Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Medicina (Buenos Aires)
versión impresa ISSN 0025-7680versión On-line ISSN 1669-9106
Medicina (B. Aires) v.67 n.5 Buenos Aires sep./oct. 2007
Dual renin-angiotensin system blockade plus oral methylprednisone for the treatment of proteinuria in IgA nephropathy
Hernán Trimarchi1, Alexis Muryan2, Pablo Young3, Mariano Forrester1, Alejandro Iotti4, Horacio Pereyra1, Fernando Lombi1, Omar Seminario1, Mirta Alonso2, Roberto Iotti4
1Division of Nephrology;
2Division of Biochemistry;
3Division of Internal Medicine;
4Division of Pathology, Department of Medicine, Hospital Británico, Buenos Aires
Postal address: Dr. Hernán Trimarchi, Hospital Británico, Perdriel 74, 1280 Buenos Aires, Argentina. Fax: (54-11) 4304- 3393 e-mail: htrimarchi@hotmail.com
Abstract
Renin-angiotensin system inhibition is a widely accepted approach to initially deal with proteinuria in IgA nephropathy, while the role of immunosuppressants remains controversial in many instances. A prospective, uncontrolled, open-label trial was undertaken in patients with biopsy-proven IgA nephropathy with proteinuria > 0.5 g/day and normal renal function to assess the efficacy of a combination treatment of angiotensin converting enzyme inhibitors plus angiotensin receptor blockers enalapril valsartan coupled with methylprednisone to decrease proteinuria to levels below 0.5 g/day. Twenty patients were included: Age 37.45 ± 13.26 years (50% male); 7 patients (35%) were hypertensive; proteinuria 2.2 ± 1.86 g/day; serum creatinine 1.07 ± 0.29 mg/dl; mean follow-up 60.10 ± 31.47 months. IgA nephropathy was subclassified according to Haas criteria. Twelve patients (60%) were class II; seven (35%) were class III and one (5%) class V. All patients received dual reninangiotensin system blockade as tolerated. Oral methylprednisone was started at 0.5 mg/kg/day for the initial 8 weeks and subsequently tapered bi-weekly until the maintenance dose of 4 mg was reached. Oral steroids were discontinued after 24 weeks (6 months) of therapy but renin-angiotensin inhibition remained unchanged. At 10 weeks of therapy proteinuria decreased to 0.15 ± 0.07 g/day (P < 0.001) while serum creatinine did not vary: 1.07 ± 0.28 mg/dl (P=ns). After a mean follow-up of 42.36 ± 21.56 months urinary protein excretion (0.12 ± 0.06 g/day) and renal function (serum creatinine 1.06 ± 0.27 mg/dl) remained stable. No major side effects were reported during the study. Renin-angiotensin blockade plus oral steroids proved useful to significantly decrease proteinuria to < 0.5 g/day in patients with IgA nephropathy without changes in renal function.
Key words: IgA nephropathy; Corticosteroids; Renin-angiotensin system; Enalapril; Valsartan; Proteinuria; Glomerulonephritis
Resumen
Doble bloqueo del sistema renina-angiotensina más metilprednisona oral para el tratamiento de la proteinuria en la nefropatía por IgA. El doble bloqueo del sistema renina-angiotensina con inhibidores de la enzima convertidora de angiotensina junto a bloqueadores del receptor tipo I de angiotensina II es aceptado como tratamiento en la proteinuria de la nefropatía por IgA, ya que el rol de los inmunosupresores continúa siendo controvertido. Estudio prospectivo, no controlado, abierto para pacientes con nefropatía por IgA con proteinurias >0.5 g/día y creatininas séricas <1.4 mg/dl, para evaluar la eficacia de tratamiento de enalapril más valsartán asociado a metilprednisona vía oral para disminuir las proteinurias a <0.5 g/día. Fueron incluidos 20 pacientes: Edad: 37.45 ± 13.3 años (50% hombres); 7 pacientes (35%) eran hipertensos; proteinuria inicial 2.2 ± 1.86 g/día; creatinina inicial 1.07 ± 0.29 mg/dl; seguimiento promedio: 60.10 ± 31.47 meses (5 ± 2.62 años). La nefropatía por IgA fue subclasificada según Haas: 12 pacientes (60%) clase II; 7 (35%) clase III y 1 (5%) clase V. Todos recibieron enalapril más valsartán según tolerancia más metilprednisona vía oral en dosis de 0.5 mg/kg/día durante las primeras 8 semanas y subsecuentemente se redujo cada dos semanas hasta llegar a 4 mg. Se discontinuaron los esteroides luego de 24 semanas (6 meses). La inhibición del sistema renina angiotensina prosiguió indefinidamente. A las 10 semanas la proteinuria disminuyó de 2.2 ± 1.86 g/día a 0.15 ± 0.7 g/día (p<0.001); la creatinina no varió significativamente (1.07 ± 0.29 mg/dl vs. 1.07 ± 0.28 mg/dl) (P=ns). Luego de 10 semanas y con un seguimiento de 42.36 ± 21.56 meses la proteinuria (0.12 ± 0.006 g/día) y la función renal (creatinina 1.06 ± 0.27mg/dl) permanecieron estables. No se informaron efectos adversos durante el estudio. El doble bloqueo del sistema renina angiotensina más bajas dosis de metilprednisona resultó útil para reducir rápidamente la proteinuria a <0.5 g/día en la nefropatía por IgA sin deterioro en la función renal. Si bien la administración de esteroides fue por sólo 6 meses, la proteinuria permaneció <0.5 g/día durante todo el seguimiento (42.36 ± 21.56 meses).
Palabras clave: Nefropatía por IgA; Corticosteroides; Sistema renina-angiotensina; Enalapril; Valsartan; Proteinuria; Glomerulonefritis
IgA nephropathy (IgAN) is the commonest glomerulopathy worldwide. Its clinical course can be quite varied, ranging from chronic isolated microscopic hematuria to nephrotic range proteinuria or nephritic rapidly progressive glomerulonephritis1. Moreover, IgAN is an important cause of progressive kidney disease with nearly 30% of patients developing end-stage renal disease within 20 years of diagnosis2 . Patients at greatest risk of progressive renal impairment are those with hypertension, persistent proteinuria above 0.5 to 1 g/day, and reduced glomerular filtration rate at diagnosis3-9. An initial approach to treat one of these risk factors, proteinuria, is to retard renal failure progression by the dual blockade of the reninangiotensin system with angiotensin-converting enzyme inhibitors and angiotensin type I receptor blockers, to reach blood pressures of approximately 125/75 mm Hg. However, this pharmacological strategy, albeit useful, may not be well tolerated by patients due to hemodynamical symptoms, mainly hypotension. In addition, proteinuria levels may not be completely managed regardless of a satisfactory blood pressure control. Finally, the pathophysiological mechanisms involved in the genesis of proteinuria in IgA nephropathy may be quite diverse, involving hemodynamic and immunologically-mediated factors, all eventually producing structural changes such as loss of negative charges in the glomerular basement membrane, pore size and number or sclerosis6, 10. Focusing therapy strategies only on the hemodynamically-mediated proteinuric mechanisms (mainly the renin-angiotensinsystem) may not be sufficient particularly in the long term, rendering immunological mechanisms untreated. Thus, it is not surprising that a single therapeutic treatment has not yet been established. In this regard, we decided to undertake a prospective, uncontrolled, open-label trial in 20 IgAN patients with urinary protein excretions > 0.5 g/ day to assess the effects of the renin-angiotensin axis inhibition coupled with a six-month course of oral steroids on proteinuria, and its efficacy to reach a protein excretion < 0.5 g/day.
Materials and Methods
Between January 1996 and December 2004, 20 patients with biopsy-proven IgAN and a daily urinary protein excretion > 0.5g were enrolled in this prospective trial at the Nephrology Section of the Hospital Británico, Buenos Aires.
Twenty adult Caucasian patients were included in this trial (Table 1). Inclusion criteria consisted of biopsy proven IgAN with protein excretions > 0.5 g/day. Ten patients (50%) were male and 8 patients (40%) were hypertensive. Initial protein excretions ranged between 0.8 and 9 g/day, initial albumin levels: 3.8 ± 0.41 g/dl. Two patients (10%) had nephrotic syndrome. Mean follow-up: 60.1 ± 31.47 months.
TABLE 1.- Patient characteristics

Proteinuria was measured after 24-hour urine collection. Initial protein excretion: 2.2 ± 1.86 g/day. Renal function was assessed by serum creatinine blood levels. Baseline serum creatinine: 1.07 ± 0.29 mg/dl. Hematuria was quantified using light microscopy and considered abnormal if > 5 red blood cells per high power field. Mean red blood cell count per high power field: 25.4 ± 15.0 cells. In all patients a renal ultrasound was performed, being normal in all cases. Other causes of chronic renal disease other than hypertension were not present in the patients included in the trial. A history of previous or concomitant malignancy was an exclusion criteria. Patients on antihypertensive drugs were also excluded.
Study design
Visit 1: The patient was evaluated at the nephrology clinic due to dysmorphic hematuria and proteinuria.
Visit 2: Blood and urinary results and renal sonography were analyzed.
Visit 3: A CT scan-guided percutaneous renal biopsy was performed.
Visit 4: The diagnosis of IgAN was confirmed by biopsy and dual blockade of the renin-angiotensin system plus steroids were started (day 0).
Patients were later followed up once a month until week 24 and subsequently followed every other month to check blood pressure, renal function and 24-hour urinary protein excretions. At the beginning of therapy, oral methylprednisone was started on day 0 at 0.5 mg/kg/day for the initial 8 weeks and subsequently tapered bi-weekly to 20 mg/day (tenth week), 14 mg/day (twelveth week), 10 mg/day (fourteenth week), 8 mg/day (sixteenth week) until the maintenance dose of 4 mg was reached on week eighteenth. Thereafter patients were kept on such steroid dose until week twenty-fourth, when finally discontinued. Dual renin-angiotensin system blockade consisted of enalapril (Renitec®, Merck, Sharpe & Dohmme, Whitehouse Station, New Jersey, USA) and valsartan (Diovan®, Novartis, Basel Switzerland) prescription to reach systolic blood pressures between 140 mm Hg and 110 mm Hg and diastolic blood pressures between 90 mmHg and 70 mm Hg as tolerated (Table 2). The combined therapy was prescribed as follows: Normotensive patients were initiated on day 0 on enalapril 2.5 mg/day and increased up to 5 mg/day one week later. If the patient referred no complaints, valsartan 80 mg/day was added to the regimen. If the patient's systolic blood pressure was < 100 mm Hg or hypotensive symptoms emerged, enalapril dose was diminished to 2.5 mg/day. Hypertensive patients were all treated with enalapril 10 mg/day and valsartan 80 mg/day was added one week later.
TABLE 2.- Subgroups results according to Haas histologic stages

Patients were classified as hypertensive if the 4 officemeasured blood pressure controls during the enrollment phase visits were in average > 140/90 mm Hg. All other patients were considered normotensive. Seven patients were hypertensive. Four patients belonged to Stage I and three patients had Stage II hypertension. These patients had no previous history of high blood pressure and therefore were not receiving antihypertensive medication. Hypertension appeared to have arisen secondary to the glomerular disorder.
All patients received calcium carbonate 1250 mg/day plus 0.25 mg 1,25-dihydroxy cholecalcipherol during the whole trial.
Histologic classification
Patient characteristics were later grouped according to IgAN Haas classification (6) (Table 2). According to Haas classification, 12 patients belonged to class II, 7 to class III and I to class V. The two patients with nephrotic syndrome were female and belonged to Class III (serum albumin 3.0 g/dl; protenuria 9 g/day) and Class V (serum albumin 3.1 g/dl; proteinuria 5.2 g/dl).
Bioethics
The institutional Teaching and Research Committee was informed about the protocol and the final results were reported to it. All patients gave informed consent to have their results included in the present manuscript.
Statistical analysis
Results are expressed as the mean ± SD. Wilcoxon signed rank test was employed to assess treatment differences; p values of < 0.05 were considered to be significant.
Results
Proteinuria
Results are depicted in Tables 3 and 4. As mentioned before, all patients were on blockade of the renin-angiotensin system. At ten weeks of steroid therapy proteinuria decreased from 2.2 ± 1.86 g/day to 0.15 ± 0.07 g/day (P< 0.001). Methylprednisone dose was gradually decreased until stopped at week 24, but urinary protein excretion still remained low and non-statistically different from the one achieved at week 10: proteinuria 0.12 ± 0.06 g/day.
TABLE 3.- General final results

TABLE 4.- Evolution of proteinuria according to Haas stages
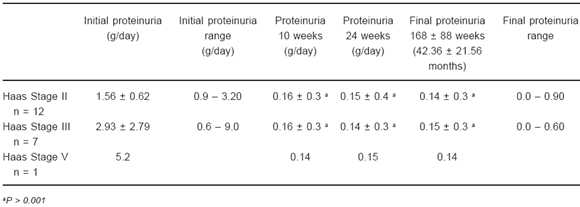
Renal function
As shown in Table 3, initial serum creatinine was 1.07 ± 0.29 mg/dl and did not show significant changes during the whole study: At week 10 serum creatinine was 1.07 ± 0.28 mg/dl and at week 24 it was 1.06 ± 0.27 mg/dl.
Side effects
Renin-angiotensin blockade
No significant side effects were reported during the study that would have derived in the interruption of treatment. Initial monotherapy with enalapril was well-tolerated in all patients. Cough episodes were not reported. When valsartan was added, 3 normotensive female patients (15%) referred headaches and hypotension-related symptoms. In these cases, enalapril was decreased to 2.5 mg/ day but valsartan was continued and symptoms resolved.
Methylprednisone
No major adverse events were reported. Two patients (10%) referred flu-like symptoms during the initial phase of the study, and symptomatic medicaction was transiently prescribed. Steroid-related symptoms consisted on mild cushingoid fascies in 6 cases (30%), facial and trunk acne in 8 patients (40%). Body weight showed no significant changes during the trial: Initial and final weights were 72.47 ± 6.59 kg and 75.62 ± 3.45 kg, P=ns. Patients were encouraged to practice physical exercise. No specific recommendations were made with respect to the diet, other than to keep a salt intake of approximately 3 grams per day.
Blood pressure control
The aim of the study in this respect was to reach systolic blood pressures between 140 mm Hg and 110 mm Hg and diastolic blood pressures between 90 mm Hg and 70 mm Hg (Table 2). All patients completed the dual blockade strategy. Normotensive patients received enalapril 5 mg/day plus valsartan 80 mg/day. Three female patients received half enalapril dose plus valsartan 80 mg/day due to hypotension. Initial and final blood pressure measurements were not different: initial 114 ± 34/76 ± 6 mm Hg vs final 108 ± 16/70 ± 6 mm Hg, P=ns. Hypertensive patients were all treated with enalapril 10 mg/day plus valsartan 80 mg/day. Initial blood pressure was 154 ± 26/96 ± 4 mm Hg and final 122 ± 8/82 ± 6 mm Hg. All hypertensive patients tolerated this pharmacological treatment.
Discussion
IgAN is defined by the predominant deposition of IgA in the glomerular mesangium, and is the commonest pattern of idiopathic glomerulonephritis in all countries where renal biopsy is widely practised2. IgAN is also an important cause of progressive kidney disease with 25-30% of patients developing end-stage renal disease within 20 years of diagnosis2,7. Therefore, effective therapies are mandatory to control the progression of this entity.
Clinical, histologic and perhaps genetic factors are all determinants of the likelihood of developing progressive disease. Clinical predictors include elevated serum creatinine concentration at diagnosis, hypertension, and/or persistent protein excretion above 0.5 to 1 g/day3-9. The relationship between increasing proteinuria and a worse prognosis is in part probably a refection of proteinuria being a marker for the severity of glomerular disease. In IgAN, prominent IgA-containing deposits accumulate in the mesangium. Mesangial deposits have access to the systemic circulation, and complement-containing complexes can attract neutrophils, monocytes, and macrophages, leading to a potentially marked inflammatory response1,11. Locally released cytokines and autacoids, including TNF-alpha, TGF-beta, IL-1 and IL-6 among others upregulate the expression of adhesion molecules both on the endothelium and on circulating inflammatory cells, resulting in a florid inflammatory process11,12. In addition, hemodynamic factors also influence the ultrafiltration of macromolecules through the glomerulus. Vasoactive hormones such as angiotensin II and norepinephrine can also cause capillary hypertension increasing proteinuria in IgAN, probably by stimulation of TGF-beta excretion13,14.
Therefore, the rationale for using a combined therapy is to use steroids in order to suppress the triggered immunologic cascade that leads to proteinuria via cytokines and growth factors, and a dual blockade of the renin-angiotensin system with angiotensin converting enzyme inhibitors plus angiotensin II receptor blockers to modulate the hemodynamic components of hyperfiltration and glomerular hypertension that exist in IgAN. Whilst there is still no successfull approach capable of modifying mesangial deposition of IgA, which may initiate the hemodynamic and inflammatory complications of IgAN at the glomerular level, many strategies have been assessed to control these factors. A number of studies have provided evidence that angiotensin converting enzyme inhibitors plus angiotensin receptor blockers may be more effective than other antihypertensive drugs in slowing the progressive decline in glomerular filtration rate in IgAN by producing a further antiproteinuric effect15,16. The clinical observation that although proteinuria may be controlled with such therapy but immediate relapses occur after discontinuation of these antihypertensive drugs, strengthens our hypothesis that the dual blockade of the reninangiotensin system may be able to control hemodynamic variables and to stop the release of inflammatory cytokines, but within certain limits13,15,16. In the data available, the degree of proteinuria in IgAN is generally partially controlled with the combination of an angiotensin converting enzyme inhibitor plus an angiotensin receptor blocker, reaching a reduction in proteinuria between 30 and 70%15,16.
Steroids are drugs capable of abrogating the genesis of the inflammatory cascade, helping to achieve a longerlasting antiproteinuric effect as in the present study. Moreover, the percentage of proteinuria reduction we achieved was virtually 100%. Therefore, a role for immunosuppressants for the treatment of proteinuria in IgAN must be seriously considered, due to the fact that prolonged proteinuria, regardless of its amount causes chronic increased tubular protein reabsorption with secondary tubular dysfunction and damage and irreversible interstitial scarring and sclerosis, culminating in renal insufficiency17. In all the studies in which the dual reninangiotensin system was blocked, the amount of proteinuria achieved has never been as low as in our study. We believe steroids are responsible for such significant decrease in proteinuria15,16.
Steroids have been employed to treat proteinuria in IgA, and have provided dramatic remissions of the nephrotic syndrome in patients with normal appearing glomeruli18. Corticosteroid therapy for 18 to 36 months may be associated with less proteinuria and perhaps a better outcome. It should be noted, however, that these studies were performed prior to the current widespread use of aggresive antihypertensive and antiproteinuric therapy blocking the renin-angiotensin system to slow the rate of progression of proteinuric chronic renal disease19-21. In other instances, steroids have given mixed results. Most of the studies are not prospective, randomized, or controlled, and have failed to show conclusive results. One randomized controlled trial by Katafuchi et al randomized 90 IgAN patients with normal renal function to receive either oral prednisone or placebo over 2 years and although was less in the steroid group, renal survival was identical (85%) at 84 months21. On the other hand, Pozzi et al randomized IgAN patients with serum creatinine < 1.5 mg/day and 1-3.5 g/day proteinuria to receive six cycles of pulse methylprednisolone at the beginning of each month followed by alternate day oral steroids vs. placebo (1 g pulse of methylprednisolone at the beginning of months 1, 3, and 5 while continously treating with oral prednisone 0.5 mg/kg every other day for 6 months). After 6 years of followed-up renal survival was better in the steroid group than in the placebo group (97 vs 53%)22. More recently, Pozzi et al analyzed these long-term results and showed that the benefits persisted for at least 10 years. Most of the subjects in this trial also received angiotensin II inhibition23. As in our trial, the short course of treatment led to few side effects and a prolonged time without relapses of proteinuria or worsening of renal function. A recent meta-analysis supports the use of corticosteroids in reducing proteinuria and preventing progression to endstage renal disease24.
This prospective uncontrolled open-label trial suggests that the combined blockade of the renin-angiotensin axis plus a six-month course with an initiating relatively low dose of 0.5 mg/kg of oral methylprednisone may be useful to reduce proteinuria in patients with IgAN to levels below 0.5 g/day. Pulses of steroids may not be necessary in patients with relatively normal renal function. Blood pressure control was successfully achieved with the dual blockade of the renin-angiotensin system and could have probably contributed to the decrease in urinary protein excretion, albeit previous studies demonstrate that the antiproteinuric effect of the dual blockade of the renin angiotensin system is independent of blood pressure control15,16. In addition, proteinuria remained negative for a prolonged period of time after steroids were discontinued. However, results must be interpreted with caution. The present study is uncontrolled. The number of patients included is small the amount of daily urinary protein excretions ranged from severely nephrotic to a mild level. Only one patient had nephrotic range proteinuria. Moreover, only patients with serum creatinine ≤ 1.8 mg/dl have been recruited; although most patients were normotensive, a small subset of individuals were hypertensive and not all normotensive patients tolerated the antihypertensive medication in the same manner. Finally, the followup time frame was quite diverse, ranging from 10 years to 1 year. However, in the present study the combination of parallel hemodynamic and immunologic strategies to treat proteinuria is complementary and proves to be useful to correct proteinuria and to achieve or maintain normotension at 10 weeks of treatment.
References
1. Barratt J, Feehally J. IgA nephropathy. J Am Soc Nephrol 2005: 16: 2088-97.
2. Barratt J, Feehally J. Treatment of IgA nephropathy. Kidney Int 2006; 69: 1934-8.
3. D'Amico G. Influence of clinical and histological features on actuarial renal survival in adult patients with idiopathic IgA nephropathy, membranous nephropathy, and membranoproliferative glomerulonephritis: Survey of the recent literature. Am J Kidney Dis 1992; 20: 315-22.
4. Alamartine E, Sabatier JC, Guerin C, et al. Prognostic factors in mesangial IgA glomerulonephritis: An extensive study with univariate and multivariate analyses. Am J Kidney Dis 1991; 18: 12-7.
5. Johnston PA, Brown JS, Braumholtz DA, et al. Clinicopathological correlations and long-term follow-up in 253 United Kingdom patients with IgA nephropathy: A report from the MRC glomerulonephritis registry. Q J Med 1992; 84: 619-24.
6. Haas M. Histologic subclassification of IgA nephropathy: A clinicopathological study of 244 cases. Am J Kidney Dis 1997; 29: 829-42.
7. D'Amico G. Natural history of idiopathic IgA nephropathy: Role of clinical and histological prognostic factors. Am J Kidney Dis 2000; 36: 227-37.
8. Bartosik LP, Lajoie G, Sugar L, et al. Predicting progression in IgA nephropathy. Am J Kidney Dis 2001; 38: 728-35.
9. Hall CL, Bradley R, Kerr A, et al. Clinical value of renal biopsy in patients with asymptomatic microscopic hematuria with and without low-grade proteinuria. Clin Nephrol 2004; 62: 267-72.
10. Hass M. IgA Nephropathy. Semin Nephrol 2004; 24: 177-89.
11. Lim ChS, Yoon HJ, Kim YS, et al. Clinicopathological correlation of intrarenal cytokines and chemokines in IgA nephropathy. Nephrology 2003; 8: 21-7.
12. Mattii L, Segnani C, Cupisti A, et al. Kidney expression of RhoA, TGF-beta1, and fibronectin in human IgA nephropathy. Nephron Exp Nephrol 2005; 101: e16-e23.
13. Song JH, Lee SW, Suh JH, et al. The effects of dual blockade of the renin-angiotensin system on urinary protein and transforming growth factor-beta excretion in 2 groups of patients with IgA nephropathy. Clin Nephrol 2003; 60: 318-26.
14. Del Prete D, Gambaro G, Lupo A, et al. Precocious activation of genes of the renin-angiotensin system and the fibrogenic cascade in IgA glomerulonephritis. Kidney Int 2003; 64: 149-59.
15. Russo D, Pisani A, Balletta MM, et al. Additive antiproteinuric effect of converting enzyme inhibitor and losartan in normotensive patients with IgA nephropathy. Am J Kidney Dis 1999; 33: 851-56.
16. Russo D, Minutolo R, Pisani A, et al. Coadministration of losartan and enalapril exerts additive antiproteinuric effect in IgA nephropathy. Am J Kidney Dis 2001; 38: 18- 25.
17. Kaysen GA, Myers BD, Couser WB, et al. Mechanisms and consequences of proteinuria. Lab Invest 1986; 54: 479-98.
18. Appel GB, Waldman M. The IgA nephropathy dilemma. Kidney Int 2006; 69: 1939-44.
19. Yoshikawa N, Ito H, Sakai T, et al. A controlled trial of combined therapy for newly diagnosed severe childhood IgA nephropathy. J Am Soc Nephrol 1999; 10: 101-9.
20. Kobayashi Y, Hiki Y, Fujii K, et al. Moderately proteinuric IgA nephropathy: Prognostic prediction of individual clinical courses and steroid therapy in progressive cases. Nephron 1989; 53: 250-6.
21. Katafuchi R, Ikeda K, Mizumasa T, et al. Controlled, prospective trial of steroid treatment in IgA nephropathy: A limitation of low-dose prednisone therapy. Am J Kidney Dis 2003; 41: 972-83.
22. Pozzi C, Bolasco PG, Fogazzi GB, et al. Corticosteroids in IgA nephropathy: A randomized controlled trial. Lancet 1999; 353: 883-7.
23. Pozzi C, Andrulli S, del Vecchio L, et al. Corticosteroid effectiveness in IgA nephropathy: Long-term results of a randomized controlled trial. J Am Soc Nephrol 2004; 15: 157-63.
24. Samuels JA, Strippolo GF, Craig JC, et al. Immunosuppressive treatments for IgA nephropathy: a metaanalysis of randomized controlled trials. Nephrology 2004; 9: 177-85. [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ]
Received: 19-03-2007
Accepted: 6-07-2007














