Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Medicina (Buenos Aires)
versión impresa ISSN 0025-7680
Medicina (B. Aires) vol.70 no.3 Ciudad Autónoma de Buenos Aires mayo/jun. 2010
ARTÍCULO ORIGINAL
Bone and mineral metabolism in primiparous women and its relationship with breastfeeding: A longitudinal study
Mariela Glerean1, Aída Furci2, Ana María Galich1, Bruno Fama3, Luisa Plantalech1
1Servicio de Endocrinología, Metabolismo y Medicina Nuclear, Hospital Italiano de Buenos Aires;
2Laboratorio Díaz Vélez, Buenos Aires;
3Servicio de Obstetricia, Hospital Italiano de Buenos Aires
Postal address: Dra. Mariela Glerean, Servicio de Endocrinología, Metabolismo y Medicina Nuclear, Hospital Italiano de Buenos Aires, Gascón 450, 1181 Buenos Aires, Argentina Fax: (54-11) 4958-4564 e-mail: mariela.glerean@hospitalitaliano.org.ar
Abstract
The aim of this study was to evaluate the changes in bone metabolism in breastfeeding women (BF). We selected 30 primiparous women and compared them to 31 nulliparous women. We assessed bone mineral density (BMD) in the lumbar spine (LS), femoral neck (FN) and trochanter (TROC), biochemical parameters of bone turnover and hormone and cytokine levels at the puerperium, 6 months and 12 months after delivery. A trend to lower BMD of LS was seen at initial evaluation in BF. BMD in LS, FN, and TROC were increased 12 months after delivery. Baseline body mass index was higher in puerperal women (p = 0.02) and correlated with an increased FN and TROC BMD one year post delivery (p = 0.001 and p = 0.003). An increase in bone remodeling markers, and lower urinary calcium was observed; after 12 months these values normalized. Prolactin, parathormone related peptide (PTHrP) and IL-6 were enhanced during the first six months of breastfeeding. We conclude that calcium for breastfeeding was obtained by transient mobilization of calcium deposits from the trabecular bone, and urinary calcium sparing induced by calciotrophic hormones and cytokines. Body weigth is an important factor in proximal femur BMD.
Key words: Breastfeeding; Bone metabolism; Bone mineral density; Serum calcium; PTHrP; Interleukin 6
Resumen
Metabolismo óseo y mineral en mujeres primíparas y su relación con la lactancia: Un estudio longitudinal. El objetivo de este estudio fue evaluar los cambios que suceden en el metabolismo óseo de mujeres que amamantan. Se seleccionaron 30 mujeres primíparas y se compararon con 30 mujeres nulíparas como grupo control. Se evaluó la densidad mineral ósea (DMO) del raquis lumbar (RL), cuello femoral (CF) y trocánter (TROC), parámetros del remodelado óseo, hormonas y citoquinas. Estos parámetros se midieron en el puerperio inmediato, y a los 6 y 12 meses post-parto. La DMO del RL de la mujeres primíparas evidenciaron una tendencia a menores valores al comienzo de la lactancia comparadas con las mujeres controles, y se observó un incremento significativo de la DMO a los 12 meses, alcanzando valores similares al grupo control. La DMO en CF y del TROC aumentó significativamente a los 12 meses post parto. El índice de masa corporal basal fue mayor en el grupo de primíparas en el puerperio inmediato (p = 0.02) y correlacionó con el incremento observado en la DMO CF y del TROC al año del post-parto (p = 0.001 y p = 0.003, respectivamente). Un alto remodelado óseo y descenso en el calcio urinario se observaron en el puerperio inmediato, y tanto los marcadores óseos como la calciuria se normalizaron a los 12 meses post-parto. Prolactina, interleuquina-6 y el PTHrP aumentaron significativamente en los primeros 6 meses. Concluimos que el calcio de la leche materna proviene de la movilización de los depósitos cálcicos del hueso trabecular y del ahorro de la pérdida renal inducido por las hormonas calcitrópicas y las citoquinas involucradas en el metabolismo óseo. El peso corporal es un factor de importancia en el incremento de DMO del fémur proximal.
Palabras clave: Lactancia; Metabolismo óseo; Densidad mineral ósea; Calcemia; PTHrP; Interleuquina 6 (IL6)
During pregnancy, postpartum and breastfeeding, the maternal skeleton undergoes mobilization of calcium deposits, to provide calcium for the mineralization of the fetal skeleton and milk production. Such changes are regulated by different hormonal systems1-4. Milk production during a 3 to 4 month-breastfeeding period extracts 30 g of calcium2. Cumulative calcium deficit during pregnancy and lactation is estimated to be about 6% of the total bone mineral content5, 6. The mechanism by which calcium requirements are met during this period involves a transient skeletal demineralization. Several authors conclude that the maternal skeleton works as a calciumbuffer system in which the low density bone and osteoid rapidly exchange minerals and contribute additional calcium to that provided by supplements in the diet3, 4.
Ferretti et al4 have described that the total mineral content of the skeleton in relation to lean body mass is higher in pre-menopausal women than in post-menopausal women, suggesting that the relative "excess" during the reproductive age ensures the preservation of maternal skeletal health during the mineralization of the fetal skeleton and lactation. Breatsfeeding produces an extraction of nutrients from the mother that favors the loss of bone mass and osteoporosis during the reproductive period5, 6.
The aim of this study was to document the changes in bone and mineral metabolism, as well as the hormonal variations related to breastfeeding.
Materials and Methods
We selected 30 women in their first pregnancy (breastfeeding women: BF) followed at the Division of Obstetrics of the Hospital Italiano de Buenos Aires. Women who were younger than 21 or older than 40 years, or had a history of renal litiasis, known bone disease, prolonged amenorrhea, diabetes mellitus, endocrine diseases, chronic alcoholism, or taking medications that modified mineral metabolism were excluded. Twenty-two primiparous women (73.3%) completed the longitudinal study. The study group was compared to 31 nulliparous women (C) who fulfilled similar exclusion criteria. A medical history was obtained and the following data were recorded: age, body mass index (BMI: kg/m2); gynecological and obstetrical history, -age at menarche (years), -weight gain during pregnancy (kg), -duration of breastfeeding (months) and post-partum amenorrhea (months); physical activity (yes or no for programmed physical activity: minimum twice a week), smoking history (yes or no), family history of osteoporosis (yes or no), weekly hours of sun exposure and calcium intake [-null, scarce (< 1 g/day), moderate (1 g/day) and high (> 1 g/day)]. Nutritional status was evaluated with serum albumin levels and BMI.
The following parameters were measured in serum: 1- Ionized calcium (ICa, normal value -nv-:1 -1.35 mmol/l); 2- Phosphorus (P, nv: 2.5-4.5 mg/dl); 3- Creatinine (Cr, nv: 0.5-1.2 mg/dl); 4- Albumin (Alb, nv: 3.5-5 g/dl)7; 5- Total alkaline phosphatase (Alk P, nv: 40-190 U/I)8 and 6- Tartrate-resistant acid phosphatase (TRAP, nv: 1.8-4.4 U/I)9.7-Fasting urinary calcium/creatinine ratio (uCa/Creat) was assessed in the morning after 500 ml water ingestion (nv: ≤ 0.1).
Parathormone-related peptide (PTHrP by IRMA method, nv: 0.2-1 pg/ml)10; prolactin (PRL)11 by RIA method (nv: 5-25 ng/ml); estradiol (E2) by RIA (nv: 50-400 pg/ml)12 and interleukin 6 (IL-6) by IRMA method (nv: 0.3 -10 pg/ml)13 were measured in only 19 women, at delivery and at 3 and 6 months thereafter.
We performed a bone mineral densitometry (BMD) of the lumbar spine (LS), femoral neck (FN) and trochanter (TROC) with a Lunar DPX densitometer at baseline of puerperium, at 6 and 12 months postpartum. BMD was expressed in g/cm2.
Statistical analysis
The SPSS statistical software, version 10F, was used for data analysis. Differences were considered to be statistically significant when the probability (P) was < 0.05. The ANOVA, Mann-Whitney and chi-square tests were used for parametric and non parametric variables, respectively. Linear regression was applied for BMI and BMD correlations.
Results
Characteristics of primiparous women at delivery and controls are shown in Table 1. Primiparous's mean age was 29.0 ± 3.5 years. The duration of breastfeeding (6.2 ± 2.6 months) matched the period of amenorrhea. Their BMI was higher than controls at initial evaluation but no significant differences were observed 12 months postpartum (not shown). No differences between groups were found with regard to physical activity, family history of osteoporosis, smoking habit, milk intake or hours of exposure to sunlight. Sixty one and fifty three percent of control and breastfeeding women, respectively, had scarce calcium intake (< 1 g/d).
TABLE 1.- Characteristics of controls (C) and breastfeeding women (BF) at delivery (mean ± SD)

Y: yes/ N: no; ns: non significant; BMI: body mass index
At delivery, ionized calcium, serum phosphorus, alkaline phosphatase and tartrate-resistant alkaline phosphatase (TRAP) were increased compared to control values, whilst uCa/Creat ratio and albumin levels were decreased in breastfeeding women. At 12 months postpartum, all variables were similar to those of the control group, except Alk P values which continued to be elevated (Table 2).
TABLE 2.- Biochemical parameters of bone and mineral metabolism in controls (C) and breastfeeding women (BF) at delivery, and at 6 and 12 months (mean ± SD)

*p < 0.05 vs. C, **p < 0.01 vs. C, ***p < 0.001 vs C
#p < 0.01 vs. at delivery BF, ^ p < 0.001 at delivery and 6 months BF.
uCa/Creat: urinary calcium/creatinine ratio; AlkP: Alkaline phosphatase; TRAP: 6-tartrate-resistant acid phosphatase.
Prolactin and PTHrP showed a marked increase at early postpartum and reached normal levels by the third month (Fig. 1). Estradiol levels were not significantly different between groups, even though there was a non-significant trend to lower levels at delivery and 3 months. IL-6 levels in women who breastfed were higher than controls throughout the study period and declined at six months vs initial evaluation (p < 0.01) (Fig. 2).
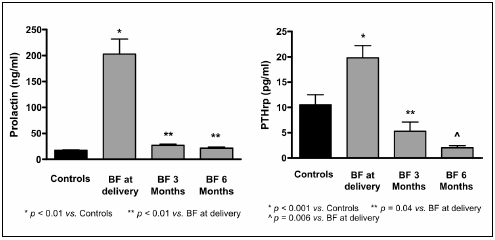
Fig 1.- Prolactin and PTHrP levels in controls and breastfeeding (BF) women at delivery, 3° and 6° months of lactation. (mean± SE)

Fig 2.- Estradiol and IL-6 levels in controls and breastfeeding (BF) women at delivery, 3° and 6° months of lactation. (mean±SE)
Mean lumbar spine BMD was reduced 5.2% in primiparous women compared to controls at initial evaluation but these values did not reach statistical significance. No significant differences were observed in FN and TROC (Table 3). An increase in BMD was observed at twelve months compared to BMD at delivery and at 6 months, in the three sites assessed (Table 3).
TABLE 3.- Bone mineral densitometry at three skeletal sites in controls (C) and breastfeeding women (BF) during the year of postpartum (mean ± SD)
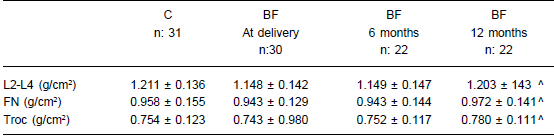
^ p < 0.001 vs. BF at delivery and six months
L2-L4: Lumbar L2-L4 vertebrae; FN: femoral neck; Troc: trochanter.
Early post partum BMI of BF women, correlated positively with BMD in the FN and TROC at one year (p = 0.001 and 0.003, respectively, Fig. 3).

Fig 3.- Correlation between basal body mass index (BMI) and bone mineral density (BMD) of the femoral neck (FN) and trochanter (TROC) one year post partum in breastfeeding women.
We did not observe any correlation between BMD changes and months of breastfeeding (not shown).
Discussion
We selected women in their third and fourth decades of life, in order to avoid the influence of physiological changes observed during the second decade when peak bone mass is attained and the fifth, when bone loss increases14, 15. All of them were primiparous, because it is well known that bone mass is affected by successive pregnancies5, 6, 16, 17. Age, risk factors for osteoporosis (family history, tobacco use, physical activity) were similar between groups. We should emphasize that calcium intake in both groups was low, and this is in agreement with previous studies from Argentina which showed that mean consumption for this age and sex was 600 mg/day18. The population studied was adequately nourished, as expressed by BMI and albumin levels. Serum albumin levels were lower in the puerperium, and this was attributed to the hemodilution observed in pregnancy. The BMI was higher in puerperal women and was attributed to the residual excess weight acquired during pregnancy.
There was an increase in bone remodeling, expressed by high biochemical markers during puerperium and the sixth months of breastfeeding, which returned to values similar to those of non-pregnant women at 12 months. However, Alk P levels did not return to normal levels in the period evaluated. It is well known that bone formation occurs after resorption and that the cycle of the remodeling unit requires 3 to 4 months. Hence, bone formation persists even after 12 months post-partum. The calcium proceeding from bone deposits and renal tubular resorption is destined to milk production1-5.
A hyperprolactinemic, hypoestrogenic amenorrheic state was showed at the beginning of breastfeeding. In our study, prolactin levels were highest during the first month post-partum, and returned to normal levels at the third month. At the same time, there was an initial increase in parathormone-related peptide or PTHrP, which then decreased gradually to normal levels in the 6th month. Prolactin is known to induce PTHrP production by the mammary gland, which stimulates bone remodeling, calcium transport in the mammary gland and maturation of the neonate's gut19. The presence of high circulating levels of PTHrP is associated with high bone resorption and low renal calcium excretion19-21. An increase in bone remodeling, induced by the PTHrP, and/or cytokines, has been postulated3, 22. Hypoestrogenism also increases bone loss mediated by cytokines (TNF, IL-1, IL-6)23-26.
We found high levels of IL-6 in serum during the first semester associated to amenorrhea. It is possible that the role of IL-6 in the stimulation of osteoclastogenesis is the link to high bone turnover found during breastfeeding23. We postulate a biphasic mechanism of high bone turnover: PTHrP action would drive the high turnover state of early puerperium, whilst persistent hypoestrogenism and associated high cytokines levels would explain negative bone balance during the subsequent months.
BMD in the lumbar spine and trochanter decreased during breastfeeding compared to baseline, and recovered at 12 months. Our observation agrees with those of other controlled and uncontrolled studies27-34. Trabecular bone undergoes a greater degree of bone remodeling than cortical bone. However, in our observational study the trochanter was not markedly affected compared to the lumbar region. In fact, we did not detect any significant differences with controls in the immediate postpartum period. We attribute this to the effect of mechanical load on the trochanter at the end of pregnancy.
Cortical bone was mobilized in a different manner during this period. The BMD of the femoral neck during the first month post-partum was similar to that of control women and increased significantly at the end of the breastfeeding period. Removal of cortical bone was poor and BMD in femoral neck increased 3%, this agrees with Naylor and Black AJ studies35, 36. The periostial remodeling induced by growth factors such as IGF1 and growth hormone, secreted by the placenta explain the positive bone balance35.
Furthermore, a direct relationship was observed between BMI at the end of pregnancy and recovery of BMD in the FN and TROC. According to current consensus the activity of the osteocytes would "sense" an increase in load, and stimulate new bone tissue formation37, 38 Pregnancy per se would have this additional effect on the proximal femur. The importance of calcium intake during breastfeeding has been evaluated in several studies39-41.
Randomized studies do not show any additional effects of calcium supplementation during pregnancy and breastfeeding on bone remodeling or BMD, although a mild improvement during the weaning period has been described40-42. In our study, fifty three percent of breastfeeding women had a calcium intake < 1gr and no differences were evidenced with the control group.
In the present study we have observed mobilization of calcium deposits from trabecular bone, with "restitution ad integrum" by the end of breastfeeding. The increase in BMD of the proximal femur depends on the body weight attained at the end of pregnancy. High bone turnover and urinary calcium sparing could be related to high levels of PTHrP in puerperium and increased levels of IL-6 during the later period of breastfeeding.
Acknowledgements: We thank María Fabiana Russo Picasso, MD, for the English translation.
Conflict of interest: None to declare.
1. Kovacs C, Kronenberg H. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium and lactation. Endoc Rev 1997; 18: 832-72. [ Links ]
2. Glerean M, Plantalech L. Osteoporosis en embarazo y lactancia. Medicina (Buenos Aires) 2000; 60: 973-81. [ Links ]
3. Sowers M. Pregnancy and lactation as risks factors for subsequent bone loss and osteoporosis. J Bone Miner Res 1996; 11 1052-60. [ Links ]
4. Ferretti JL, Capozza RF, Cointry GR, et al. Gender-related differences in the relationship between densitometric values of whole-body bone mineral content and lean body mass in humans between 2 and 87 years of age. Bone 1998; 22: 683-90. [ Links ]
5. Specker B, Bincley T. High parity is associated with increased bone size and streght. Osteoporosis Intl 2005; 16: 1969-74. [ Links ]
6. Fox KM, Magaziner J, Sherwin R, et al. Reproductive correlates of bone mass in elderly women. J Bone Miner Res 1993; 8: 901-8. [ Links ]
7. Farrel E. Methods in Clinical Chemistry. New York: Mosby, 1987. [ Links ]
8. Rosalki SB, Foo AY. Multicenter evaluation of iso ALP Kit for measurement of bone alkaline phosphatase activity in serum and plasma. Clin Chem 1993; 39:648-52. [ Links ]
9. Yung DS, Pestaner LC, Gibberman V. Effects of drugs on clinical laboratory tests. Clin Chem 1975; 21: 430-2 [ Links ]
10. Ratcliffe WA, Norbury S, Heath DA, Ratcliffe JG. Development and validation of an immunoradiometric assay of parathyrin related-protein in extracted plasma. Clin Chem 1991; 37: 678-685. [ Links ]
11. Kao PC, Jiang NS, Abboud CF. Radioimmunoassay of human homologous prolactin in serum with commercially available reagents. Clin Chem 1997; 43: 1563-8. [ Links ]
12. Thomas CM, van den Bergh RJ and Segers MF. Measurement of serum estradiol: comparison of three "direct" radioimmunoassays and effects of organic solvent extraction. Clin Chem 1987; 33: 1946-7. [ Links ]
13. Brally H, Montero-Julian FA, Zuber CE, Flavetta S, Grassi J, van Snick J. Total interleukin-6 in plasma measured by immunoassay. Clin Chem 1994; 40: 116-23. [ Links ]
14. Gilzanz V, Nelson D. Childhood and adolescence. In Primer on the Metabolic Bone Disease and Disorders of Mineral Metabolism. 5th ed. Murray J Favus and associated (eds). Washington, DC: American Society for Bone and Mineral Research 2003; 71-80. [ Links ]
15. Reid I. Menopause. In Primer on the Metabolic Bone Disease and Disorders of Mineral Metabolism 5th Ed. Murray J Favus and associated (eds). Washington, DC: American Society for Bone and Mineral Research 2003; 86-9. [ Links ]
16. Aloia JF, Cohn SH, Vaswani A, Kapoor A, Yeh JK, Colin SH. Risks factors for postmenopausal osteoporosis. Am J Med 1985; 78: 95-100. [ Links ]
17. Gur A, Nas K, Cevik R, Sarac AJ, Ataoglu S, Karakoc M. Influence of number of pregnancies on bone mineral density in postmenopausal women of different age groups. J Bone Miner Metab 2003; 21: 234-41. [ Links ]
18. Ercolano M, Drnovsek M, Moran M, Salerni H, Guadagna M, Rubin Z. Encuesta sobre ingesta de calcio en mujeres de Capital Federal y Gran Buenos Aires. Rev Argentina de Endocrinología y Metabolismo 2001, 36: 591-3. [ Links ]
19. Lippuner K, Zehnder H, Casez JP, Takkinen R, Jaeger P. PTHrP released into the mother's bloodstream during lactation: Evidence for beneficial effects on maternal calcium-phosphate metabolism. J Bone Miner Res 1996; 11: 1394-9. [ Links ]
20. Stiegler C, Leb G, Kleinert R, Warnkross H, Lipp R, Dobnig H. Plasma Levels of parathyroid hormone-related peptide are elevated in hyperprolactinemia and correlated to bone density status. J Bone Miner Research 1995; 10: 751-9. [ Links ]
21. Strewler G. The physiology of parathyroid hormone-related protein. N Engl J Med 2000; 342: 177-85. [ Links ]
22. Kovacs C, Chik C. Hyperprolactinemia caused by lactation and pituitary adenomas are associated with altered serum calcium, phosphate, parathyroid hormone (PTH) and PTHrP levels. J Clin Endocrinol Metab 1995; 80: 3036-41. [ Links ]
23. Sissel LO, Neville CW, Solveig T, Rigmor A. Cytokines in normal human pregnancy. Am J Obstet Gynecol 1993; 169: 397-404. [ Links ]
24. Jilka RL, Hangoc G, Gorasole G, et al. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science 1992; 257: 88-91. [ Links ]
25. Zinaman MJ, Hickey H, Tomai TP, Alberstson BD,Simon JA. Calcium metabolism in postpartum lactation: The effect of estrogen status. Fertil Steril 1990; 54: 465-9. [ Links ]
26. Van Houten JN, Wylsolmerski JJ. Low estrogen and high parathyroid hormone related peptide levels contribute at accelarated bone resorption and bone loss in lactating mice. Endocrinology 2003; 144: 5521-9. [ Links ]
27. Cross N, Hillman L, Allen S, Krause G,Vieira N. Calcium homeostasis and bone metabolism during pregnancy, lactation and postweaning: A longitudinal study. Am J Clin Nutr 1995; 61: 514-23. [ Links ]
28. Kent GN, Price RI, Gutteridge DH, et al. Human lactation bone loss, increased bone turnover and renal conservation of calcium and inorganic phosphate with recovery of bone mass following weaning. J Bone Min Res 1990; 5: 361-9. [ Links ]
29. Sowers M. Changes in bone density with lactation. JAMA 1993; 269: 3130-5. [ Links ]
30. Yamaga A, Taga M, Minaguchi H, Sato K. Changes in bone mass as determined by ultrasound and biochemical markers of bone turnover during pregnancy and puerperium: A longitudinal study. J Clin Endocrinol Metab 1996; 81: 752-6. [ Links ]
31. Affinito P, Tommaselli G, Di Carlo C, Guida F, Nappi C. Changes in bone mineral density and calcium metabolism in breastfeeding women: a one year follow up study. J Clin Endocrinol Metab 1996; 81: 2314-8. [ Links ]
32. Pearson D, Kaur M, San P, Lawson N, Baker P, Hosking D. Recovery of pregnancy mediated bone loss during lactation. Bone 2004; 34: 570-8. [ Links ]
33. Drinkwater B, Chesnut C. Bone density changes during pregnancy and lactation in active women. Bone Miner 1991; 14: 153-60. [ Links ]
34. Kent GN, Price RI, Gutteridge DH, et al. Effect of pregnancy and lactation on maternal bone mass and calcium metabolism. Osteoporosis Intl 1993; 1: 44-7. [ Links ]
35. Naylor KE, Iqbal P, Fledelius C, Fraser RB, Eastell R. The effect of pregnancy on bone density and bone turnover. J Bone Miner Res 2000; 15: 129-37. [ Links ]
36. Black AJ, Topping J, DurhamB, Farquharson RG, Fraser WD. A detailed assement of alterations in bone turnover calcium homeostasis and bone density in normal pregnancy. J Bone Miner Res 2000; 15: 557-563. [ Links ]
37. Nijweide PJ, Burger EH, Klein-Nulend J. The osteocyte. In Principles of Bone Biology by Bilezikian J, Raisz Land Rodan G. 2nd Ed San Diego: Academic Press 2002, p 93-107. [ Links ]
38. Lian JB SteinJS, Aubin JE. Bone formation: Maturation and functional activities of the osteoblast linage cells. In Primer on the Metabolic Bone Disease and Disorders of Mineral Metabolism. 5th Ed. Murray J Favus and associated (eds).; Washington, DC: American Society for Bone and Mineral Research 2003, p 13-28. [ Links ]
39. Cross NA, Hillman L, Allen S, Krause G. Changes in bone mineral density and markers of bone remodeling during lactation and postweaning in women consuming high amounts of calcium. J Bone Miner Res 1995; 9: 1312-20. [ Links ]
40. Kalkwarf HJ, Specker BL, Bianchi DC, Ranz J, Ho M. The effect of calcium supplementation on bone density during lactation and after weaning. N Engl J Med 1997; 337: 523-8. [ Links ]
41. Prentice A, Jarjou LM, Stirling DM, Buffestein R, Fairweather- Tait S. Biochemical markers of calcium and bone metabolism during 18 months of lactation in Gambian women accustomed to a low calcium intake and in those consuming a calcium supplement. J Clin Endocrinol Metab 1998; 83: 1059-66. [ Links ]
42. Jarjou LM, Pentrice A, Sawo Y, et al. Randomized placebo- controlled, calcium supplementation study in pregnant Gambian women: effects on breast-milk calcium concentrations and infant birth weight, growth, and bone mineral accretion in the first year of life. Am J Clin Nutr 2006; 83: 657-66. [ Links ]
Recibido: 18-9-2009
Aceptado: 13-4-2010














