Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Medicina (Buenos Aires)
versión impresa ISSN 0025-7680versión On-line ISSN 1669-9106
Medicina (B. Aires) v.67 n.3 Buenos Aires mayo/jun. 2007
Low-dose glucocorticoids in hyperandrogenism*
Leonardo Rizzo1, Viviana Dobrovsky1, Karina Danilowicz1, Martha Kral1, Graciela Cross1, Héctor A. Serra2, Oscar D. Bruno1
1 División Endocrinología, Hospital de Clínicas, y
2 1ª Cátedra de Farmacología, Facultad de Medicina, Universidad de Buenos Aires
* Partially presented at the VII Conference on Clinical Pharmacology and Therapeutics, Florence, Italy, July 2000 and to the Endocrine Society 83rd Annual Meeting, Denver, Co, USA, June 2001.
Postal address: Dr. Oscar D. Bruno, División Endocrinología, Hospital de Clínicas, Avenida Córdoba 2351, 5to piso, 1120 Buenos Aires, Argentina. Fax (54-11) 4805-0631 e-mail: divendhcli@intramed.net
Abstract
To investigate the effect of low-doses of glucocorticoids on androgen and cortisol secretion during the course of the day, we evaluated clinical signs of hyperandrogenism and total, free and bioavailable testosterone, SHBG, and cortisol following two different protocols: A) fourteen patients received betamethasone 0.6 mg/day (n=8) or methylprednisolone 4 mg/day (n=6), as single daily oral dose at 11.00 PM, during 30 days, B) fourteen patients were evaluated under betamethasone 0.3 mg in a single daily dose at 11.00 PM during six months, 11 out of whom were re-evaluated six months later. Twenty eight women with hyperandrogenism were included and seven normal females were used as control. Blood samples were taken in follicular phase at 8 AM and 7 PM to determine SHBG, cortisol, total, free and bioavailable testosterone. In both protocols, a significant morning and evening decrease in cortisol and testosterone (p<0.05 to < 0.01), which was more marked with betamethasone (p<0.05), was shown. In protocol B, morning SHBG levels showed a significant increase (p<0.05) and betamethasone also improved clinical hyperandrogenism along the trial. Although morning and evening cortisol significantly decreased during treatment, no side effects were reported. The 11 patients re-evaluated after therapy withdrawal, showed a rise in serum total testosterone and its fractions to pre-treatment values and a normalization of cortisol levels. It is concluded that glucocorticoids in low-doses effectively normalize serum androgens, independently of their origin. They may be used therapeutically, mainly whenever a hyperandrogenic woman presents with cycle irregularities or seeking fertility.
Key words: Hyperandrogenism; Glucocorticoids; Cortisol secretion
Resumen
Efecto de bajas dosis de glucocorticoides en el hiperandrogenismo. Con el objetivo de investigar el efecto de bajas dosis de glucocorticoides sobre la secreción de andrógenos y cortisol en el curso del día, evaluamos signos de hiperandrogenismo, testosterona total, libre y biodisponible y cortisol según dos protocolos diferentes: A) catorce pacientes recibieron betametasona 0.6 mg/día (n= 8) o metilprednisolona 4 mg/día (n= 6) en dosis única cotidiana, a las 23 h, durante 30 días, B) catorce pacientes fueron evaluadas bajo betametasona 0.3 mg en dosis única cotidiana a la 23 h, administrada durante 6 meses; de ellas, 11 pacientes fueron re-evaluadas 6 meses más tarde. Se incluyeron 28 mujeres con hiperandrogenismo y 7 controles normales. Se obtuvieron muestras de sangre en fase folicular a las 08:00 y 9:00 h para determinar SHBG, cortisol, testosterona total, libre y biodisponible. En ambos protocolos se observó una disminución significativa de cortisol y testosterona (p<0.05 a <0.01), más importante con betametasona (p<0.05). En el protocolo B, los niveles matutinos de SHBG aumentaron significativamente (p<0.05) y se observó mejoría clínica con el tratamiento. Aunque los niveles matutinos y vespertinos de cortisol disminuyeron significativamente durante el tratamiento, no se observaron efectos secundarios. En las 11 pacientes reevaluadas luego de suspensión de glucocorticoides se observó un aumento de testosterona y sus fracciones a los niveles pre-tratamiento con normalización de las concentraciones de cortisol. Dosis bajas de glucocorticoides normalizaron eficazmente los andrógenos séricos elevados, independientemente de su causa. Pueden emplearse terapéuticamente, en especial cuando una mujer hiperandrogénica presenta alteraciones del ciclo menstrual o busca fertilidad.
Palabras clave: Hiperandrogenismo; Glucocorticoides; Secreción de cortisol
The hyperandrogenic syndrome or hyperandrogenism (HA) is a frequent condition mainly related, among others, to polycystic ovary syndrome (PCOS), late onset congenital adrenal hyperplasia or 5a reductase hyperactivity1. Its prevalence in our country has been estimated to be as high as 17% in women between 18 to 29 years2. This disorder is characterized in most cases by hyperandrogenemia and one or more clinical signs such as acne, alopecia, hirsutism, seborrhea, menstrual cycle irregularities or infertility3-5. Prior evidence suggests that one early alteration of this syndrome could be a decrease in serum sexual hormone binding globulin (SHBG) levels6 which consequently promotes an increase in free and bioavailable testosterone.
Since the 1950s, glucocorticoids (GC) have been used to treat HA7. This therapeutic option has been reported to lead not only to normalization of serum androgen levels but also to amelioration of cutaneous symptoms8 and improvement in ovulatory function7. Prolonged treatment with low doses of GC during 1 to 2 years may result in a lasting remission of hyperandrogenism for up to 3 to 4 years9. The aim of our study was to investigate in women with hyperandrogenism the effect of low-dose glucocorticoid administration on androgen and cortisol secretion, during the course of the day.
Materials and Methods
The study included 28 women (aged 17 to 31 years) with clinical and biochemical hyperandrogenism who consulted at the outpatient ward of our Division of Endocrinology. Diagnosis was made on the basis of previous history, physical examination and serum androgen determinations. Inclusion criteria were the presence of hirsutism with a Ferriman & Gallwey score (FG)3 ³ 8 accompanied by seborrhea, and/or alopecia and/or acne and/or oligomenorrhea, and the demonstration of serum androgen concentrations above the upper normal reference values. Exclusion criteria were: patients with idiopathic hyperandrogenism (no hyperandrogenemia), those who received treatment with GC, antiandrogens and/or oral contraceptives during the last 6 months or with chronic diseases, virilizing tumors, Cushing's syndrome, pregnancy, obesity (BMI >30), dyslipidemia, diabetes and/or family history of diabetes10; no specific studies were done to exclude late-onset CAH. Seven normal females aged 19 to 29 years; BMI 21.35 ± 1.15; Ferriman & Gallwey score £ 7 and regular cycles were used as control for inclusion criteria. The study was approved by the local Ethical Committee and all the patients included were informed about the purpose of the study and gave their written informed consent.
Blood samples were taken in follicular phase at 8 AM and 7 PM to determine total testosterone by RIA (Coat-A-Count, DPC), SHBG by IRMA (ORION Diagnostica), cortisol by RIA (Coat-A-Count, DPC); free and bioavailable testosterone were calculated11. Assays were done in runs specifically dedicated to this study.
Protocols and design
For the purposes of the study, betamethasone and methylprednisolone both widely used in our country were employed. Hyperandrogenic patients were investigated following two different protocols.
Protocol A: In this protocol, we compared the effects of an intermediate (methylprednisolone) and a long (betamethasone) acting GC in doses nearly equivalent to the maximal endogenous cortisol secretion12. Fourteen females with hyperandrogenism were randomly assigned to one of two treatment groups. The first one (n=8), aged 18 to 28 years, BMI (mean ± SD) 20.97 ± 1.98 received betamethasone 0.6 mg; the other group (n=6), aged 19 to 28 years, BMI 22.50 ± 2.53 received methylprednisolone 4 mg. The drugs were administered as a single daily oral dose at 11.00 PM during 30 days. Blood samples were obtained just before and after completing treatment.
Protocol B: We used a lower dose of betamethasone (half the dosage used in Protocol A) for a longer period, in order to explore if it was able to maintain androgen suppression while inducing a less significant suppression of the ACTH-cortisol axis. Fourteen females, aged 17 to 31 years, BMI 21.97 ± 2.99 were included in this second trial. The hyperandrogenic women were treated with betamethasone 0.3 mg in a single daily dose at 11.00 PM during six months. Clinical and biochemical assessement were performed before, and after 2 and 6 months of steroid treatment. Eleven out of the 14 patients were also re-evaluated six months after therapy withdrawal.
Statistical procedures
Parametric data were expressed as means ± standard deviation (x ± SD), except for age which was expressed as mean and range. Distribution data were indicated in percentages. Student's t-test for independent variables was used to assess group demographic differences. One way ANOVA and Newman Keuls were employed to analyze the biochemical values. Non-parametric data were expressed as medians ± 95% confidence interval (95% CI). These data were analyzed by the Wilcoxon matched paired test or c2 with Yates' correction. All statistical procedures were performed using Statistica 5.5 for Windows. All tests of significance were performed at p <0.05 level.
Results
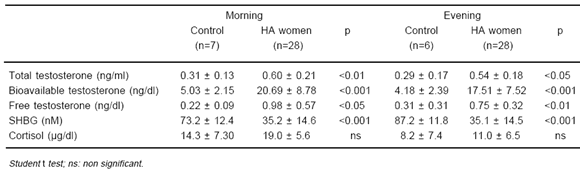
Table 1 shows a comparison of clinical parameters between control and hyperandrogenic women, whereas in Table 2 a comparison between baseline hormone variables of patients included in both protocols and control women is represented. As it was expected, main clinical and biochemical data of control and hyperandrogenic women were significantly different.
TABLE 1.- Comparison of clinical data of hyperandrogenised (HA) women with control women
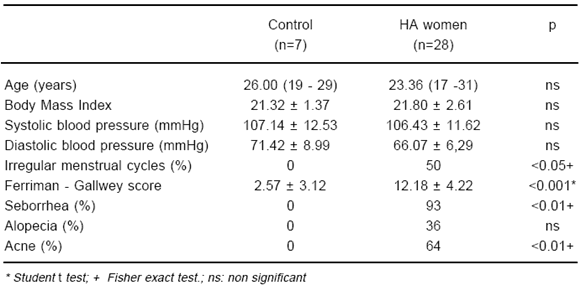
TABLE 2.- Morning (8 AM) and evening (7 PM) laboratory data of hyperandrogenised (HA) women compared with control women
Protocol A (Table 3)
TABLE 3.- Comparison of morning (8 AM) and evening (7 PM) laboratory values of hyperandrogenised women, before and under treatment with betamethasone, 0.6 mg daily (B; n=8) vs methylprednisone, 4 mg daily (MP; n=6) (Protocol A)

In treated patients, there was a significant decrease in mean total, free and bioavailable testosterone with both steroids, but this effect was more marked with betamethasone than with methylprednisolone. Morning serum cortisol concentrations were also decreased by both treatments. Evening serum levels of testosterone and cortisol were significantly lower with betamethasone than with methylprednisolone. SHBG levels were not modified by either GC in this short period of treatment. Mild untoward effects (gain weight, edema and facial plethora) were seen only in three patients treated with betamethasone.
Protocol B (Table 4)
TABLE 4.- Comparison of morning (8 AM) and evening (7 PM) laboratory values of hyperandrogenised women, before (basal), at 2nd and 6th months under treatment with betamethasone (B; n=14), 0.3 mg daily, and 6 months after stopping

Total testosterone and its fractions were significantly suppressed by betamethasone along treatment. After 6 months of treatment there was a significant decrease only in seborrhea (86%, n=12, to 14%, n=2; p<0.001) whereas improvement of acne, alopecia and menstrual cycles were reported only by few patients, and hirsutism remained unchanged. Interestingly, morning SHBG levels showed a significant increase, yet without reaching the control group level. Although not totally suppressed, morning and evening cortisol significantly decreased to subnormal values during treatment (Table 4). No side effects were seen during this trial. After six months of betamethasone withdrawal, eleven patients were re-evaluated. They showed a rise in serum total testosterone and its fractions to pre-treatment abnormally high values and a normalization of cortisol levels. Even though SHBG concentration seemed to be similar to that obtained under therapy, it was not significantly different from pretreatment levels.
Discussion
In the hyperandrogenic syndrome, an overlap between ovarian and adrenal production has been described13-16, which was attributed to the presence of excessive adrenal androgen levels in approximately 60% of PCOS patients17. Even though it is well recognized the rol of insulin resistance and hyperinsulinemia over ovarian hyperandrogenism, we did not evaluate this condition biochemically, based upon a recent consensus meeting on polycystic ovary syndrome10.
Adrenal androgen secretion is more sensitive to suppression by glucocorticoids than adrenal cortisol secretion18. Moreover, it has been suggested that the use of low doses of GC can extend the lenght of remission of hyperandrogenism after discontinuing this therapy, as previously reported by Steinberger et al.7 and confirmed by other authors, who evaluated the effectiveness of GC in HA, documenting prolonged remission of the syndrome after treatment withdrawal9, 19.
The frequency of hyperandrogenic signs was significantly higher in our patients than in controls with the exception of alopecia, probably due to the small size of the population sample. In protocol A we compared the degree of androgen and cortisol suppression between betamethasone 0.6 mg/day and methyprednisolone 4 mg/day during the course of the day. In the second protocol (B) we evaluated whether lower doses of betamethasone, 0.3 mg/day were similarly effective in normalizing morning and evening serum androgens in patients with HA, while reducing the risks of GC side effects. The long-term androgen suppression with this treatment was also analyzed during the course of the day.
Low doses of glucocorticoids rarely induce significant side-effects20. Alternatively, some investigators suggest the appearance of side effects with a low dose of dexamethasone (0.5 mg/day) given for 4 or more months21. We noticed gain weight, edema and facial plethora in three patients (protocol A) treated with betamethasone 0.6 mg/day (fairly equivalent to dexamethasone 0.5 mg/day), but in none treated with lower dose (protocol B).
Bone loss and suppression of the adrenal axis are well-recognized and severe side effects induced by GC. The decrease of bone mineral density is directly related to the length of glucocorticoid therapy as well as the cumulative dose22. Some authors describe loss of bone mineral density with the use of low doses of corticosteroids23. In contrast, in a previous study we failed to find changes in bone mineral content after long-term low-dose glucocorticoid therapy24.
It is widely recognized that high corticosteroids doses employed to treat chronic diseases induce a suppression of the hypothalamic-pituitary-adrenal axis. Fujieda et al. 25 also found this effect by using 5 mg of prednisone in hyperandrogenic women. Conversely, we and others did not find this result with low doses of glucocorticoids as treatment of hyperandrogenism24, 26. However, as Redmond has cautioned the use of even slightly excessive dosis of glucocorticoids is potentially harmful27. Therefore, it is advisable to titrate low doses, beginning with 0.3 or 0.15 mg of betamethasone, in order to avoid cushingoid signs and adrenal suppression. These doses are in the "physiological" range, equivalent or less than the corresponding amount of endogenous cortisol secretion12.
In close agreement with GC androgen suppression, marked clinical improvement of the hyperandrogenic syndrome has been widely observed7, 14, 15, 20, 21. Commonly, there is a substantial decrease in seborrhea and acne8, and menstrual irregularity is normalized in 30-60% of oligo-ovulatory patients14, 28 with GC alone or associated with clomiphene29, 30. However, amelioration of hirsutism is at best moderate, and better results are obtained with the use of antiandrogens such as spironolactone31, 32.
In the second part of this study, most patients manifested a prompt and significant decrease in seborrhea, while improvement of acne and menstrual cycles were reported by some of them; hirsutism remained unchanged. Nevertheless, treatment length was too short to draw valuable clinical conclusions. After discontinuing treatment, clinical signs worsened in some of the patients but the limited sample size again precluded drawing any valid conclusion. In contrast with other observations33, we found a significant decrease in serum testosterone and its fractions with low dose betamethasone, attaining the control group levels by the end of the trial. Long-acting glucocorticoids, such as betamethasone, seem to suppress androgen secretion more effectively than methylprednisolone during the course of the day. Betamethasone therapy in low-doses (0.3 mg/day) suppresses serum androgens without adverse effects, whereas higher doses are liable to promote them in a few cases. Interestingly, although without reaching the control group level, morning SHBG levels have significantly increased at the end of treatment, despite the well-known negative effect of GC on this globulin. This increase could have been due to an increment in endogenous estradiol levels (not measured) as ovarian function ameliorated and/or to the fall in androgen levels leading to the elimination of its suppressive action on SHBG synthesis. The rise in SHBG levels during treatment also suggests that a deleterious effect on insulin sensitivity might not be expected with low doses of GC, since a decrease of SHBG has been considered as an early marker of insulin resistance34, 35. While not significantly different from pre-therapy figures, such increased levels persisted 6 months later despite a rebound increment in serum androgens to altered pre-treatment levels, apparently at variance with data showing lasting remission of hyperandrogenism, up to 4 years, in patients treated with GC for 1-2 years9. However, in our patients androgen rebound after only 6 months of interrupting steroid therapy may not be comparable with more prolonged GC treatment. Finally, whereas cortisol levels were significantly inhibited by treatment, the dose employed in this study failed to induce a severe suppression of the hypothalamic-pituitary-adrenal axis. After 6-month of treatment withdrawal, serum cortisol concentrations fully recovered pre-treatment levels, in agreement with our previous observations showing a normalization of response to metyrapone as early as one month after discontinuing administration of low-dose methylprednisolone during 12 months24.
Although in the present study no tests were done to exclude non classical CAH, the uniform and significant decrease in serum androgens that we observed with low doses of GC cannot be merely explained by the inclusion of patients with this diagnosis since it is not expected to represent more than about 5-10% of total patients consulting for hyperandrogenism36.
From these data, we conclude that low-dose glucocorticoid administration is an effective way to normalize serum hyperandrogenemia in the usual cases of hyperandrogenic syndrome. This favorable outcome may be reached without inducing a marked suppression of endogenous cortisol secretion by employing doses with the so-called "physiological" range. As a primary therapeutic approach, this schedule might be particularly promising whenever a woman with a hyperandrogenic syndrome presents with cycle irregularities or is seeking fertility.
References
1. McKenna TJ, Cunningham SK. The pathogenesis of adrenal and extra adrenal hyperandrogenism. J Steroid Biochem Mol Biol 1993; 45: 117-21.
2. Bruno OD, Arebalo-Cross G, Kral M, et al. Hyperandrogenic syndrome II. Prevalence and biochemical alterations (abstract). Medicina (Buenos Aires) 1989; 49: 453-4.
3. Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab 1961; 21: 144-7.
4. Rosner W. Steroid hormones: Synthesis, metabolism and action in health and disease. Endocrinol Metab Clin North Am 1991; 20: 697-720.
5. Redmond GP. Androgenic disorders of women: Diagnostic and therapeutic decision making. Am J Med 1995; 98: 120S-129S.
6. Caufriez A, Arebalo-Cross G, Kral M, et al. A decrease in sex hormone-binding-globulin levels is a sensitive marker of hirsutism in adolescent girls. 74th Annual Meeting of the Endocrine Society San Antonio, Texas. June 24-27, 1992.
7. Steinberger E, Rodríguez-Rigaud LJ, Petak SM, Weidman ER, Smith KD, Ayala C. Glucocorticoid therapy in hyperandrogenism. Baillieres Clin Obstet Gynaecol 1990; 4: 457-71.
8. Nader S, Rodriguez-Rigau LJ, Smith KD, Steinberger E. Acne and hyperandrogenism: impact of lowering androgen levels with glucocorticoid treatment. J Am Acad Dermatol 1984; 11: 256-9.
9. Carmina E, Lobo RA. The addition of dexamethasone to antiandrogen therapy for hirsutism prolongs the duration of remission. Fertil Steril 1998; 69: 1075-9.
10. The Rotterdam ESRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19: 41-7.
11. Södergard R, Bäckström T, Shanbag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17beta to human plasma proteins at body temperature. J Steroid Biochem 1982; 16: 801-8.
12. Harter JG. Corticosteroids: their physiologic use in allergic diseases. N Y State J Med 1966; 66: 827-40.
13. Loughlin T, Cunningham S, Moore A, Culliton M, Smyth PP, McKenna TJ. Adrenal abnormalities in polycystic ovary syndrome. J Clin Endocrinol Metab 1986; 62: 142-7.
14. McKenna TJ. Pathogenesis and treatment of polycystic ovary syndrome. N Eng J Med 1988; 318: 558-62.
15. Carmina E, Lobo RA. Adrenal hyperandrogenism in the pathophysiology of polycystic ovary syndrome. J Endocrinol Invest 1998; 21: 580-8.
16. Azziz R, Black VY, Knochenhauer ES, Hines GA, Boots LR. Ovulation after glucocorticoid suppression of adrenal androgens in the polycystic ovary syndrome is not predicted by the basal dehydroepiandrosterone sulfate level. J Clin Endocrinol Metab 1999; 84: 946-50.
17. Baumann EE, Rosenfield RL. Polycystic ovary syndrome in adolescence. The Endocrinologist 2002; 12: 333-48.
18. Rittmaster RS, Loriaux DL, Cutler GB. Sensitivity of cortisol and adrenal androgens to dexamethasone suppression in hirsute women. J Clin Endocrinol Metab 1985; 61: 662-6.
19. Devoto E, Aravena L, Gaete X. Prolonged remission of female hyperandrogenism after discontinuing glucocorticoid therapy. Rev Med Chil 1995: 123: 207-14.
20. Redmond GP, Gidwani GP, Gupta MK, et al. Treatment of androgenic disorders with dexamethasone: Doseresponse relationship for suppression of dehydroepiandrosterone sulfate. J Am Acad Dermatol 1990; 22: 91-3.
21. Azziz R. Glucocorticoid suppression in the treatment of androgen excess. In: Azziz R, Nestler JE, Dewailly D (eds). Androgen Excess Disorders in Women. Philadelphia: Lippincott-Raven 1997, pp 737-46.
22. Libanati CR & Baylink DJ. Prevention and treatment of glucocorticoid-induced osteoporosis. Chest 1992; 102: 1426-34.
23. Buckley LM, Leib ES, Cartularo KS, Vacek PM, Cooper SM. Calcium and vitamin D3 supplementation prevents bone loss in the spine secondary to low-dose corticosteroids in patients with rheumatoid arthritis. Ann Intern Med 1996; 125: 961-8.
24. Contreras LN, Rizzo L, Gómez RM, et al. Long-term low-dose glucocorticoid therapy in hyperandrogenized women: Utility and effects on bone mineral content and hypothalamic-pituitary-adrenocortical function. Horm Res 1991; 35: 142-5.
25. Fujieda K, Reyes FI, Blankstein J, Faiman C. Pituitaryadrenal function in women treated with low doses of prednisone. Am J Obstet Gynecol 1980; 137: 962-5.
26. Smith K, Rodriguez-Rigau L, Steinberger E. Response of the adrenal to adrenocorticotropic hormone (ACTH) in hyperandrogenic women treated chronically with low doses of prednisone. Fertil Steril 1982; 38: 202-6.
27. Redmond GP. Treatment of androgenic disorders. In: Redmond GP. Androgenic disorders, New York: Raven Press 1995, ch 13, pp 279-99.
28. Rodriguez-Rigau LJ, Smith KD, Tcholakian RK, Steinberger E. Effect of prednisone on plasma testosterone levels and on duration of phases of the menstrual cycle in hyperandrogenic women. Fertil Steril 1979; 32: 408-13.
29. Lobo RA, Paul W, March CM, Granger L, Kletzky OA. Clomiphene and dexamethasone in women unresponsive to clomiphene alone. Obstet Gynecol 1982; 60: 497-501.
30. Daly DC, Walters CA, Soto-Albors CE, Tohan N, Riddick DH. A randomized study of dexamethasone in ovulation induction with clomiphene citrate. Fertil Steril 1984; 41: 844-8.
31. Crosby PDA, Rittmaster RS. Predictors of clinical response in hirsute women treated with spironolactone. Fertil Steril 1991; 55: 1076-81.
32. Carmina E, Lobo RA. Peripheral androgen blockade versus glandular suppression in the treatment of hirsutism. Obstet Gynecol 1991; 78: 845-9.
33. Rittmaster RS. Medical treatment of androgen-dependent hirsutism. J Clin Endocrinol Metab 1995; 80: 2559-63.
34. Nestler JE. Sex hormone-binding globulin: a marker for hyperinsulinemia/or insulin resistance. J Clin Endocrinol Metab 1993; 76: 273-4.
35. Jayagopal V, Kilpatrick ES, Jennings PE, Hepburn DA, Atkin SL. The biological variation of testosterone and sex hormone-binding globulin (SHBG) in polycistic ovarian syndrome: Implications for SHBG as a surrogate marker of insulin resistance. J Clin Endocrinol Metab 2003; 88: 1528-33.
36. Ehrmann DA, Barnes RB, Rosenfield RL. Hyperandrogenism, hirsutism, and the polycystic ovary syndrome. In: DeGroot LJ, Jameson JL. (eds) Endocrinology. 5th Ed. Philadelphia: Elsevier Saunders, 2006, ch 157, pp 2963-82.
Received: 14-09-2006
Accepted: 19-03-2007














