Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Medicina (Buenos Aires)
versión impresa ISSN 0025-7680versión On-line ISSN 1669-9106
Medicina (B. Aires) v.67 n.4 Buenos Aires jul./ago. 2007
Outcome of sporadic amyotrophic lateral sclerosis treated With non-invasive ventilation and riluzole
Martín Sívori1, Gabriel E. Rodríguez2, Daniel Pascansky1, César Séenz1, Roberto E. P. Sica2
1 Unidad Neumotisiología,
2 División Neurología, Hospital, José M. Ramos Mejía, Buenos Aires
Postal address: Dr. Gabriel Rodríguez, División Neurología, Hospital Ramos Mejía, Urquiza 609, 1221 Buenos Aires, Argentina Fax (54-11) 4127-0280 e-mail: gerodrig@intramed.net.ar
Abstract
Sporadic amyotrophic lateral sclerosis (sALS) is a progressive degenerative motor neuron disorder lacking specific treatment. Riluzole is the only drug able to modestly slow down the course of the disease. Respiratory insufficiency is the main cause of death; non invasive ventilation (NIV) has shown to improve survival. Our aim was to evaluate the effect of NIV and riluzole on survival. Ninety seven patients with a diagnosis of sALS were assessed and followed up for 60 months. Twenty nine patients received NIV and 68 did not (nNIV). Overall median survival In the NIV group was 15.41 ± 7.78 months vs. 10.88 ± 7.78 months in the nNIV group (p= 0.028). Median survival time was not different in patients receiving riluzole (n=44), as compared with those who did not (n=53), although at month 4th and 5th riluzole treated patients showed a modest benefit. In those who only received NIV (n=11) or only riluzole (n=26), survival time was 13.45 ± 13.44 months and 11.19 ± 7.79 months, respectively. Patients who received both NIV and riluzole (n=18) had a median survival time of 16.61 ± 10.97 months vs. 10.69 ± 7.86 months for those who received only supportive treatment (n=42) (p= 0.021). NIV improved survival in our series of patients. Riluzole did not show any significant impact on survival when employed as the only therapy. Patients receiving both treatments simultaneously had a significant longer survival.
Key words: Amyotrophic lateral sclerosis; Survival; Non invasive ventilation; Riluzole
Resumen
Sobrevida en pacientes con esclerosis lateral amiotrófica esporádica tratados con ventilación no invasiva y riluzole. La esclerosis lateral amiotrófica esporádica (sALS) es una enfermedad degenerativa para la que no existe tratamiento etiológico eficaz. El riluzole prolonga poco la sobrevida. La principal causa de muerte es la insuficiencia respiratoria. Uno de los tratamientos para esta última es la ventilación asistida no invasiva (NIV) con equipos de doble nivel de presión. El objetivo de este trabajo fue determinar el impacto en la sobrevida de estos enfermos combinando ventilación no invasiva y riluzole. Se evaluaron y siguieron durante 60 meses 97 pacientes con diagnóstico de sALS, según criterios definidos en El Escorial modificados, y fueron seguidos por 60 meses. Veintinueve pacientes recibieron NIV y 68 no (nNIV). En el grupo NIV la sobrevida media fue de 15.41 ± 7.78 meses vs. 10.88 ± 7.78 meses en nNIV (p= 0.028). La sobrevida media de los pacientes que recibieron riluzole (n=44) no fue diferente de la que no lo recibieron (n=53), aunque en el 4° y 5° mes los pacientes tratados con riluzole mostraron un escaso beneficio. Los pacientes que recibieron NIV y riluzole (n=18) tuvieron una sobrevida media de 16.61 ± 10.97 meses vs. 10.69 ± 7.86 meses para los que sólo recibieron tratamiento sintomático (n=42) (p= 0.021). La NIV prolongó significativamente la sobrevida en este grupo de pacientes. El riluzole, empleado como única terapéutica, no lo hizo. Los pacientes que combinaron los dos tratamientos tuvieron la mayor sobrevida.
Palabras clave: Esclerosis lateral amiotrófica;Sobrevida; Ventilación no invasiva; Riluzole.
Sporadic amyotrophic lateral sclerosis (sALS) is a fatal progressive degenerative motor neuron disorder, which combines upper and lower motor neurons symptoms1. Currently, there is no disease-specific therapy. Riluzole is the only approved drug which has been shown to slow down the course of the disease2. Symptomatic treatment includes non-invasive ventilation (NIV), which has been shown to prolong survival as well3, 4.
Ventilatory insufficiency in sALS is caused by respiratory muscle weakness, being the major cause of death (84%) in these patients5, 6. Dyspnea on exertion is the first respiratory symptom, eventually progressing to hypoventilation and leading to chronic respiratory failure7. Patients with sALS show a restriction pattern insufficiency on pulmonary function tests. Forced vital capacity is reduced. The most sensitive tests employed to assess global inspiratory and expiratory muscle strength are the static maximum pressures measured at the mouth8. Peak flow also appears to be useful to monitor expiratory muscle weakness9.
We hypothesized that non-invasive ventilatory support may prolong survival either in those patients receiving conventional treatment or in those others who, for different reasons, were not under the current pharmacological therapy employed for this disease. Therefore, the aim of our study was to evaluate survival in a series of sALS patients, assessing the efficacy of NIV and riluzole, when independently used, and combining both therapies. Also a group of patients receiving just supportive treatment was studied for comparison.
Materials and Methods
The study involved 97 patients with a diagnosis of sALS based on the El Escorial (modified at Arlie House) criteria10, 11 who were assessed at the Hospital J.M. Ramos Mejía División Neurología, Sector Enfermedades de Neurona Motora, from December 1999 to December 2004 (60 months). There were 62 men (63.9%). Average age was 54.2 ± 13.12 years, ranging between 22 and 78 years.
Patients were studied by a neurologist and a pneumonologist. The initial evaluation included clinical assessment, spirometry, assessment of maximum pressures measured at the mouth and measurement of arterial blood gases (pO2, pCO2, pH, HCO3, Sat O2). The evaluation was repeated every 3 months. The American Thoracic Society predictive spirometry values were used as the normal standards12, 13.
Criteria for submitting patients to NIV were based on the "Consenso Argentino para el diagnóstico y tratamiento de la esclerosis lateral amiotrofica"14 and international guidelines15: These included symptomatic ventilatory impairment (dyspnea, morning headache, fatigue) and one of the following: PaCO2 > 45 mm Hg or nocturnal oxygen saturation by pulse oximeter £ 88% for 5 continues minutes or maximal inspiratory pressure (PImax) < 60 cm H2O or forced vital capacity (FVC) < 50%. All the patients when fulfilling these criteria were offered NIV and sixty eight patients refused.
Those patients who acepted NIV were treated with non invasive positive-pressure ventilation (with bilevel ventilation), using a nasal mask. Inspiratory pressures were individually adjusted until O2 saturation was above 92% (positive inspiratory pressure of 13-25 cm H20 with expiratory pressures between 5-9 cm H20. Patients were admitted into the ward to start NIV, and caregivers were given instructions for its proper use. Riluzole was offered to all patients but only 44 acepted to employ it.
Statistical analysis
Data are expressed as means ± 1 standard deviation (SD). Survival time was estimated as the time elapsed since the diagnosis to death or december 2004 and was calculated by employing the Kaplan-Meier method (16). Comparisons between groups were calculated by the chi-square and ANOVA for continuum variables tests; p < 0.05 was considered statistically significant. All statistics were carried out using SPSS software version 11.1 (SPSS Inc. Chicago, Il).
Results
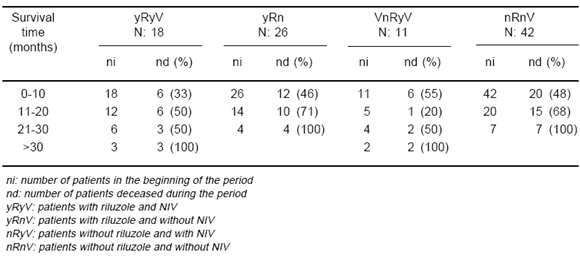
Eighteen patients received NIV and riluzole (yRyV). Twenty six received only riluzole (yRnV), 11 received only NIV (nRyV) and 42 did not receive either riluzole or NIV (nRnV). This last group received only supportive care (Table 1).
TABLE 1.- Clinical characteristics of patients

The average survival times for each group are shown in Tables 2 while Kaplan Meier survival curves are depicted in Fig. 1 (Table 3). The largest significant difference in the average survival time was found when comparing groups yRyV and nRnV (yRyV= 16.61± 10.97; nRnV= 10.69 ± 7.86, p= 0.021) favouring the yRyV group.
TABLE 2.- Overall median survival for different modalities of treatment


Fig. 1.- Impact of NIV and riluzole on survival time. yRyV: patients with riluzole and NIV yRnV: patients with riluzole and without NIV nRyV: patients without riluzole and with NIV nRnV: patients without riluzole and without NIV
TABLE 3.- Impact of NIV and riluzole on survival time
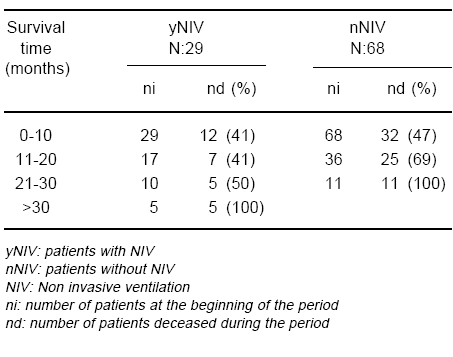
Twenty nine patients were submitted to NIV (yNIV), while 68 were not (nNIV); independently of any associated pharmacological therapy, the average survival was 15.41 ± 11.83 months for the yNIV group and 10.88 ± 7.78 months for the other (nNIV) (p= 0.028). Statistical significant difference (p= 0.045) between groups appeared at month 16th along the follow-up (Fig. 2, Table 4).

Fig. 2.- Impact of non invasive ventilation on survival. yNIV: patients with NIV nNIV: patients without NIV
TABLE 4.- Impact of non invasive ventilation on survival
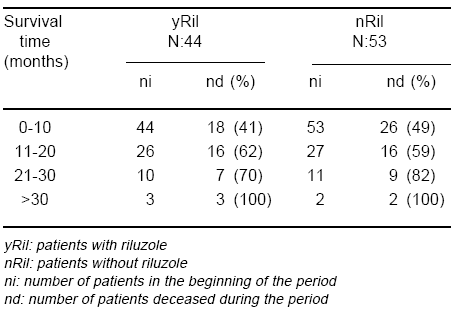
Forty four patients were given riluzole (yRil) and 53 were not (nRil), independently of NIV use. The average survival was 13.41 ± 9.49 months for the yRil group and 11.26 ± 9.2 months for the other (nRil). A weak statistical significant difference (p= 0.013), favouring yRil, was found at months 4th and 5th (Fig. 3, Table 5).

Fig. 3.- Impact of riluzole on survival. yRil: patients with riluzole nRil: patients without riluzole
TABLE 5.- Impact of riluzole on survival
Discussion
In our sALS patients cohort, the use of NIV was associated with longer survival, a finding which agrees with previous reports in the literature3, 4. When the patients were divided into two groups, yNIV and nNIV, independently of any other associated treatment, survival time was 15.41 ± 11.83 months for the yNIV group, while for the nNIV group mean survival was 10.88 ± 7.78 (p= 0.028). This significant difference appeared at month 16th after diagnosis, and was not related to the eventual pharmacological treatment received by the patients.
Riluzole, when employed as the only therapy, showed only a modest beneficial effect on survival at month 4th and 5th. This observation is partially at variance with the findings of Lacomblez and Bensimon17, 18 who found that the major effect of the drug was observed after month 18th. This disagreement may be due to differences in the studied populations.
For a more detailed analysis we divided the patients in four groups: yRyV, yRnV, nRyV and nRnV. We observed that patients who received both riluzole and NIV had the longest median survival time when compared with those others who received only one modality of treatment or were only symptomatically treated. This findings was not reported before.
Therefore, NIV, a usually well tolerated procedure, may substantially prolong survival in sALS patients, either when employed as the only treatment modality or when combined with pharmacological therapy, being this last strategy the most convenient medical attitude.
References
1. Dubrovsky A, Sica REP. Esclerosis lateral amiotrófica. Definición y criterios diagnósticos. En Sica REP, Dubrovsky A, eds. Esclerosis lateral amiotrófica y enfermedades relacionadas. Buenos Aires: Científica Interamericana, 2001, p. 27-36.
2. Miller RG; Mitchell JD; Lyon M; Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev 2002; (2): CD001447.
3. Pinto AC, Evangelista T, Carvalho M, Alves MA, Sales ML. Respiratory assistance with a noninvasive ventilator (BiPAP in MND/ALS) patients: survival rates in controlled trial. J Neurol Sci 1995; 129: 19-26.
4. Aboussouan LS, Khan SU, Meeker DP, Stelmach K, Mitsumoto H. Effect of noninvasive positive pressure ventilation on survival in amyotrophic lateral sclerosis. Ann Int Med 1997; 127: 450-3.
5. Gay P, Westbrook P, Daube JR, Litchy WJ, Windebank AJ, Iverson R. Effects of alterations in pulmonary function and sleep variables on survival in patients with Amyotrophic Lateral Sclerosis . Mayo Clin Proc 1991; 66: 686-94.
6. Caroscio TJ, Mulvihill MN, Sterling R, Abrams B. Amyotrophic Lateral Sclerosis: its natural history. Neurol Clin 1987; 5: 1-8.
7. Bach J. Amyotrophic Lateral Sclerosis: predictors for prolongation of life by noninvasive respiratory aids . Arch Phys Med Rehabil 1995; 76: 828-32.
8. De Vito E, Suárez A. Compromiso respiratorio en la esclerosis lateral amiotrófica. En Sica REP, Dubrovsky A, eds. Esclerosis lateral amiotrófica y enfermedades relacionadas. Buenos Aires: Científica Interamericana, 2001, p 215-77.
9. Suárez AA, Pessolano FA, Monteiro SG, et al. Peak flow and peak cough flow in the evaluation of expiratory muscle weakness and bulbar impairment in patients with neuromuscular disease. Am J Phys Med Rehabil. 2002; 81: 506-11.
10. World Federation of Neurology Research Group on Neuromuscular Diseases. El Escorial World Federation of Neurology Criteria for the diagnosis of Amyotrophic Lateral Sclerosis. J Neurol Sci 1994; 124S: 96-107.
11. Brooks B, Miller G, Swash M, Munsat T. for the World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial Revisited: Revised Criteria for the Diagnosis of Amyotrophic lateral Sclerosis. (A Consensus Conference held at Airlie House, Warrenton, Virginia, April 2-4, 1998). Amyotroph Lateral Scler Other Motor Neuron Disord 2000; 1: 293-9.
12. American Thoracic Society. Standardization of Spirometry. 1994 Update. Am. J Respir Crit Care Med 1995; 152: 1107-1136.
13. Black LF, Hyatt RE. Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis 1969; 99: 696-702.
14. Dubrosky A, Sica R, Aguilera N, et al. Consenso Argentino para el Diagnóstico y Tratamiento de la Esclerosis Lateral Amiotrófica. Rev Neurol Arg 2001; 26: 93-101.
15. Clinical indications for the noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation: a consensus report . Chest 1999; 116: 521-34.
16. Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. Journal of the American Statiscal Association 1958; 53: 437-81.
17. Bensimon G, LaComblez L, Meininger V, and the ALS/ Riluzole study group. A controlled trial of riluzole in amyotrophic lateral sclerosis. N Engl J Med 1994; 330: 585-91.
18. Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V, and the Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Lancet 1996; 347: 1425-31. [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ]
Received: 21-09-2006
Accepted: 7-03-2007














