collaborated with Georges Canetti et al25. After his return, the simplified variant of the PM method in Löwenstein Jensen was implemented at the Muñiz Hospital, and in 1963 Canetti visited the TB laboratory26.
Laboratory examinations for TB were performed following strictly medical requests. All specimens were investigated by AFB (acid fast bacilli) microscopy, cultured, and those that resulted culture positive for mycobacteria were submitted to first line susceptibility testing. DST was performed directly from the specimen when the patient was sputum smear positive8-10. Upon medical request, SLD were also included in the test. First and second line DST were repeated when respiratory specimens continued to be culture positive after 4 months of treatment, even when resistance to one or more drugs had already been documented. These procedures were time and work demanding, especially when DST were required on monthly basis.
The knowledge gained in those years at this referral hospital was not widely disseminated, as the reports produced by the medical and laboratory teams were published only locally. The information communicated in those times provide an invaluable opportunity to retrospectively analyze the utility of DST to SLD. The aim of this report was to disclose some pioneer evidence regarding the relation between in vitro susceptibility of Mycobacterium tuberculosis isolates to SLD and the clinical outcome of patients under treatment with these drugs.
Materials and Methods
Clinical trials on DR-TB conducted at Muñiz Hospital during the 1960's decade whose results were published in Argentinean journals or congress proceedings were reviewed. The studies presenting results of in vitro SLD susceptibility testing obtained by the PM were selected21-23. All three eligible trials provided results corresponding to M. tuberculosis isolates obtained from chronic pulmonary TB in-patients. They had received irregular "standard chemotherapy" with INH, SM and PAS, continued bacteriologically positive, developed resistance to at least the first two drugs and were then submitted to treatment with SLD. The PM was performed on Löwenstein Jensen containing 20 mg/ml of KM and ETH and 30 mg/ml of CS and VM. Isolates were classified as resistant if 10% or more of the bacterial colony forming units inoculated onto the drug containing medium survived8-10.
Study 121
This report presents the first results of SLD susceptibility tests obtained at the Muñiz Hospital corresponding to 100 chronic in-patients. At the admission they were classified as "not previously treated" (NPT) or "previously treated" (PT) in relation to each SLD. So the same patient could be included in the group of patients PT with one drug, and simultaneously among NPT cases in relation to other one. Subsequently they received ETH, CS, KM, and VM, on the basis of clinical assessment, without considering DST results to these drugs. After at least 24 months follow-up DST results were retrospectively analyzed in the light of history of treatment with SLD and the clinical outcome.
Study 222
The report presents the clinical and bacteriological outcome of 1292 in-patients and focuses on 212 cases whose isolates showed different patterns of resistance to SLD during hospitalization. At the admission, 48 of these cases were affected by M. tuberculosis susceptible to SLD, and 164 excreted bacilli that presented resistance to at least one of these drugs. In vitro, the isolates of 28 of these 212 patients showed newly acquired resistance to a single or multiple SLD, while receiving second line treatment at the hospital. The order of appearance of this resistance was documented. The resistance patterns determined by the last test available for each case were analyzed.
Study 323
This study included 2000 TB in-patients. DST was performed at hospital admission and repeated at least once during treatment. The authors analyzed retrospectively the DST results of 195 cases whose isolates presented resistance to one or more SLD, in relation to their clinical and bacteriological outcome. The last DST result available for each case was included in the study. Follow-up was conducted either until culture negativization, decease, or for at least 2 years if the patients continued to be culture positive.
Statistical analysis
Differences in frequency of drug resistance among groups of patients were evaluated by the c2 test and considered significant if p values were less than 0.05. Yates correction was applied when there was only one degree of freedom (df). Odds ratio (OR) was used to estimate chances of persistent culture positive TB according to drug resistance pattern.
Results
Study 121
Table 1 shows DST results of isolates according to prior treatment with each SLD tested. In vitro DR to ETH, CS and KM were observed in respectively 13.2, 4.8, and 4.2% of the cases for whom there was no evidence of prior treatment with each of these drugs, and in 83.0, 46.6 and 17.9% of the patients for whom treatment with each drug was documented. Hence, frequency of bacterial resistance to ETH, CS and KM was near 6, 9 and 4 times higher among treated cases in that order. It was demonstrated strong association between previous treatment with ETH or CS and in vitro bacterial resistance to these drugs. Table 2 separates the results of PT cases according to the duration of chemotherapy with the secondary drugs at the time of DST. In vitro DR to all three drugs was detected early, within the first three months of treatment. Increase in frequency of drug resistance among patients treated for longer periods was not evidenced in this experience. Among all treated patients resistance to ETH was detected significantly more often than resistance to any of the drugs CS and KM (p[2df]=0.00000001). Resistance to VM was not observed in any of 7 patients treated with this drug (data not shown).
TABLE 1.- Study 1: Frequency of second-line drug resistance among 100 chronic pulmonary tuberculosis cases according to history of treatment with each drug tested
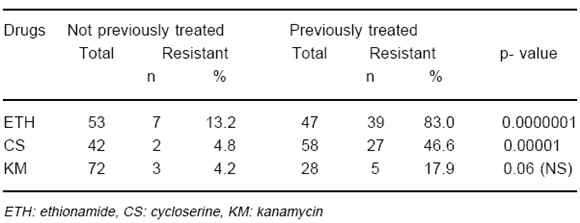
TABLE 2.- Study 1: Number of patients investigated by drug susceptibility tests (DST) to each of the drugs ethionamide (ETH), cycloserine (CS), and kanamycin (KM), after different periods of time (in months) under treatment, and number (percentage) of drug resistance results to the drug tested
Study 222
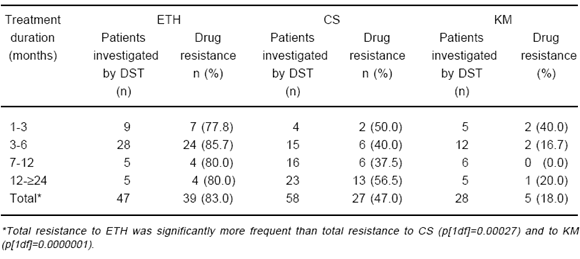
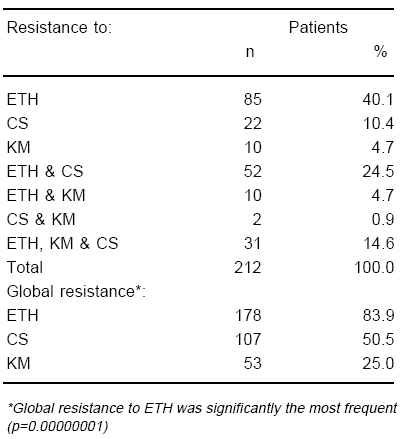
Table 3 displays the patterns of drug resistance corresponding to the 212 cases monitored in this study. Global resistance to ETH was the most frequently observed (p[2df]=0.00000001). Both global resistance to ETH and to CS, were significantly more frequent than resistance to KM (p[1df]=0.00000001). Thirty one out of the total 212 patients (14.6%) presented DR to the three drugs here tested. On the other hand, resistance to ETH was the first to appear among 21 of the 28 (75%) of the cases for whom serial DST results were reported.
TABLE 3.- Study 2: Frequency of in vitro second-line drug resistance pattern among 212 chronic tuberculous patients treated with ethionamide (ETH), cycloserine (CS), and kanamycin (KM)
Study 323
Lack of reproducibility was seen while testing isolates of 6.2% (12/195) of patients evaluated. In effect, isolates of 7, 4 and 1 patients "reverted" resistance to ETH, CS, and KM, respectively, as evidenced by DST performed on subsequent isolates obtained after detecting resistance to any of these drugs. The results of these patients were excluded from further analysis.
Drug susceptibility tests results corresponding to the remaining 183 in-patients are summarized in Table 4. Accumulative percentages of resistance by drug in the entire group show again the significantly higher frequency of ETH resistance (152/183 patients, 83%), followed by CS (70 patients, 38.3%) and KM (47 patients, 25.7%), (p[2df]=0.0000001). Differences in frequency between ETH and CS resistances, and between CS and KM resistances also achieved significance (p[1df]=0.0000001; and p(1df)=0.02 respectively). Nearly 9% (16/183) of the chronic cases analyzed in this series were resistant to the three drugs.
TABLE 4.- Study 3: Second line drug resistance pattern and bacteriological evolution after at least 6 months treatment among 183 patients with chronic pulmonary tuberculosis
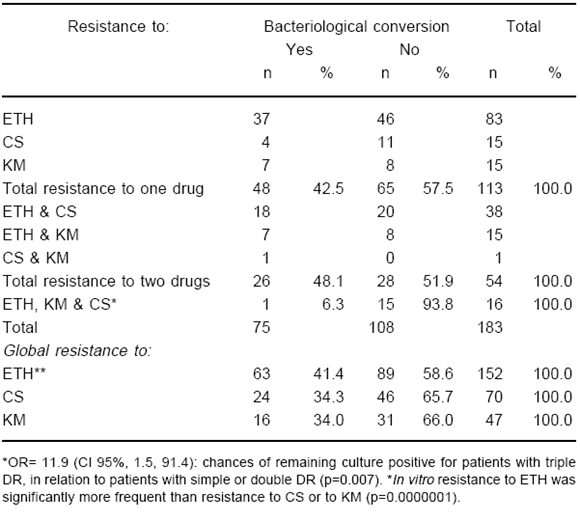
Only 75 of these 183 patients (41%) were discharged from hospital with at least one negative culture. The proportion of patients who converted to negative was not significantly different among those whose isolates were resistant to one or to two SLD (42.5 vs. 48.1%, p=0.60). However, the proportion of bacteriological failures among patients affected by isolates resistant to 3 SLD was significantly higher than that found among patients with resistance to one or to two drugs (93.8% vs. 44.3%, p=0.007). Only one out of 16 patients with resistance to three drugs achieved bacteriological negativization. According to the odds ratio (OR= 11.9; 95% confidence interval [CI]= 1.5, 91.4) patients with resistance to the 3 SLD were nearly 12 times more likely to remain culture positive during the follow-up period than those affected by strains showing in vitro resistance to one or to two of these drugs (Table 4). Among patients with mono-resistance either to ETH, CS or KM, no significant differences were found in the proportion of those achieving bacteriological conversion to negative (p[2df]=0.41). A similar situation was observed with resistances to two drugs, in combinations ETH & CS, or ETH & KM (p[1df]=0.79). There was only one case with resistance to CS & KM.
Discussion
At usual doses, the current first line drugs INH and RFP can attain in vivo concentrations considerably higher than those needed to inhibit M. tuberculosis growth in vitro (MIC). Substandard regimens usually select resistant mutants able to survive at high concentrations of these drugs. Thus INH and RFP susceptible and resistant strains can be separated into clear cut groups, and in vitro DST results are usually unequivocal. On the other hand, therapeutic doses of SLD, must be limited to levels that produce tissue concentrations relatively close to MICs because of toxic effects. Several hours after the drug intake the concentration may be even lower than the MIC and then clinically ineffective. Thus, resistant mutants to SLD are selected at a relatively low concentration of the drug. This phenomenon has been signaled as the cause of the limited efficiency, reproducibility and predictive value of DST for ETH and other SLD8, 27.
The apparent resistance reversion found in 6.2% of tests while monitoring sequential respiratory specimens in Study 3 shows some inconsistency of SLD susceptibility tests results. As it is usually the case, the authors had not observed this incident when testing first-line drugs23.
However, generalization on the poor credibility of this examination might mask some certitude and support that SLD susceptibility testing may provide. For instance, in vitro resistance to ETH and CS is not a contingent phenomenon but it is strongly associated to history of treatment with these drugs as showed by Studies 1 and 2 and recently corroborated by Rich et al 28. Another interesting finding is related to the accumulation of resistance. While resistance to one or two SLD may not be predictive of the treatment outcome, simultaneous in vitro resistance to ETH, CS and KM seems to be a good marker of treatment failure as demonstrated in Study 3.
The information here analyzed showed that resistance to ETH emerged earlier in the course of treatment and was consistently the most frequent, followed by CS and then by KM resistance. The relatively low KM dosage then used could probably have influenced the results. In recent times the neat predominance of ETH resistance was also described among MDR cases, while the resistance to aminoglycosides, nowadays administered in higher dosages, was found to be more frequent than reported in the early studies at the Muñiz Hospital2.
The percentage of isolates resistant to SLD among patients apparently not previously treated with these drugs observed in Study 1 is in concordance with the results of Canetti et al., who found 6% of DR to ETH, 4% to CS and 10% to KM, among patients treated with these SLD for less than one month8. The information is also consistent with that communicated by Rich et al. who have recently observed 18.6% of resistance to ETH, and 11.6% to KM, in a group of patients that failed with WHO Category I treatment which does not include these drugs28. The level of initial ETH resistance could be attributed to cross resistance between this drug and the structural analogs INH or thioacetazone, as in those times the front-line schemes included these last two drugs8. Consistently with this hypothesis nowadays it is known that these three drugs are mycolic acid synthesis inhibitors and that co-resistance between INH and ETH can be originated by dominant mutations in the target gene inhA13, 29, 30. On the other hand, previous administration of SM might have generated KM cross-resistance in the group of patients that had not been treated with SLD8, 13, 31.
Knowledge on the level of SLD resistance found among strains circulating in a determined scenario can be useful for the programmatic management of MDR TB.
Encouraging evidence has been furnished in the last decade on the cure of MDR TB, based on appropriate management of new fluoroquinolones, linezolid, and some of the SLD analyzed in these studies. With the limitations above discussed, quality assured laboratory data on SLD could support clinical decision making and help to prevent the emergence of further drug resistance in patients with MDR TB. On these bases, Guidelines for DST for second line anti-TB drugs for DOTS-PLUS were produced in 200132.
Concerning the standardization and evaluation of new DST methods proposed to evaluate the activity of SLD and newer anti-tuberculosis drugs, it is relevant to determine the stability of drugs in culture media, critical concentrations and breakpoints to distinguish drug resistant from drug sensitive isolates. This knowledge should be gained by testing a representative number of wild strains and strains obtained from treatment failures. So did Canetti et al.8, in their classic study evaluations, Lefford and Mitchison27 (more recently reviewed by Mitchison)33. The wild strain population was composed of isolates from patients apparently never treated with any anti-tuberculosis drug, while the presumably drug resistant isolates came from failure cases already treated with the drug. Animal models might help to precise the predictive value of DST to SLD. These models enable the evaluation of the in vivo activity of an individual drug in animals infected with strains that are in vitro susceptible or resistant to the same drug.
Then standardized inter-laboratory proficiency tests of DST to SLD and new drugs used in the treatment of MDR cases will be needed. The external quality program already established for first-line anti-TB drugs by the WHO/IUATLD Global Supranational Laboratory Network could be extended to these drugs. The inter-laboratory efficiency that can be achieved while testing each second-line and new drugs must still be known.
In summary, efficiency of DST to SLD is limited by their pharmacokinetics. Further knowledge and external quality control is eagerly needed to precise and improve the value of these laboratory tests. Despite this limitation, this report based on very early experiences made with the classical proportion method on Löwenstein Jensen, documents that in vitro SLD resistance emerges as consequence of antibiotic pressure during treatment, that accumulation of resistance to several SLD is associated to treatment failure with schemes composed by these drugs, and that the tests to SLD are consistent enough to estimate prevalence of DR in a setting.
Acknowledgements: The authors wish to thank Dr. Adalbert Laszlo (WHO Consultant in Tuberculosis, Ottawa, Canada), for his original suggestion of reviewing these pioneer works.
References
1. Centers of Disease Control. Emergence of Mycobacterium tuberculosis with extensive resistance to secondline drugs-worldwide, 2000-2004. MMWR Morb Mortal Wkly Rep 2006; 55: 301-5.
2. Toungoussova OS, Mariandyshev AO, Bjune G, Caugant DA, Sandven P. Resistance of multidrug-resistant strains of Mycobacterium tuberculosis from the Archangel oblast, Russia, to second-line anti-tuberculosis drugs. Eur J Clin Microbiol Infect Dis. 2005; 24: 202-6.
3. World Health Organization. Anti-tuberculosis drug resistance in the world. Report 3. The WHO/IUATLD Global Project on Anti-tuberculosis Drug Resistance Surveillance. WHO/HTM/TB/2004.343. Geneva: WHO, 2004, 299 pp.
4. Mitnick C, Bayona J, Palacios E, et al. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. NEJM 2003; 348: 119-28.
5. Grosset J. Systematic drug susceptibility testing: a necessary component of the "DOTS Plus" strategy? Int J Tuberc Lung Dis 1999; 3: 549-50.
6. Heifets LB, Cangelosi GA. Drug susceptibility testing of Mycobacterium tuberculosis: a neglected problem at the turn of the century. Int J Tuberc Lung Dis 1999; 3: 564-81.
7. Laszlo A, Rahman M, Espinal M, et al. Quality assurance program for drug susceptibility testing of Mycobacterium tuberculosis in the WHO/IUATLD Supranational Reference Laboratory Network: five rounds of proficiency testing, 1994-1998. Int J Tuberc Lung Dis 2002; 6: 748-56.
8. Canetti G, Rist N, Grosset J. Mésure de la sensibilité du bacille tuberculeux aux drogues antibacillaires par la méthode des proportions. Rev Tuberc Pneum 1963; 27: 217-72.
9. Canetti G, Fox W, Khomenko A, et al. Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull World Health Organ 1969; 41: 21-43.
10. Canetti G, Grosset J. Téchniques et Indications des Examens Bactériologiques en Tuberculose. St. Mandé (France): Editions de la Tourelle, 1969, 192 pp
11. NCCLS. Susceptibility testing of Mycobacteria, Nocardia, and other aerobic Actinomycetes; Tentative Standard-Second Ed. NCCLS document M24-T2[ISBN1-56238-423-6]. Wayne, Pennsylvania: NCCLS, 2000, 81 pp.
12. Pfyffer GE, Bonato DA, Ebrahimzade A, et al. Multicenter laboratory validation of susceptibility testing of Mycobacterium tuberculosis against classical second-line and newer antimicrobial drugs by using the radiometric BACTEC 460. Technique and the proportion method with solid media J Clin Microb, 1999; 37: 3179-86.
13. Heifetz LB. Drug susceptibility in the chemotherapy of mycobacterial infections. Boca Raton: CRC Press, 1991, 212 pp.
14. Rüsch-Gerdes S, Pfyffer GE, Casal M, Chadwick M, Siddiqi S. Multicenter laboratory validation of the BACTEC MGIT 960 technique for testing susceptibilities of Mycobacterium tuberculosis to classical second-line drugs and newer antimicrobials. J Clin Microbiol 2006; 44: 688-92.
15. Barreto AM, Araujo JB, Melo Medeiros RF, Souza Caldas PC. Evaluation of indirect susceptibility testing of Mycobacterium tuberculosis to the first- and second-line, and alternative drugs by the newer MB/BacT System. Mem Inst Oswaldo Cruz 2003; 98: 827-30.
16. Martin A, Camacho M, Portaels F, Palomino JC. Resazurin microtiter assay plate testing of Mycobacterium tuberculosis susceptibilities to second-line drugs: Rapid, simple, and inexpensive method. Antimicrob Agents Chemother 2003; 47: 3616-9.
17. Morcillo N, Di Giulio B, Testani B, Pontino M, Chirico C, Dolmann A. A microplate indicator-based method for determining the susceptibility of multidrug-resistant Mycobacterium tuberculosis to antimicrobial agents Int J Tuberc Lung Dis 2004; 8: 253-9.
18. Parsons, L. M., Somoskovi, A., Urbanczik, R., Salfinger, M. Laboratory diagnostic aspects of drug resistant tuberculosis. Frontiers in Bioscience 2004; 9: 2086-105.
19. Huang TS, Lee SS, Tu HZ, et al. Use of MGIT 960 for rapid quantitative measurement of the susceptibility of Mycobacterium tuberculosis complex to ciprofloxacin and ethionamide. J Antimicrob Chemother. 2004; 53: 600-3.
20. Kim SJ, Espinal MA, Abe C, et al. Is second-line anti-tuberculosis drug susceptibility testing reliable? Int J Tuberc Lung Dis 2004; 8: 1157-8.
21. Rey JC, Cetrángolo A, Dubra FA. Resistencia bacteriana a las drogas antituberculosas subsidiarias. Medicina (Buenos Aires) 1964; 24: 273-6.
22. Cetrángolo A, Di Lonardo M, de Kantor IN. Resistencia del Mycobacterium tuberculosis a algunas drogas antituberculosas subsidiarias o de segunda línea. Actas Xo. Congreso Argentino de Tisiología y Neumonología, Mar del Plata, Argentina, noviembre 1965, p162-5.
23. Cetrángolo A, Di Lonardo M, de Kantor IN, Isola NC. Bacteriología de los enfermos tuberculosos hospitalizados eliminadores de Mycobacterium tuberculosis resistentes a drogas subsidiarias. Actas del XI Congreso Argentino de Tisiología y Neumonología, Buenos Aires, octubre 1967, p 273-5.
24. World Health Organization. Guidelines for the pro-grammatic management of drug-resistant tuberculosis. WHO/HTM/TB/2006.361. In: whqlibdoc.who.int/publications/2006/9241546956_eng.pdf Consulted Jan 25, 2007.
25. Canetti G, Rist N, Grosset J, Cetrángolo A. Une simplification technique de la méthode des proportions pour tests de sensibilité du bacille tuberculeux: l'emploi d'une anse de platine. Arch Inst Pasteur Alger, 1964, 42: 14-8.
26. Canetti G. Bases biológicas para el tratamiento de la tuberculosis con las drogas menores (Conferencia, traducción al castellano por G.Amadio). Actas del IX Congreso Argentino de Tisiología y Neumonología, Octubre 1963, Córdoba, Argentina, p 543-8.
27. Lefford MJ, Mitchison DA. Comparison of methods for testing the sensitivity of Mycobacterium tuberculosis to ethionamide. Tubercle, Lond. 1966; 47: 250-62.
28. Rich ML, Socci AR, Mitnick CD, et al. Representative drug susceptibility patterns for guiding design of retreatment regimens for MDR-TB. Int J Tuberc Lung Dis 2006; 10: 290-6.
29. Vilcheze C, Weisbrod TR, Chen B, et al. Altered NADH/NAD+ ratio mediates co-resistance to isoniazid and ethionamide in mycobacteria. Antimicrob Agents Chemother 2005; 49: 708-20.
30. Morlock GP, Metchock B, Sikes D, Crawford JT, Cooksey RC.ethA, inhA, and katG loci of ethionamide-resistant clinical Mycobacterium tuberculosis isolates Antimicrob Agents Chemother. 2003; 47: 3799-805.
31. McClatchy JK, Kanes W, Davidson PT, Moulding TS. Cross-resistance in Mycobacterium tuberculosis to kanamycin, capreomycin and viomycin. Tubercle. 1977; 58: 29-34.
32. Communicable Diseases/World Health Organization. Guidelines for drug susceptibility testing for second-line anti tuberculosis drugs for DOTS-PLUS. Treatment of Multidrug-Resistant Tuberculosis. Geneva: WHO/CDS/TB/2001.288, 2001. En: www.who.int/infectious-disease-news/IRCcatalogue/PDF/irccat2001-1.pdf Consulted Jan 8, 2007.
33. Mitchison DA. Drug resistance in tuberculosis. Eur Respir J 2005; 25: 376-9.
Received: 13-02-2007
Accepted: 21-03-2007














