Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Medicina (Buenos Aires)
versión impresa ISSN 0025-7680versión On-line ISSN 1669-9106
Medicina (B. Aires) v.69 n.4 Ciudad Autónoma de Buenos Aires sep./oct. 2009
ARTÍCULO ORIGINAL
Adult height in Turner syndrome girls after long-term growth hormone treatment
Analía Morín1, 2, Luis M. Guimarey1, 2, 3, María Apezteguía3, Zulma C. Santucci1, 2
1Sala de Endocrinología, Hospital de Niños Sor María Ludovica, La Plata;
2Fundación de Endocrinología, Nutrición Infantil y Crecimiento (FUNDENIC);
3Comisión de Investigaciones Científicas de la Provincia de Buenos Aires (CIC)
Dirección postal: Dra. Analía Morin, Sala de Endocrinología y Crecimiento, Hospital de Niños Sor María Ludovica, Calle 14 N° 1631, 1900 La Plata, Buenos Aires, Argentina Fax: (54-221) 4572135 e-mail: morinanalia@yahoo.com.ar
Abstract
We studied the adult height (AH) outcome, and factors likely to influence it, in Turner Syndrome (TS) girls treated with growth hormone (GH). A total of 25 TS girls treated with GH were compared with 10 TS girls not treated with GH. The percentage of girls who achieved normal third percentile was determined. Projected AH (PAH) was calculated according to height standard deviation score (HSDS) at the beginning of the treatment. Gain in height was determined as: AH - pretreatment PAH. The percentage of girls who achieved target range (midparental height±2 SD) was determined. Multiple linear regression models were fitted on baseline variables- chronological age (CA), midparental height (MPH) and HSDS; and treatment variablesduration of oestrogen-free GH therapy and duration of GH therapy+oestrogens. As for baseline data: median CA was 13.0 years (5.6-15.8). Mean HSDS was 0.25±1.1 SDS. PAH was 139.2±5.6 cm. MPH was 160.0±5.0 cm. As for follow up data: Median CA at onset oestrogens was 15.1 years (13.2-16.6). Median duration of GH therapy was 3.8 years (2.1-10.3). Median oestrogen-free GH period was 2.0 years (0.7-7.8), and median GH+oestrogens period, 1.8 years (1.0-3.2). Adult height: Mean AH was 150.4±7.0 cm in treated patients and 140.8±7.2 cm in the group not treated with GH (p=0.001). Fourteen (56%) girls achieved normal third percentile compared with an initially predicted 1 (4%). Gain in height was 11.2±3.7 cm. Thirteen (59%) girls reached an AH within target range. HSDS at the beginning of the treatment was the variable most strongly related to AH and duration of oestrogen-free GH period was the variable most strongly related to gain in height.
Key words: Turner syndrome; Growth; Growth hormone; Adult height; Oestrogen
Resumen
Talla adulta en pacientes con síndrome de Turner tratadas con hormona de crecimiento a largo plazo. Se estudió la talla adulta (TA) y los factores que pudieran influenciarla en niñas con síndrome de Turner (ST) tratadas con hormona de crecimiento (HC). Se compararon 25 pacientes con ST tratadas con HC y 10 niñas no tratadas. Se determinó: el porcentaje de niñas que alcanzó el tercer percentilo de la curva de normalidad, la talla adulta proyectada (TAP) de acuerdo al score de desvío estándar de talla (SDST) al inicio del tratamiento, la ganancia en talla (TA - TAP pretratamiento) y el porcentaje de niñas que alcanzó el rango genético (talla media parental ± 2 DS). Se ajustaron modelos de regresión múltiple sobre variables basalesedad cronológica (EC), talla media parental y SDST; y variables durante el tratamiento- duración del tratamiento con GH sin estrógenos y con GH+estrógenos. Resultados: datos basales: la EC mediana fue 13.0 años (5.6- 15.8), el SDST 0.25 ± 1.1 SDS, la TAP 139.2 ± 5.6 cm y la talla media parental 160.0 ± 5.0 cm. Datos en el seguimiento: la EC mediana al inicio del estrógeno fue 15.1 años (13.2-16.6), la duración mediana del tratamiento con GH 3.8 años (2.1-10.3), del tratamiento con GH y sin estrógenos 2.0 años (0.7-7.8), y del tratamiento con GH + estrógenos 1.8 años (1.0-3.2). Talla adulta: la TA media fue 150.4 ± 7.0 cm en pacientes tratadas y 140.8 ± 7.2 cm en el grupo no tratado (p = 0.001). 14 niñas (56%) alcanzaron el tercer percentilo comparado con la predicción inicial de una niña (4%). La ganancia en talla fue 11.2 ± 3.7 cm. 13 niñas (59%) alcanzaron una TA dentro del rango genético. La variable que más se relacionó con la TA fue el SDST al inicio del tratamiento y con la ganancia en talla, la duración del tratamiento con GH libre de estrógenos.
Palabras clave: Síndrome de Turner; Hormona de crecimiento; Talla adulta; Estrógenos
Turner Syndrome (TS) is one of the most common human genetic disorders, affecting approximately one of every 2500 live born females1, 2. Growth failure is a cardinal feature of TS3. The mean adult height (AH) of Argentinian TS women not treated with growth hormone (GH) is 137.9 ± 5.1 cm4, 21.1 cm below that of the general female population5. The short stature characteristic of TS is believed to result at least in part from happloinsufficiency of the short stature homeobox-containing gene, located in the pseudoautosomal region of the X and Y chromosomes at Xp22.3 and Yp11.3, respectively6, 7.
Recombinant human GH therapy has been widely used to enhance growth and thus to increase AH. Short-term results revealed a clear acceleration of height gain velocity when these patients were treated with supra-physiological doses of GH8. However, data about long-term height attainment have shown a variable outcome2.
Because of ovarian dysgenesis, the induction of puberty is required in most of TS girls. Some authors have concluded that the addition of oestrogens to GH treatment did not affect AH2, 9. Other studies, however, have shown that oestrogen administration could decrease AH10-12, particularly when started soon after initiation of GH therapy. Although it has been suggested that oestrogen replacement therapy should be postponed as late as possible to prolong the growth period9, delaying puberty could have adverse psychological consequences13 and may result in a decrease bone mineralization14,15. Starting GH treatment at a young age allows a longer period of oestrogen- free GH treatment permitting the induction of puberty at an age-appropriate time16, 17. Despite these considerations, the optimal ages at which GH and oestrogens should be started are not clear at present.
We studied AH outcome and factors likely to influence it in a group of Argentinian TS girls treated with GH during childhood and followed-up until AH.
Materials and Methods
Twenty five TS girls followed longitudinally at the Endocrinology Unit of La Plata Children Hospital were studied. Patients fulfilling the following inclusion criteria were selected: 1) having karyotipe of TS, 2) having been treated with daily injections of GH for at least 2 years, 3) having undergone induced puberty, 4) having reached their AH, i.e. height velocity during the preceding year of less than 1 cm or a growth during the last 6 months of less than 0.5 cm.
The karyotipe distribution was: 45,X = 10 (40%); 45,X/46,Xi(Xq) = 6 (24%); 45,X/46,XX = 4(16%); 46,Xi(Xq) = 2 (8%); other karyotypes = 3 (12%).
All patients were treated with different registered trade marks of human recombinant GH at a dose of 0.33 ± 0.06 mg/kg/week, administered as daily sc injections. The GH dose was adjusted every 6 months according to body weight to keep the dose as close as possible to 0.33 mg/kg/week. The maximum GH dose was fixed at 2 mg/day.
Puberty was induced with low doses of echinus conjugated oestrogens (CEs). Starting dose was 0.625 mg, 2 days/week. The CEs were progressively increased every 6 months to reach an adult dose of 0.625 mg/day after 1 or 2 years of treatment. Cyclic progestagen therapy (medroxiprogesterone acetate, 10 mg daily for 10 days each month) was added 2.0 ± 0.7 years after the onset of CEs therapy to induce regular menstrual cycles.
Ten TS patients never treated with GH were matched to the TS girls treated with GH. There were no differences in the age of initiation of CEs treatment between patients and controls (15.2 ± 1.0 and 14.3 ± 2.3 years, respectively).
Height standard deviation scores (HSDS) were derived from published Argentinian standards for TS girls4.
According to the Lyon height projection model18, the HSDS at the beginning of the treatment was used to derive projected AH (PAH) on the assumption that in girls with TS there is a close relationship between height in the first decade and the AH.
The gain in height as a consequence of GH therapy was determined by subtracting the pre-treatment PAH from the recorded AH.
AH was defined when the height gain velocity during the preceding year was less than 1 cm or growth during the last 6 months less than 0.5 cm.
The midparental height (MPH) was calculated as: (father's height + mother's height)/2-6.0 cm. The target height range was defined as: MPH ± 2 SD. The percent of girls who achieved the target range was calculated. The remaining height deficit was considered to be: MPH-AH.
Bone age (BA) was determined at yearly intervals using the Greulich and Pyle method19.
The effect of GH treatment was evaluated by: 1) comparing the AH of GH-treated patients with the AH of Argentinean TS standards and the historical controls not treated with GH, but supplemented with CEs, 2) calculating the percent of the girls who achieved the height of 149.2 cm considered to be a threshold for normal stature (Argentinean standard third percentile)5, 3) considering the gain in height during GH treatment, 4) calculating the percent of the girls who attained the target range and the remaining height deficit (MPH - AH).
Two types of variables likely to influence the effect of GH treatment were calculated: baseline variables -chronological age (CA), BA, CA-BA difference, MPH and HSDS; treatment variables- duration of oestrogen-free GH therapy and duration of GH therapy + CEs.
The results are expressed as median (range) or mean (± SD).
The Student t test was used for comparisons between patients and controls.
Multiple linear regression models with backward selection were fitted to predict AH, gain in height and remaining height deficit. CA, CA-BA, MPH, HSDS, duration of oestrogen-free GH therapy and duration of GH therapy + CEs were tested as independent variables
Results
Twenty five TS girls started GH treatment at a median CA of 13.0 (Mn = 5.6-Mx = 15.8) years. The median BA was 11.0 (Mn = 3.0-Mx = 13.0) years, with a mean CA-BA difference of 2.4 ± 1.3 years. The mean HSDS matching Argentinian Turner height standards was 0.25 ± 1.1 SDS. Fig. 1 presents the individual CAs and heights of the girls at the start of therapy.
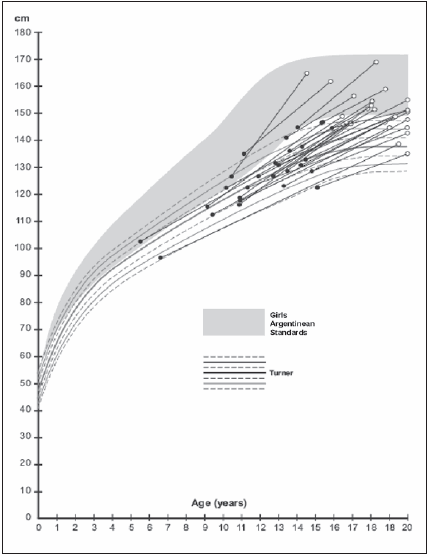
Fig. 1.- Height at start of growth hormone treatment and adult height in Turner Syndrome girls.
According to the Lyon projected method adapted to Argentinian TS standards, PAH at the start of GH therapy was 139.2 ± 5.6 cm.
The MPH calculated from normal population standards was 160.0 ± 5.0 cm.
The median CA at the onset of CEs replacement was 15.1 (Mn = 13.2-Mx = 16.6) years.
The median duration of GH therapy was 3.8 (Mn = 2.1-Mx = 10.3) years. The median CEs-free GH therapy period was 2.0 (Mn = 0.7-Mx = 7.8) years, and the median GH + CEs period, 1.8 (Mn = 1.0-Mx = 3.2) years.
Fig. 1 shows the individuals AHs of the TS girls in the present experimental group. The mean AH was 150.4 ± 7.0 cm.
Expressed as SDS, the AH was -1.8 ± 1.1 with respect to the Argentinian normal standards, and 2.4 ± 1.4 Argentinian TS standards. In comparison with the Argentinian TS girls not treated with GH, the mean AH was 12.5 ± 7.0 cm higher (p = 0.000), with an HSDS increase of 2.4 ± 1.4 (p = 0.000).
The TS historical group not treated with GH reached a mean AH of 140.8 ± 7.2 cm, a value which was 9.6 ± 2.6 cm shorter than the AH of treated patients (p = 0.001).
On the basis of the PAH calculated by means of the Lyon PAH method, adapted to Argentinean TS girls not treated with GH, the gain in height during GH treatment (AH-PAH) was 11.2 ± 3.7 cm (p = 0.000), (5.88 ± 2.97 cm during CEs-free GH therapy period and 5.35 ± 2.38 cm during GH + CEs period).
With respect to the arbitrary height of 149.2 cm as a threshold for normal stature, 14 (56%) girls achieved this target compared to an initially predicted 1girl (4%).
The remaining height deficit (MPH-AH) was 10.2 ± 5.5 cm. Fifty percent of the girls reached an AH within target range (MPH ± 2SD).
In 20 patients, three multiple linear regression models were fitted to establish the influence of baseline variables (CA, CA-BA difference, HSDS, MPH) and treatment variables (duration of oestrogen-free GH therapy and duration of GH therapy + CEs) on AH, gain in height, and remaining height deficit (Table 1 a, b, c). The HSDS at the beginning of GH treatment, CA at the beginning of the treatment and the duration of oestrogen-free GH therapy period were related positively to the AH and inversely to the remaining height deficit. The MPH was related positively to the remaining height deficit. The CA at the beginning of the treatment, MPH, and the duration of oestrogen-free GH therapy period were positively related to the gain in height. The HSDS was the variable most strongly related to AH (p = 0.000) and to the remaining height deficit (p = 0.000) while the duration of the oestrogen- free GH therapy period was the variable most strongly related to gain in height (p = 0.019) The CA-BA difference and the duration of GH therapy + CEs were not related to the AH, the gain in height or the remaining height deficit (not shown).
TABLE 1.- (a) Factors related to adult height, (b) Gain in height and (c) Remaining height deficit
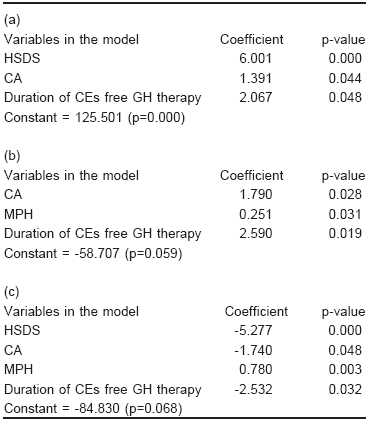
HSDS: height standard deviation score; CA: chronological age; CEs: conjugated oestrogens; MPH: midparental height
Discussion
Growth hormone administration has become a common treatment for the short stature of the Turner syndrome. Although GH stimulates linear growth in TS patients, the effect on AH has remained uncertain. The present study shows that girls with TS treated with GH at a dose of 0.33 mg/kg/week have a significant increase in AH compared to TS women not treated with GH: the mean AH of our TS treated patients was 150.4 ± 7.0 cm, a value which is about 12.5 ± 7.0 cm taller than the AH reported in Argentinean Turner women not treated with GH (137.9 ± 5.1 cm)4. Our results are consistent with data from the literature for similar GH doses, in which the mean AH varied between 146.8 and 152.2 cm2, 9, 12, 16, 17, 20-26. To evaluate the effect of GH treatment, we also compared the AH to a historical control TS group. We found that the height of GH treated TS patients was 9.6 ± 2.6 cm taller than the controls. Furthermore, on the basis of the arbitrary height of 149.2 cm as a threshold for normal stature, 56% of the TS girls achieved this target compared with an initially predicted 4%. In addition, 50% the TS girls attained a height within the target range. Comparison of their AHs to the MPH revealed a remaining height deficit of 10.2 ± 5.5 cm. This result is similar to the observations of Massa et al22 who found a remaining height deficit of 9.8 ± 6.4 cm. Taken together, our data and those from the literature clearly show a significant effect of GH treatment on the AH of TS girls2, 9, 12, 16, 17, 20-26.
Although GH treatment seems to be effective in improving the AH in TS girls, a variability in the magnitude of the response to GH has long been recognised22. In the present study, we also found a large inter-individual variability with respect to the AH (range: 136.8-168.9 cm). A number of factors could potentially contribute to this variability, including pre-treatment features, such as the chronological or bone age at the initiation of therapy and the baseline height, as well as therapeutic details, such as the dose or duration of GH and the timing of oestrogen replacement. In agreement with other reports10, 20-22 we found that height at the beginning of the treatment, parameter which is related to parental height, is one of the most important variables that could influence the AH. Hence, shorter TS girls at the beginning of GH treatment will end up with a shorter AH than will the taller TS girls. These findings demonstrate the importance of the genetic influences on growth. Indeed, TS girls may suffer intrauterine growth retardation. Accordingly, it has been described26 that girls treated with higher doses of GH, corresponding to those used in non TS children with intrauterine growth retardation, the best results in terms of AH were obtained. Therefore, in view of the large variability in the GH response, GH treatment should be individualized in girls with TS syndrome in order to optimize the AH22, 27, 28.
One of the key influences associated with improvement in the AH of TS girls is the age at the beginning of GH therapy. In accordance with the findings of Massa et al22 and Pasquino et al24, however, we did not observe that TS girls treated at a younger age could achieve a better AH. This discrepancy may be due to the delayed initiation of GH therapy in most of our patients. In fact, in this study the median age at the beginning of GH treatment was 13.0 (Mn=5.6-Mx=15.8) years. Such late initiation of therapy has diverse consequences: the patient spends most of her childhood as a short child, thus bearing the psychological burden of short stature with respect to her peers. Furthermore, height deviates progressively from normal with greater height deficit and shorter available time for therapy. In addition, because it has been shown that the duration of free-estrogen GH therapy period could influence height gain2, 12, 16, 17, 22, 26, 29, 30, there is a tendency to postpone initiation of estrogen replacement. For these reasons, GH treatment should be started as early as possible in order both to prevent progressive deterioration in height and to prolong pre-estrogen therapy period without causing a delay in puberty. Nearly all prior reports that have shown relatively modest gains in height have involved the study of patients with oestrogen replacement introduced shortly after the start of GH therapy. For example, the patients described by Taback et al31 who had a gain in height of less than 5 cm, began oestrogens only 1.3 years after the initiation of GH therapy. Likewise, the 3 cm gain in height reported by Van de Broeck et al32 was in patients beginning oestrogen therapy soon after starting GH therapy. In contrast, in our study, the gain in height was on the average 11.2 ± 3.7 cm. When we performed a linear regression analysis between factors that could influence gain in height, we found that the duration of free-oestrogen GH treatment period was the most important parameter involved. Our results are consistent with those reported in the literature which show that patients who received oestrogens early on relative to GH initiation fared worse in terms of their AH2, 12, 16, 17, 22, 26, 29, 30. Therefore, Turner syndrome should be early recognized in order to start GH therapy at a younger age allowing a longer free-oestrogen GH treatment period. Nevertheless, more data are required to delineate the optimal age to start GH treatment, taking into account the auxological as well as the psychological status of each individual TS girl.
In conclusion, this study has shown that GH treatment improves AH in TS girls, but with a wide variability on the response. The duration of the pre-oestrogen GH treatment period was a strong predictor for height gain. Therefore, early diagnosis of TS permits longer oestrogens-free GH treatment improving AH outcome and allowing a more age-appropriate initiation of oestrogen replacement.
Moreover, since the HSDS at the beginning of the GH treatment, which is related to the parental height, was a strong predictor for AH, this study demonstrates the importance of the genetic factors on GH treatment outcome.
Conflictos de interés: Ninguno a declarar.
1. Hook EB, Warburton D. The distribution of chromosomal genotypes associated with Turner syndrome: livebirth prevalence rates and evidence for diminished fetal mortality and severity in genotypes associated with structural X abnormalities or mosaicism. Hum Genet 1983; 64: 24-7. [ Links ]
2. Quigley CA, Crowe BJ, Anglin DG, Chipman JJ and the U.S. Turner Syndrome Study Group. Growth hormone and low dose estrogen in Turner syndrome: results of a Unites states multi-center trial to near-final height. J Clin Endocrinol Metab 2002; 87: 2033-41. [ Links ]
3. Davenport ML, Punyasavatsut N, Gunther D, Savendahl L, Stewart PW. Turner syndrome: a pattern of early growth failure. Acta paediatr 1999; 433(Suppl): 118-21. [ Links ]
4. García-Rudaz C, Martínez AS, Heinrich JJ et al. Growth of Argentinian girls with Turner syndrome. Ann Hum Biol 1995; 22: 533-44. [ Links ]
5. Lejarraga H, Orfila G. Estándares de peso y estatura para niñas y niños argentinos desde el nacimiento hasta la madurez. Arch Arg Pediatr 1987; 85: 209-22. [ Links ]
6. Rao E, Weiss B, Fukami M, et al. Pseudoautosomal deletions encompassing a novel homeobox gene cause growth failure in idiopathic short stature and Turner syndrome. Nat Genet 1997; 16: 54-63. [ Links ]
7. Ellison JW, Wardak Z, Young MF, Gehron Robey P, Laig- Webster M, Chiong W. PHOG, a candidate gene for involvement in the short stature of Turner syndrome. Hum Molec Genet 1997; 6: 1341-7. [ Links ]
8. Morín A, Guimarey L, Santucci Z, Apezteguía M. Predicción de la estatura final en niñas con síndrome de Turner tratadas con hormona de crecimiento. Medicina (Buenos Aires) 2000; 60: 551-4. [ Links ]
9. Johnston DI, Betts P, Dunger D, et al. A multicentre trial of recombinant growth hormone and low dose oestrogen in Turner syndrome: near final height analysis. Arch Dis Child 2001; 84: 76-81. [ Links ]
10. Cacciari E, Mazzanti L, and the Italian Study Group for Turner Syndrome, Final height of patients with turner syndrome treated with growth hormone (GH): indications for GH therapy alone at high doses and late estrogen therapy. J Clin Endocrinol Metab 1999; 84: 4510-5. [ Links ]
11. Nilsson KO, for the Swedish Study Group of Growth Hormone Treatment Timing of oestrogen therapy in girls with Turner syndrome: the Swedish experiences and a review. In: Saenger P, Pasquino AM, eds. Optimizing health care for Turner patients in the 21 st century. Amsterdam: Elsevier Science BV; 2000: p 185-97. [ Links ]
12. Chernausek SD, Attie KM, Cara JF, Rosenfeld RG, Frane J, and the Genentech, Inc., Collaborative Study Group. Growth hormone therapy of Turner syndrome: the impact of age of estrogen replacement on final height. J Clin Endocrinol Metab 2000; 85: 2439-45. [ Links ]
13. Ross JL, McCauley E, Roeltgen D, et al. Self-concept and behavior in adolescent girls with Turner syndrome: potential estrogen effects. J Clin Endocrinol Metab 1996; 81: 926-31. [ Links ]
14. Carrascosa A, Gussinye M, Terradas P, Yeste D, Audi L. Vicens-Calvet E. Spontaneous, but not induced, puberty permits adequate bone mass acquisition in adolescent Turner syndrome patients. J Bone Miner Res 2000; 15: 2005-10. [ Links ]
15. Costa AM, lemos-Marini SH, Baptista MT, Morcillo AM, Maciel-Guerra AT, Guerre Jr G. Bone mineralization in Turner syndrome: a transverse study of the determinant factors in 58 patients. J Bone Miner Metab 2002; 20: 294-7. [ Links ]
16. Reiter EO, Blethen SL, Baptista J, Price L. Early initiation of growth hormone allows age-appropiate estrogen use in Turner syndrome. J Clin Endocrinol Metab 2001; 86: 1936-41. [ Links ]
17. Morín A, Guimarey L, Apezteguía M, Santucci Z. Efecto del tratamiento con estrógenos sobre el crecimiento en niñas con síndrome de Turner tratadas con hormona de crecimiento. Medicina (Buenos Aires) 2001; 61: 271-4. [ Links ]
18. Lyon AJ, Preece MA, Grant DB. Growth curve for girls with Turner¢s syndrome. Arch Dis Child 1985; 60: 932-5. [ Links ]
19. Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. Stanford: Stanford University Press, 1959. [ Links ]
20. Hochberg Z, Zadik Z. Final height in young women with Turner syndrome after GH therapy: an open controlled study. Eur J Endocrinol 1999; 141: 218-24. [ Links ]
21. Schweizer R, Ranke MB, Binder G, et al. Experience with growth hormone therapy in Turner syndrome in a single centre: low total height gain, no further gains after puberty onset and unchanged body proportions. Horm Res 2000; 53: 228-38. [ Links ]
22. Massa G, Heinrichs C, Verlinde S, et al. in collaboration with the Belgian Study Group for Pediatric Endocrinology. Late or delayed induced or spontaneus puberty in girls with Turner syndrome treated with growth hormone does not affect final height. J Clin Endocrinol Metab 2003; 88: 4168-74. [ Links ]
23. Gracia Bouthelier R, Oliver Iguacel A, Gonzalez Casado I, Alcalde de Alvare A. Optimization of treatment in Turner´s syndrome. J Pediatr Endocrinol Metab 2004;17 (Suppl 3): 427-34. [ Links ]
24. Pasquino AM, Pucarelli I, Segni M, Turani L, Calcaterra V, Larizza D. Adult height in sixty girls with Turner syndrome treated with growth hormone matched with an untreated group. J Endocrinol Invest 2005; 28: 350-6. [ Links ]
25. Soriano-Guillen L, Coste J, Ecosse E, et al. Adult height and pubertal growth in Turner syndrome after treatment with recombinant growth hormone. J Clin Endocrinol Metab 2005; 90: 5197-204. [ Links ]
26. Van Pareren YK, de Muinck Keizer-Schrama SM, Stijnen T, et al. Final height in girls with Turner syndrome after long-term growth hormone treatment in three dosages and low dose estrogens. J Clin Endocrinol Metab 2003; 88: 1119-25. [ Links ]
27. Saenger P, Wikland KA, Conway GS et al. Recommendations for the diagnosis and management of Turner syndrome. J Clin Endocrinol Metab 2001; 86: 3061-9. [ Links ]
28. Tauber M. Optimizing growth in Turner syndrome: rationale to use dose escalation studies in GH treatment. In: Saenger P, Pasquino AM (eds). Optimizing health care for Turner patients in the 21st century. Amsterdam: Elsevier Science BV; 2000: p 191-200. [ Links ]
29. Lee Y-J. Growth hormone therapy in Turner syndrome. Acta paediatr 2000; 41: 292-3. [ Links ]
30. Sas TC, de Muinck Keizer-Schrama SM, Stijnen T, et al. Final height in girls with Turner´ syndrome treated with once or twice daily growth hormone injections. Dutch Advisory Group on Growth Hormone. Arch Dis Child 1999; 80: 36-41. [ Links ]
31. Taback SP, Collu R, Deal CL. Does growth-hormone supplementation affect adult height in turner syndrome? Lancet 1996; 348: 25-7. [ Links ]
32. Van den Broeck J, Massa GG, Attanasia A. Final height after long-term growth hormone treatment in Turner syndrome. J Pediatr 1995; 127: 729-35. [ Links ]
Received: 25-10-2008
Accepted: 12-2-2009














