Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Medicina (Buenos Aires)
versión impresa ISSN 0025-7680versión On-line ISSN 1669-9106
Medicina (B. Aires) v.69 n.1 Ciudad Autónoma de Buenos Aires ene./feb. 2009
ORIGINAL ARTICLE
Protocol for thyroid remnant ablation after recombinant TSH in thyroid carcinoma
Fabián Pitoia1, Elías El Tamer2, María Eugenia Salvai1, Hugo Niepomniszcze1
1División de Endocrinología,
2Centro de Medicina Nuclear - UBA-CNEA, Hospital de Clínicas José de San Martín, Facultad de Medicina, Universidad de Buenos Aires
Postal address: Dr. Fabián Pitoia, Esmeralda 961, 1007 Buenos Aires, Argentina Fax: (54-11) 43129891 e-mail: fpitoia@intramed.net
Abstract
In some countries, in order to perform rhTSH-aided thyroid remnant ablation (TRA) after surgery, it is generally necessary to confirm that thyroidectomy has been almost complete. Otherwise, the nuclear medicine specialist will not administer a high radioiodine dose because it might be hazardous due to the possibility of thyroid remnant actinic thyroiditis. Considering this, it would be necessary to use two rhTSH kits (one for diagnostic purposes and the other one to administer the 131I dose). In this study, we used an alternative protocol for TRA with the use of one kit of rhTSH in twenty patients diagnosed with low risk papillary thyroid carcinoma. All patients had negative titers of anti-thyroglobulin antibodies. Successful thyroid remnant ablation was confirmed with an undetectable rhTSH stimulated thyroglobulin level (< 1 ng/ml) in all 20 patients between 8 to 12 months after radioiodine administration. The use of this protocol combining scintigraphy with the subsequent administration of a therapeutic dose following the administration of one kit of rhTSH would avoid the need of using 2 kits to perform the ablation and would decrease the costs associated with its use while significantly enhancing the quality of life of patients with thyroid cancer.
Key words: Recombinant human TSH; Cancer; Thyroid; Ablation
Resumen
Protocolo para ablación de remanentes tiroideos luego de TSH recombinante en el cáncer diferenciado de tiroides. En algunos países, para realizar la ablación de los remanentes tiroideos con radioyodo después de la cirugía, generalmente se requiere confirmar que la tiroidectomía fue casi completa, ya que de otra manera el especialista en medicina nuclear no administrará una dosis elevada de radioyodo, considerando que esto puede ser dañino para el paciente debido a la posibilidad de generar una tiroiditis actínica. De acuerdo con esto, sería necesario administrar 2 kits de rhTSH (uno para diagnóstico y otro para la dosis de radioyodo). En este estudio, empleamos un protocolo alternativo para la ablación luego de la administración de un único kit (2 ampollas) de rhTSH en 20 pacientes con antecedentes de un carcinoma papilar de bajo riesgo. Todos los pacientes presentaban títulos negativos de anticuerpos anti-tiroglobulina. La ablación exitosa de remanente tiroideo se confirmó con un nivel no detectable de tiroglobulina (<1 ng/ml) al estímulo por rhTSH en los 20 pacientes, entre 8 a 12 meses luego de la ablación. El uso de este protocolo que combina la posibilidad de realizar un centellograma diagnóstico y la ablación luego del uso de un solo kit de rhTSH, facilita su empleo, disminuye los costos asociados, a la vez que permite una mejor calidad de vida de los pacientes con cáncer de tiroides.
Palabras clave: TSH recombinante; Cáncer; Tiroides; Ablación
Differentiated thyroid carcinoma (DTC), which includes the papillary and follicular subtypes, shows an excellent prognosis after initial treatment which usually includes total thyroidectomy and radioiodine ablation of post-surgical thyroid remnants1. For the administration of radioiodine it is necessary to obtain the appropriate uptake level after surgery by the remnant thyroidal tissue, whether cancerous or not. To achieve this, the thyroid hormone suppression therapy (THST) must not be started after surgery, or it is necessary to withdraw thyroid hormone. The discontinuation or the delay in initiation of the thyroid hormone therapy after the total thyroidectomy brings about the progressive increase of the TSH endogenous levels. Most investigators consider that serum TSH levels needed to obtain this stimulus of the thyroid tissue is achieved 4 to 6 weeks after the discontinuation of THST (TSH levels higher than 25-30 mUI/l)2, 3.
The thyroid hormone withdrawal may be associated with signs and symptoms of severe hypothyroidism, which are generally poorly tolerated. The use of rhTSH prevents the discontinuation of the THST, while it allows an efficient follow-up of the DTC patients.
Due to the drug availability, between 1997 and 2007 more than 30 centers around the world reported over 400 DTC patients who were administered rhTSH before a radioiodine dose for the ablation of normal remnants or for the treatment of local or metastatic disease. Luster et al4 analyzed and summarized the data submitted so far in relation to the use of rhTSH as adjunctive therapy for DTC. Recently, rhTSH was approved by the Food and Drug Administration in the US for thyroid remnant ablation.
In order to perform post-surgical rhTSH-aided thyroid remnant ablation (TRA), it is generally not necessary to confirm that thyroidectomy has been almost complete. This is true when patients are operated on by specialized surgeons with extensive experience. But in many occasions, patients are referred for ablation after a surgical procedure performed in centers where we do not know who made the operation. In these cases, it is necessary to evaluate the size of the thyroid remnant, otherwise, a high radioiodine dose might cause actinic thyroiditis of the remnants.
For such a reason, it would be necessary to use two rhTSH kits (4 injections): one kit for diagnostic purposes and the other one to administer the ablative radioiodine dose.
This study was performed with the aim to present an alternative scheme for ablation with a single kit (two 0.9 mg injections) of rhTSH and for evaluating the ablation effectiveness after applying this protocol.
Materials and Methods
Twenty female patients diagnosed with low risk classic thyroid papillary carcinoma, mean age 32 years old (range 21- 42 years old) were included (Table 1). Total thyroidectomy was performed in all patients. Additionally, six patients were subjected to level VI lymph node dissection without evidence of lymph node metastatic disease. After written consent these patients were enrolled consecutively to receive thyroid remnant ablation using the protocol described below. Low risk papillary thyroid cancer was defined according to the following criteria: absence of lymph nodes or distant metastases; all the macroscopic tumor had to be eliminated, absence of tumor invasion of locoregional tissues or structures, and absence of aggressive histology (e.g., tall cell, insular, columnar cell carcinoma) or vascular invasion.
TABLE 1.- Characteristics of the twenty patients with low risk classic papillary thyroid carcinoma who received rhTSH-aided ablation, included in the study

After total thyroidectomy, the replacement therapy with triiodothyronine at a dose of 40 μg/day was initiated. This treatment was performed until two days after the administration of the ablative dose of 131I, when the treatment with levothyroxine was initiated. According to our own and other experiences, this treatment maintains TSH levels between undetectable and in the normal range5.
When the presence of neoplasia was confirmed by the result of the anatomical pathology, a low iodine diet was initiated 10 days before the administration of the radioiodine dose. An intramuscular dose of 0.9 mg of rhTSH (Thyrogen®, Genzyme Corp. - MA) was administered on the first day (Day 1), and another dose of 0.9 mg of rhTSH was administered on Day 2. On Day 2, each patient was administered a tracer dose of 100 μCi 131I. On the third day, a thyroid scintigraphy was performed (Fig. 1). At the confirmation of the presence of small post-surgical remnants in the thyroid bed (in general we expected a radioiodine uptake lower than 3-5%), an ablative dose of 100 mCi 131I was immediately administered to all patients, except one (Patient number 13, Table 1), who received a lower dose (50 mCi 131I), due to a big thyroid remnant showed by the scintigraphy (10% uptake) (Fig. 1). The thyroglobulin (Tg) and antithyroglobulin antibodies (Tg-Ab) levels were measured three days after the second injection of rhTSH. One week after the administration of the ablative dose of 131I, a post dose whole-body scan (WBS) was performed (Fig. 2).
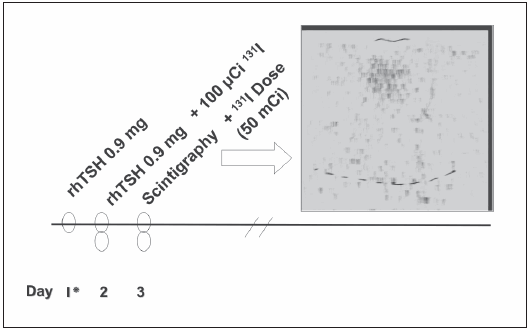
Fig. 1.- Thyroid scintigraphy observed in patient number 13 (see Table 1) showing an elevated (10%) uptake after the administration of 100 μCi of radioiodine. rhTSH: recombinant human TSH.
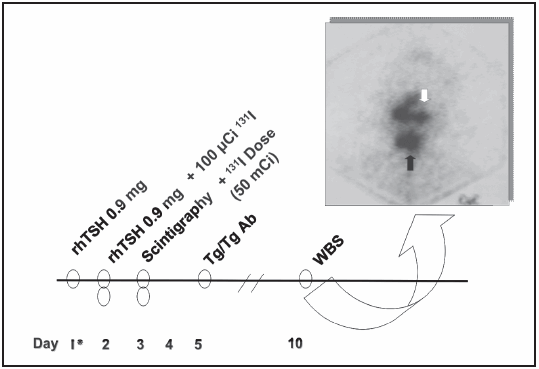
Fig. 2.- Protocol for the post-surgical ablation with recombinant TSH (rhTSH). Whole-body scan (WBS) after the administration of 50 mCi 131I to patient number 13 (see Table 1) that confirms the big thyroid remnant in the neck. Black arrow: thyroid bed uptake. White arrow: nasal and salivary glands radioiodine uptake. rhTSH: recombinant human TSH; Tg: Thyroglobulin; Tg Ab: anti-thyroglobulin antibodies.
RhTSH stimulated Tg levels were re-measured between 8-12 months after remnant ablation to confirm the success of the procedure. Thyroglobuline levels were measured by quimio luminiscence (ICMA) with a functional sensitivity of 0.5 ng/ml. Tg Antibodies (Tg-Ab) were measured by an inmmunoradiometric assay with a detection limit lower than 0.5 UI/l.
Results
The mean stimulated level of Tg at the moment of ablation was 6.3 ng/ml (range 1.2 to 26 ng/ml) (Table 1). All patients had negative titers of Tg Ab. The initial thyroidal scintigraphy showed the existence of small remnants in the thyroid bed in all (mean radioiodine uptake 3% range 2-5%) (Table 1), except for one patient in whom a big remnant was evidenced (131I uptake of 10%). Radioiodine dose was decreased from 100 to 50 mCi in this last case (Figure 1 and 2).
Thyroid remnants were later confirmed by the WBS after the administration of the radioiodine ablative dose (50 or 100 mCi 131I).
A new rhTSH stimulated Tg level measured 3 days after the second injection, and performed between 8 to 12 months after radioiodine ablation showed an undetectable Tg level in all patients confirming the success of the radioiodine remnant ablation performed after rhTSH. Due to the fact that the stimulated levels of Tg at that moment were undetectable, associated with an ultrasonography showing unaltered lymph nodes, these patients were considered free from disease and correctly ablated by the rhTSH-aided radioiodine dose.
Discussion
It has been shown that diagnostic radioiodine doses produces thyroid "stunning" It occurs most prominently with 131I doses of 5 to10 mCi5, and when there is an increased time between the diagnostic dose and radioiodine therapy6. It is generally not visually appreciated at lower doses (1-3 mCi).
Although it is not usually necessary to perform a diagnostic WBS after surgery, before ablation, it should be considered in some circumstances, such as uncertainty concerning the extent of thyroidectomy. In such a case, a low activity of 131I (100 to 300 μCi) could be used in order to reduce thyroid stunning7. Besides, in our country it is very frequent that the nuclear medicine specialist will require having a thyroid scintigraphy to administer the first radioiodine dose in order to certify the size of the thyroid remnant after surgery, because a high radioiodine dose could originate an actinic thyroiditis of the remnant. Following this approach, when rhTSH is used for ablation, it would be necessary to administer two kits (4 injections), one for the diagnostic WBS and the other one for the administration of the ablative radioiodine dose. With the present results we are showing that a diagnostic scintigraphy can be performed simultaneously with the administration of the radioiodine dose by using only one kit of rhTSH (2 injections).
According to the experiences published up to date, the ablation after rhTSH administration has been effective in most of the cases when a dose of 30 mCi 131I or higher was used8-15. Pacini et al15 published the results of the first prospective randomized multicenter study after the administration of 100 mCi 131I in two situations: following rhTSH or after the discontinuation of the thyroid hormone replacement therapy. The published results showed that the ablation percentages were similar in both situations and the quality of life maintained in patients ablated after rhTSH compared to that observed in the hypothyroid patients. Recently, the Food and Drug Administration approved the use of rhTSH to perform radioiodine ablation in patients with thyroid cancer in the United States. On the other hand, Pacini et al11 had previously demonstrated that a single standard dose of 30 mCi of 131I was less effective for the ablation of thyroid remnants when patients had been prepared with rhTSH (54%) than when they had been prepared after hormone discontinuation (84%). These authors explained the difference on the grounds of the accelerated clearance of the radioiodine usually observed in euthyroid patients, which would probably lead to the decrease of the therapeutic effect of radioactive iodine in such cases. Our personal opinion is that the main reason for such difference may be related to the content of organic iodine in the synthetic hormone of levothyroxine. This organic iodine would compete with the radioiodine and might decrease its effectiveness16. Therefore, when we administer a radioiodine dose after rhTSH, we always change levothyroxine to T3 about one month in advance, due to the fact that the latter contains less stable iodine than levothyroxine. This would probably lead to a higher uptake in the thyroid remnants and, as a consequence, would produce higher rates of successful ablation. Recently, Barbaro et al17 concluded that the impact of the amount of 131I to be administered and the influence of iodine intake is still a theme of debate. Therefore, when rhTSH is used for ablation, radioiodine activities in the 90 to 100 mCi 131I range are recommended, although a recent publication found good ablation results using 50 mCi 131I and no change in thyroid hormone replacement was required18.
Another benefit of performing ablation after rhTSH, besides the quality of life, is the greater permanence of radioiodine in the remnants, as well as a lower systemic effect after performing dosimetric studies19.
In conclusion, the post-surgical ablation after the administration of rhTSH seems to be an effective method. The use of this innovative protocol combining scintigraphy with the subsequent administration of a therapeutic dose following the administration of rhTSH would avoid the need of using 2 kits (four rhTSH injections) to perform the ablation and would decrease the costs associated with its use while will significantly enhance the quality of life of the DTC patients.
Conflict of interests: Fabian Pitoia is an external medical advisor and speaker bureau for Genzyme Corporation.
Maria Eugenia Salvai, Elias El Tamer and Hugo Niepomniszcze have nothing to declare regarding conflict of interest.
1. Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med 1998; 338: 297-306. [ Links ]
2. Goldman JM, Line BR, Aamodt RL, Robbins J. Influence of triiodothyronine withdrawal time on 131I uptake postthyroidectomy for thyroid cancer. J Clin Endocrinol Metab 1980; 50: 734-9. [ Links ]
3. Schlumberger M, Charbord P, Fragu P, et al. Relationship between thyrotropin stimulation and radioiodine uptake in lung metastases of differentiated thyroid carcinoma. J Clin Endocrinol Metab 1983; 57: 148-51. [ Links ]
4. Luster M, Lippi F, Jarzab B, et al. rhTSH-aided radioiodine ablation and treatment of differentiated thyroid carcinoma: a comprehensive review. Endocr Relat Cancer 2005; 12: 49-64. [ Links ]
5. Leboeuf R, Perron P, Carpentier AC, Verreault J, Langlois MF. L-T3 preparation for whole-body scintigraphy: a randomized-controlled trial. Clin Endocrinol (Oxf) 2007; 67: 839-44. [ Links ]
6. Park HM, Park YH, Zhou XH. Detection of thyroid remnant/metastasis without stunning: An ongoing dilemma. Thyroid 1997; 7: 277-80. [ Links ]
7. Hilditch TE, Dempsey MF, Bolster AA, et al. Self-stunning in thyroid ablation: evidence from comparative studies of diagnostic 131I and 123I. Eur J Nucl Med Mol Imaging 2002; 29: 783-8. [ Links ]
8. Gauna A. Abalovich M, Gerez A, et al. Primer consenso argentino sobre patologías endocrinas: Tiroides. Rev Arg Endocrinol Metab 2006; 43: 124-43. [ Links ]
9. Robbins RJ, Tuttle RM, Sonenberg M, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin. Thyroid 2001; 11: 865-9. [ Links ]
10. Robbins RJ, Larson SM, Sinha N, et al. A retrospective review of the effectiveness of recombinant human TSH as preparation for radioiodine thyroid remnant ablation (brief communication). J Nucl Med 2002; 43: 1482-8. [ Links ]
11. Berg G, Lindstedt G, Suurküla M, Jansson S. Radioiodine ablation and therapy in differentiated thyroid cancer under stimulation with recombinant human thyroid-stimulating hormone (rhTSH). J Endocrinol Invest 2002; 25: 4-52. [ Links ]
12. Pacini F, Molinaro E, Castagna MG, et al. Ablation of thyroid residues with 30 mCi 131I: a comparison in thyroid cancer patients prepared with recombinant human TSH or thyroid hormone withdrawal. J Clin Endocrinol Metab 2002; 87: 4063-8. [ Links ]
13. Barbaro D, Boni G, Meucci G, et al. Radioiodine treatment with 30 mCi after recombinant human thyrotropin stimulation in thyroid cancer: effectiveness for postsurgical remnants ablation and possible role of iodine content in L-thyroxine in the outcome of ablation. J Clin Endocrinol Metab 2003; 88: 4110-5. [ Links ]
14. Kovatcheva RD, Hadjieva TD, Kirilov GG, Lozanov BS. Recombinant human TSH in radioiodine treatment of differentiated thyroid cancer. Nucl Med Rev Cent East Eur 2004; 7: 13-9. [ Links ]
15. Pitoia F, Tamer EE, Schere DB, Passerieu M, Bruno OD, Niepomniszcze H. Usefulness of recombinant human TSH aided radioiodine doses administered in patients with differentiated thyroid carcinoma. Medicina (Buenos Aires) 2006; 66: 125-30. [ Links ]
16. Pacini F, Ladenson PW, Schlumberger M, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. J Clin Endocrinol Metab. 2006; 91: 926-32 [ Links ]
17. Pitoia F, Degrossi OJ, Niepomniszcze H. Why should the radioiodine dose be different in patients with differentiated thyroid carcinoma prepared with recombinant human TSH? Eur J Nucl Med Mol Imaging 2004; 31: 924; author reply: 924-5. [ Links ]
18. Barbaro D, Boni G. Radioiodine ablation of post-surgical thyroid remnants after preparation with recombinant human TSH: why, how and when. Eur J Surg Oncol. 2007; 33: 535-40. [ Links ]
19. Pilli T, Brianzoni E, Capoccetti F, et al. A comparison of 1850 MBq (50 mCi) and 3700 MBq (100 mCi) 131- iodine administered doses for recombinant TSH-stimulated postoperative thyroid remnant ablation in differentiated thyroid cancer. J Clin Endocrinol Metab. 2007; 92: 3542-6. [ Links ]
20. Luster M, Sherman SI, Skarulis MC, et al. Comparison of radioiodine biokinetics following the administration of recombinant human thyroid stimulating hormone and after thyroid hormone withdrawal in thyroid carcinoma. Eur J Nucl Med Mol Imaging 2003; 30: 1371-7. [ Links ]
Received: 13-3-2008
Accepted: 16-9-2008














