Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Medicina (Buenos Aires)
versión impresa ISSN 0025-7680versión On-line ISSN 1669-9106
Medicina (B. Aires) v.69 n.5 Ciudad Autónoma de Buenos Aires set./oct. 2009
ARTÍCULO ORIGINAL
Glomerular filtration rate, cardiovascular risk factors and insulin resistance
Martín R. Salazar1, Horacio A. Carbajal1, Alberto G. Marillet2, Delfina M. Gallo2, María L. Valli2, Miguel Novello2, Raúl F. Echeverría1
1Centro de Referencia en Hipertensión Arterial, Servicio de Clínica Médica, H.I.G.A. Gral. San Martín, La Plata,
2Municipalidad de San Andrés de Giles, Buenos Aires
Postal address: Dr. Martín R. Salazar, Calle 14 N° 320, 1900 La Plata, Argentina Fax: (54-221) 483-329 e-mail: salazarlandea@gmail.com
Abstract
The aim of this paper was to study the estimated glomerular filtration rate (eGFR), its changes with age, and its association with systolic blood pressure (SBP) and diastolic BP (DBP), indicators of obesity, dyslipemia, insulin resistance and inflammation on a random population sample. BP, weight, size and waist circumference (WC) were recorded at home. Fasting morning blood samples were analysed. The eGFR was calculated with MDRD (eGFR-MDRD), Cockroft-Gault (eGFR-CG) adjusted to 1.73 m2 and reciprocal of serum creatinine (100/serum cretinine). A total of 1016 individuals, 722 females (41.97 ± 0.66 years old) and 294 males (42.06 ± 0.99 years old), completed the laboratory tests. The mean of 100/Scr was 115.13 ± 0.60 (dl/mg), the mean eGFR-CG was 98.48 ± 0.82 ml/min/1.73 m2; the mean eGFR-MDRD was 85.15 ± 0.58 ml/min/1.73 m2. The eGFR-MDRD decreased with age and with the number of risk factors in both sexes. The eGFR-MDRD < 60 ml/min/1.73 m2 adjusted prevalence was 6.2 per 100 inhabitants (CI 95%, 4.7-7.7), 3.6 (CI 95%, 1.5-5.7) in males and 8.6 (CI 95%, 6.6-10.6) in females. The bivariate analysis showed that the eGFR-MDRD correlates inversely with age, SBP, DBP WC, BMI, serum glucose, serum total cholesterol, LDL cholesterol, serum triglycerides, serum uric acid and, in males, with C-reactive-protein. There was no correlation with either insulinemia or HOMA.The mean eGFR value, its association with cardiovascular risk factors and the prevalence of eGFR < 60 ml/min/1.73 m2 found in a rural population of Argentina are similar to those found in other parts of the world.
Key words: Chronic kidney disease; Epidemiology; Glomerular fibration rate estimate; Cardiovascular risk factors
Resumen
Filtrado glomerular, riesgo cardiovascular y resistencia a la insulina. El objetivo fue evaluar en una muestra poblacional aleatoria el filtrado glomerular estimado (FGe), sus cambios con la edad y su asociación con presión arterial sistólica (PAS) y diastólica (PAD), indicadores de obesidad, dislipemia, resistencia a la insulina e inflamación. En cada domicilio fueron medidos presión arterial, peso y talla y perímetro de la cintura (PC). Se analizaron muestras de sangre en ayunas y fue calculado el FGe usando las fórmulas de MDRD (FGe-MDRD) y Cockroft-Gault (FGe-CG) ajustado a 1.73 m2, y la inversa de la creatinina sérica (100/CrS). Completaron el protocolo de laboratorio 1016 sujetos, 722 mujeres (41.97 ± 0.66 años) y 294 varones (42.06 ± 0.99 años). La media de 100/Crs fue 115.13 ± 0.60 (dl/mg), la del FGe-CG 98.48 ± 0.82 ml/min/1.73 m2 y la del FGe-MDRD 85.15 ± 0.58 ml/min/1.73 m2 (CI 95% 84.00-86.29). El FGe-MDRD disminuyó con la edad y con el número de factores de riesgo cardiovascular en ambos sexos. La prevalecencia ajustada de FGe-MDRD < 60 ml/min/1.73 m2 fue 6.2 por 100 habitantes (CI 95%, 4.7-7.7); 3.6 (CI 95%, 1.5-5.7) en varones y 8.6 (CI 95%, 6.6- 10.6) en mujeres. El análisis bivariado mostró correlación inversa del FGe-MDRD con edad, PAS, PAD, PC, IMC, glucemia, colesterolemia total, colesterol-LDL, trigliceridemia, uricemia y, en varones, con la proteina-C-reactiva. No hubo correlación con insulinemia u HOMA. La media del FGe, su asociación con factores de riesgo cardiovascular y la prevalecencia de FGe < 60 ml/min/1.73 m2 fueron similares a los hallados en otras partes del mundo.
Palabras clave: Enfermedad renal crónica; Epidemiología; Filtrado glomerular estimado; Factores de riesgo cardiovascular
The Glomerular Filtration Rate (GFR) decreases with age1. This fact, although considered as part of the ageing process, could actually predict cardiovascular morbidity and mortality2, 3. The prevalence of GFR deterioration can vary according to the geographic area, and being able to quantify the factors associated with decreased GFR may help prevent not only renal function deterioration but also cardiovascular disease. In order to plan the preventive measures for the cardiovascular as well as the renal diseases, it is important to reach an estimation of the affected population. There is little information available in Argentina on the prevalence of renal function deterioration. On the other hand, an agreement3, 4 has been reached on the convenience of evaluating the renal function clinically by means of estimated glomerular filtration (eGFR).
The aim of this paper was to study the eGFR, its changes with age, and its association with systolic blood pressure (SBP), diastolic blood pressure (DBP), indicators of obesity, dyslipemia, insulin resistance and inflammation in a random population sample.
Materials and Methods
As starting point of a community intervention programme on cardiovascular risk factors (PROCER) in the city of San Andres de Giles in the province of Buenos Aires, an epidemiological study on BP, renal disease and other risk factors was conducted between September and December 2007 on a random population sample of e ≥ 15 years of age. The universe was the inhabitants of the urban area of San Andres de Giles between 15 and 75 years of age. This city lies in the province of Buenos Aires, 34° 26' 30.52'' south latitude and 59° 26' 37.29'' west longitude. According to the last national census available (2001), there were 15 056 inhabitants in the urban area of San Andres de Giles, 13 922 between 15 and 75 years of age. No abnormalities had been shown since 2001 to assume changes in the population. The survey was performed on simple random sample of subjects living in the addresses of the chosen blocks. Since the socio-economic features and the number of inhabitants were similar, a proportional probability was not taken into consideration. The interviewers were carefully selected nurses from the San Andrés de Giles Hospital, who had been previously trained. The sample had a total of 1617 cases, and 1591 interviews (98.4%) were considered to be valid (1091 females and 500 males). All of the subjects provided written informed consent.
The BP measurements, the weight, size and waist circumference and an epidemiological file were recorded at home on each subject. The BP was measured while the individual was sitting down and after a minimum resting period of five minutes. The right arm was laid out at the heart level and the BP was measured using a mercury sphygmomanometer whose cuff was applied on the upper arm 3 cm above the elbow fold. Phase I and V (disappearance) Korotkoff sounds were used to identify SBP and DBP, respectively. Three separate measurements were carried out, each after a 5-minute interval. The SBP and DBP values taken into account were an average of the three measurements' values. Weight measurement was carried out using a balance which was calibrated before each measurement, with the individual wearing light clothes and no shoes. The height was also measured with no shoes on and using a metallic metric tape. The waist circumference was measured with a relaxed abdomen using a metallic metric tape on a horizontal plane above the iliac crest. The body mass index (BMI) was calculated using the weight/height2 formula. Also, at the San Andrés de Giles Hospital's laboratory, tests for the following items were run, after 12-hour fasting:
- Fasting serum glucose: Enzymatic method. Glucose oxidase / peroxidase trinder. Reagent: Wiener.
- Serum total cholesterol: Enzymatic method. Lipase / cholesterol oxidase / peroxidase / trinder. Reagent: Wiener.
- Serum uric acid: Enzymatic method. Urease / peroxidase / trinder. Reagent: Biosystem.
- Serum creatinine: Jaffe's method without deproteinization. Automatized kinetic method. Reagent: solution prepared in laboratory containing sulfanilic acid and picric acid.
- LDL cholesterol: without pre-treatment. Reagent: LDL-C plus 2nd generation (Roche). MOPS buffer, HSDA, ascorbate oxidase, peroxidase, 4-amino antipyrine, cholesterol oxidase, cholesterol esterase.
- Serum triglycerides: Enzymatic Trinder Method. Reagent: Biosystem.
- C-Reactive Protein (CRP): Turbidimetric method. Latex particles carrying human C-reactive antiprotein antibodies were used and the agglutination was quantified by means of turbidimetry.
- Also, insulinemia levels were determined using EIA by chemiluminiscence (Immunite 1000), sensitivity 2 μIU/ml, CV of less than 8%, proinsuline cross-reactivity of less than 8.5%.
The eGFR was calculated from serum creatinine levels using the following formulas:
1. Abbreviated MDRD5 (eGFR-MDRD) (ml/min/1.73 m2), without correcting the equation for race;
2. Cockroft-Gault (eGFR-CG)6, 7 adjusted to 1.73 m2 using the Dubois-Dubois8 body surface area formula (ml/min/1.73 m2).
3. Reciprocal of serum creatinine: 100/serum creatinine (100/sCr) (dl/mg)
HOMA (Homeostasis Model Assessment)9 was calculated as insulin resistance indicator (HOMA-IR) using the formula ([Insulin (μU/ml) x Glycemia (mg/100 ml)/18]/22.5).
The cardiovascular risk factors considered were defined as being: 1) Hypertension: BP ≥ 140/90 mm Hg or undergoing antihypertensive treatment; 2) Diabetes Mellitus: fasting serum glucose ≥ 126 mg/dl or undergoing insulin or oral antidiabetic agents; 3) Obesity: BMI ≥ 30 Kg/m2; 4) Dyslipemia: serum total cholesterol ≥ 200 mg/dl, or serum triglycerides ≥ 150mg/dl, or undergoing hypolipemiant treatment; 5) Smoking: reported smoking ≥ 100 cigarettes and currently smoked.
The continuous variables were expressed as mean ± SE or CI 95% and the prevalences as cases per 100 inhabitants. The 2001 census data was used to adjust by age and sex. The differences between continuous variables were determined using a t-test for independent samples and analysis of variance (ANOVA). The association between eGFR and the continuous variables was evaluated using bivariate correlation. The variables which were shown to have significant correlation by the bivariate analysis were included as z-score in two models (with and without considering age) of stepwise multiple regression analysis. P value < 0.05 was considered significant. The data was processed using SPSS.
Results
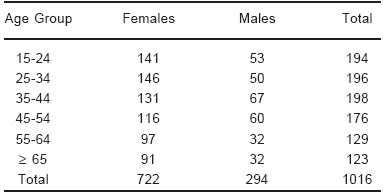
A total of 1016 individuals, 722 females (41.97 ± 0.66 years old) and 294 males (42.06 ± 0.99 years old), completed the laboratory tests. Table 1 shows the sample distribution according to sex and age.
TABLE 1.- Sample distribution according to sex and age
The prevalence of the cardiovascular risk factors was: hypertension 28.1, diabetes mellitus 6.6, obesity 30.0, dyslipemia 53.0 and smoking 24.6.
The mean reciprocal of serum creatinine was 115.13 ± 0.60 (dl/mg), the mean eGFR-CG was 98.48 ± 0.82 ml/min/1.73 m2; the mean eGFR-MDRD was 85.15 ± 0.58 ml/min/1.73 m2 (CI 95% 84.00-86.29). The correlation between eGFR-CG and eGFR-MDRD was r = 0.83 (p < 0.01). The correlation between reciprocal of serum creatinine and eGFR-MDRD was r = 0.68 (p < 0.01). The correlation between reciprocal of serum and eGFR-CG was r = 0.65 (p < 0.01). The eGFR-MDRD showed a normal distribution, it decreased with age (Figure 1, Table 2) and with the increase in the number of risk factors (Figure 2) in both sexes. The eGFR-MDRD was greater in males (p < 0.001) and the reciprocal of serum creatinine gave higher values for females than for males (p < 0.001) (Table 2). 35.4% of the sample showed an eGFR-MDRD ≥ 90 ml/min/1.73 m2, 59.1% between < 90 and < 60 ml/min/1.73 m2, and 5.5% < 60 ml/min/1.73 m2.
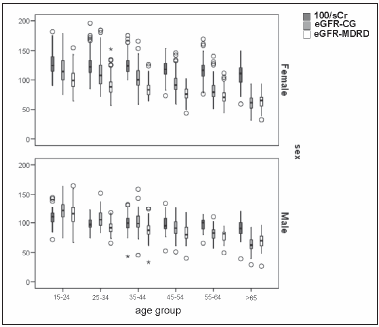
Fig. 1.- Box-plot of eGFR-MDRD, eGFR-CG (ml/min/1.73 m2) and reciprocal of serum creatinine (100/sCr, dl/mg) (y-axis) stratified by gender and age groups (in years) (x-axis). The decrease with age was significant in females (p = 0.000) and in males (p = 0.000).
TABLE 2.- Estimated Glomerular Filtration* using the MDRD formula (eGFR-MDRD ml/min/1.73 m2), the Cockcroft-Gault formula adjusted to body surface area (eGFR-CG, ml/min/1.73 m2) and reciprocal of serum creatinine (100/sCr, dl/mg) according to sex and age group
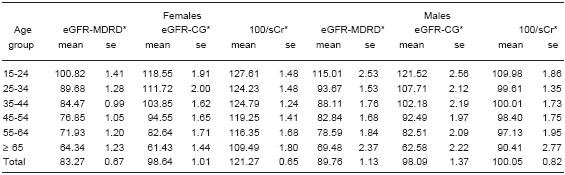
*ANOVA by age group p < 0.001

Fig. 2.- Box-plot of eGFR-MDRD (ml/min/1.73 m2) (y-axis) according to the number of cardiovascular risk factors (xaxis). The decrease with number of risk factors was significant in females (p = 0.000, unadjusted) and in males (p = 0.000, unadjusted).
The eGFR-MDRD < 60 ml/min/1.73 m2 adjusted prevalence was 6.2 (CI 95%, 4.7-7.7); 3.6 (CI 95%, 1.5-5.7) in males and 8.6 (CI 95%, 6.6-10.6) in females. The eGFR-CG adjusted prevalence was 8.4 (CI 95%, 6.7- 10.1); 7.4 (CI 95%, 4.4-10.4) in males and 9.3 (CI 95%, 7.2-11.4) in females. The prevalence of eGFR-MDRD < 60 ml/min/1.73 m2 increased with age in both sexes from the age of 45 and it was of 0.6, 2.2 and 18.8 in males < 45, 45-65 and > 65 years old, and of 0.2, 7.0 and 34.1 in females of the same age groups. Using the eGFR-CG, the prevalences of < 60 ml/min/1.73 m2 for the same age groups were of 0.6, 2.2 and 43.8 in males, and 0.2, 2.4 and 43.3 in females.
The bivariate analysis showed that the eGFR-MDRD correlates inversely with age, SBP, DBP, waist circumference, BMI, fasting serum glucose, serum total cholesterol, LDL cholesterol, serum triglycerides, serum uric acid and, in males, with CRP. There was no significant correlation with either insulinemia or HOMA. The eGFR-CG correlates inversely in both sexes with SBP, DBP, serum total cholesterol, HLD cholesterol levels and serum uric acid; and it correlates inversely with fasting serum glucose in females and with CRP in males. The reciprocal of serum creatinine correlates inversely with age, SBP, DBP, serum total cholesterol, LDL cholesterol, serum uric acid; and it correlates inversely with serum glucose in females and with waist circumference, BMI, CRP and serum triglycerides in males. In general, the correlations with eGFR-MDRD were stronger (Table 3).
TABLE 3.- Bivariate correlations with the estimated glomerular filtration rate calculated using the abbreviated MDRD study formula (eGFR-MDRD) and Cockcroft-Gault formula (eGFR-CG) adjusted to body surface area (ml/min/1.73 m2) and with the reciprocal of serum creatinine (100/sCr, dl/mg)

WC: waist circumference; BMI: body mass index; HOMA-IR: homeostasis model assessment-insulin resistance.
Using the stepwise multiple regression analysis without considering age as a covariate, the eGFR-MDRD was correlated in males with SBP (r -0.336; p = 0.019), with waist circumference (r -0.499; p = 0.000), with BMI (r - 0.376; p = 0.007), with serum total cholesterol (r -0.384; p = 0.000), with serum uric acid (r -0.415; p = 0.000), and with CRP (r -0.162; p = 0.016). In females, eGFR-MDRD was correlated with SBP (r -0.371; p = 0.000), with waist circumference (r -0.191; p = 0.018), with fasting serum glucose (r -0.201; p = 0.003), with serum total cholesterol (r -0.374; p = 0.000), with LDL cholesterol (r -0.332; p = 0.024), with serum triglycerides (r -0.085; p = 0.000), and with serum uric acid (r -0.401; p = 0.000). When we included age as covariant only the serum total cholesterol (p = 0.005) and the serum uric acid (p = 0.000) in males, and the serum total cholesterol (p = 0.004), serum triglycerides (p = 0.017), waist circumference (p = 0.000) and serum uric acid (p = 0.000) in females maintained the statistical significance.
Discussion
As far as we know, this is the first assessment of renal function in a random population sample of Argentina. In the USA the prevalence of eGFR < 60 ml/min/1.73 m2 was estimated by the National Health and Nutrition Examination Survey (NHANES) in 5.6 in 1988-1994, and in 8.1 in 1999-200410, which shows that the tendency is growing. In Europe there have been prevalence descriptions of 4.5 in Norway11, 5.1 in Spain12, 4.7 and 11.5 in Iceland13, for male and female individuals respectively. In England, a laboratory sample study of almost 100 000 subjects determined a prevalence of 4.014. In Asia, there have been prevalence descriptions of 4.6 in Thailand15, 4.9 in China16, and even higher prevalences (~15) in Japan17. In Australia, a prevalence of 11.2 was reported18. Part of the differences could be explained by the fact that there are different population pyramids, so comparisons need to be done on the basis of standardized population pyramids. In a systematic review of 26 studies a prevalence of eGFR < 60 ml/min was 7.2 in individuals over 30 years old19. The adjusted by age and sex prevalence of eGFR < 60 ml/min/1.73 m2 found in San Andrés de Giles is in the middle of the above mentioned studies, and the eGFR value (85.15 ml/min/1.73 m2) is quite close to gender-specific reference values of estimated GFR in Caucasians20 (85 ml/min/1.73 m2 for men and 83 ml/min/1.73 m2 for women). The prevalence of eGFR < 60 ml/min increased after the age of 45 and was highly prevalent in patients over 65, what agrees with the data from other sample population studies. More than half the population showed eGFR of between 60-90 ml/min/1.73 m2, what also agrees with data from other studies13.
We estimated glomerular filtration in three ways, by means of the abbreviated formula of the MDRD5 study, by means of the Cockroft-Gault6 formula, which was adjusted to the body surface area21 and by the reciprocal of serum creatinine. Although the correlation between the results of both formulas was high, the operational characteristics of both formulas differ in many aspects. In general, up to 65 years of age, the mean eGFR was higher with the Cockroft-Gault formula. However, the prevalence of individuals with eGFR < 60 ml/min/1.73 m2 was higher with the Cockcroft-Gault formula. Those patients who had eGFR of < 60 ml/min/1.73 m2 with the Cockroft-Gault formula but not with the abbreviated MDRD study formula were older, of an average age of ~75. The reciprocal of serum creatinine differed from the other equations in that females had higher eGFR than males. This may not come as a surprise since males tend to have higher serum creatinine values than females. In addition, there was little difference in the reciprocal of serum creatinine between age groups. This behaviour is similar to that observed in other studies on population samples13, 22.
The analysis of the relationship between eGFR and cardiovascular risk factors is complex, partly because of the effects of age on most variables (it is actually part of the eGFR calculation formula), and partly because of the complex interactions between variables. The eGFR decreased in both sexes as the number of cardiovascular risk factors present increased. In general, the eGFR calculated using the MDRD formula had a better correlation with factors associated to atherosclerosis than eGFR calculated using the Cockroft-Gault formula. In the bivariate correlation the magnitude of the association of eGFR with age, BP and dyslipemia indicators was similar in both sexes; on the other hand, the association with obesity indicators was greater in males. The eGFR had a greater correlation with SBP than with DBP, and the correlation was also greater with the waist circumference than with BMI. In the bivariate analysis, serum total cholesterol was the cardiovascular risk factor which had the highest correlation with eGFR, and it kept its correlation in the stepwise multiple regression analysis, in both sexes, even after age was considered. This high correlation of eGFR and serum total cholesterol may be explained by the high prevalence of dyslipemia that was found in the sample (53.0). Thus, there is a clear association between ageing, eGFR decrease, and the appearance of cardiovascular risk factors, facts which highlight the possible role of intrarenal atherosclerosis in the decrease of renal function with age. On the other hand, low eGFR is a risk factor independent from traditional factors and from albumin excretion23, which suggests a two-way relationship between atherosclerosis and renal function.
Amongst the non-traditional risk factors, CRP (inflammation indicator) had a weak and limited relationship with eGFR in males, and HOMA-IR (insulin resistance indicator) did not have a correlation with low eGFR, even though the used model does not rule out non-linear correlations.
Thus, the mean eGFR value, its association with cardiovascular risk factors and the prevalence of eGFR < 60 ml/min/1.73 m2 found in a sample of a rural population of Argentina are similar to those found in other parts of the world. The eGFR calculated with the abbreviated MDRD study formula shows a better correlation to cardiovascular risk factors.
Acknowledgements: This study could not have been conducted without the help of the nurses from the San Andrés de Giles Hospital.
Conflict of interest: None to declare
1. Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 2003; 41: 1-12. [ Links ]
2. Shik J, Parfrey PS. The clinical epidemiology of cardiovascular disease in chronic kidney disease. Curr Opin Nephrol Hypertens 2005; 14: 550-7. [ Links ]
3. Brosius FC 3rd, Hostetter TH, Kelepouris E, et al. Detection of chronic kidney disease in patients with or at increased risk of cardiovascular disease. Circulation 2006; 114: 1083-7. [ Links ]
4. de Jong PE, Gansevoort RT. Screening techniques for detecting chronic kidney disease. Curr Opin Nephrol Hypertens 2005; 14: 567-72. [ Links ]
5. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461-70. [ Links ]
6. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31-41. [ Links ]
7. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145: 247-54. [ Links ]
8. DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Int Med 1916; 17: 863-71. [ Links ]
9. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004; 27: 1487-95. [ Links ]
10. Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298: 2038-47. [ Links ]
11. Hallan SI, Coresh J, Astor BC, et al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol 2006; 17: 2275-84. [ Links ]
12. Otero A, Gayoso P, Garcia F, de Francisco AL. Epidemiology of chronic renal disease in the Galician population: results of the pilot Spanish EPIRCE study. Kidney Int Suppl 2005; (99): S16-9. [ Links ]
13. Viktorsdottir O, Palsson R, Andresdottir MB, Aspelund T, Gudnason V, Indridason OS. Prevalence of chronic kidney disease based on estimated glomerular filtration rate and proteinuria in Icelandic adults. Nephrol Dial Transplant 2005; 20: 1799-807. [ Links ]
14. Quinn MP, Rainey A, Cairns KJ, et al. The practical implications of using standardized estimation equations in calculating the prevalence of chronic kidney disease. Nephrol Dial Transplant 2008; 23: 542-8. [ Links ]
15. Chittinandana A, Chailimpamontree W, Chaloeiphap P. Prevalence of chronic kidney disease in Thai adult population. J Med Assoc Thai 2006; 89 (Suppl 2): S112-20. [ Links ]
16. Li ZY, Xu GB, Xia TA, Wang HY. Prevalence of chronic kidney disease in a middle and old-aged population of Beijing. Clin Chim Acta 2006; 366: 209-15. [ Links ]
17. Iseki K, Kohagura K, Sakima A, et al. Changes in the demographics and prevalence of chronic kidney disease in Okinawa, Japan (1993 to 2003). Hypertens Res 2007; 30: 55-62. [ Links ]
18. Chadban SJ, Briganti EM, Kerr PG, et al. Prevalence of kidney damage in Australian adults: The AusDiab kidney study. J Am Soc Nephrol 2003; 14 (Suppl 2): S131-8. [ Links ]
19. Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health 2008; 8: 117. [ Links ]
20. Wetzels JF, Kiemeney LA, Swinkels DW, Willems HL, den Heijer M. Age- and gender-specific reference values of estimated GFR in Caucasians: the Nijmegen Biomedical Study. Kidney Int 2007; 72: 632-7. [ Links ]
21. Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med 2006; 354: 2473-83. [ Links ]
22. Nitsch D, Felber Dietrich D, von Eckardstein A, et al. Prevalence of renal impairment and its association with cardiovascular risk factors in a general population: results of the Swiss SAPALDIA study. Nephrol Dial Transplant 2006; 21: 935-44. [ Links ]
23. Cirillo M, Lanti MP, Menotti A, et al. Definition of kidney dysfunction as a cardiovascular risk factor: use of urinary albumin excretion and estimated glomerular filtration rate. Arch Intern Med 2008; 168: 617-24. [ Links ]
Recibido: 29-12-2008
Aceptado: 29-5-2009














