Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Medicina (Buenos Aires)
Print version ISSN 0025-7680
Medicina (B. Aires) vol.70 no.3 Ciudad Autónoma de Buenos Aires May/June 2010
COMUNICACIÓN BREVE
Aberrant expression of glucagon receptors in adrenal glands of a patient with Cushing's syndrome and ACTH-independent macronodular adrenal hyperplasia
Valeria de Miguel1, María Ana Redal2, María Lorena Viale1, Mariano Kahan2, Mariela Glerean1, Axel Beskow3, Patricia Fainstein Day1
1Servicio de Endocrinología, Metabolismo y Medicina Nuclear,
2Unidad de Medicina Molecular y Genómica,
3Departamento de Cirugía, Hospital Italiano de Buenos Aires
Postal address: Dra. Patricia Fainstein Day, Servicio de Endocrinología, Metabolismo y Medicina nuclear, Hospital Italiano, Av. Gascón 450, 1181 Buenos Aires, Argentina Fax: (5411) 4959-0323 e-mail: patricia.fainstein@hospitalitaliano.org.ar
Abstract
Adrenocorticotropin (ACTH) independent bilateral macronodular adrenal hyperplasia (AIMAH) is a rare cause of Cushing´s syndrome, characterized by bilateral adrenal lesions and excess cortisol production despite ACTH suppression. Cortisol synthesis is produced in response to abnormal activation of G-protein- coupled receptors, such as gastric inhibitory peptide, vasopressin, beta adrenergic agonists, LH/hCG and serotonin receptors. The aim of this study was to analyze the expression of glucagon receptors in adrenal glands from an AIMAH patient. A patient with ACTH-independent Cushing´s syndrome and bilateral macronodular adrenal hyperplasia was screened for altered activation of adrenal receptors by physiological (mixed meal) and pharmacological (gonadotrophin releasing hormone, ACTH and glucagon) tests. The results showed abnormally high levels of serum cortisol after stimulation with glucagon. Hypercortisolism was successfully managed with ketoconazole treatment. Interestingly, a 4-month treatment with a somatostatin analogue (octreotide) was also able to reduce cortisol secretion. Finally, Cushing's syndrome was cured after bilateral adrenalectomy. Abnormal mRNA expression for glucagon receptor in the patient´s adrenal glands was observed by Real-Time PCR procedure. These results strongly suggest that the mechanism of AIMAH causing Cushing´s syndrome in this case involves the illicit activation of adrenal glucagon receptors. This is the first case reported of AIMAH associated with ectopic glucagon receptors.
Key words: Cushing´s syndrome; Adrenocorticotropin; ACTH-independent bilateral macronodular adrenal hyperplasia (AIMAH); Glucagon receptor; Illicit receptor
Resumen
Expresion aberrante de receptores de glucagón en tejido adrenal de un paciente con síndrome de Cushing e hiperplasia adrenal macronodular indedependiente de ACTH. La hiperplasia adrenal macronodular bilateral independiente de ACTH (HAMIA) es una causa infrecuente de Síndrome de Cushing, caracterizada por lesiones adrenales bilaterales, hipercortisolismo y ACTH plasmática suprimida. La síntesis de cortisol estaría regulada a través de ligandos de receptores asociados a proteína G que se expresan en forma aberrante en la corteza de las glándulas adrenales. El objetivo de este trabajo es analizar la presencia del receptor de glucagón en tejido adrenal de un paciente con diagnóstico de Síndrome de Cushing asociado a hiperplasia adrenal bilateral independiente de ACTH. Se realizaron tests de estímulos fisiológicos y farmacológicos para evaluar la respuesta en la secreción de cortisol. Como resultado se observó respuesta significativa del cortisol posterior al estímulo con glucagón. El paciente presentó buena evolución clínica y bioquímica al tratamiento con ketoconazol. La administración del análogo de somatostatina (octreotide) redujo los niveles de cortisol. Finalmente, la curación se logró posteriormente a la adrenalectomía bilateral. Mediante el estudio de PCR en Tiempo Real se halló la presencia del receptor de glucagón en tejido adrenal del paciente. Según nuestro conocimiento, es el primer paciente descripto de HAMIA vinculado a la expresión ilícita de receptores de glucagón.
Palabras clave: Síndrome de Cushing; Síntesis de cortisol; Adrenocorticotrofina; Hiperplasia adrenal bilateral no dependiente de ACTH; Receptores de glucagón
Cushing´s syndrome (CS) results from chronic exposure to high serum cortisol levels and other adrenal steroids. Bilateral adrenal lesions occur in 10-15% of adrenal CS, including corticotropin (ACTH)-independent macronodular adrenal hyperplasia (AIMAH)1. The excess cortisol secretion, which leads to progressive inhibition of ACTH release is under control of ligands that bind to illegitimate G-protein-coupled receptors (GPCRs) in the adrenal glands (AG). The expression of ectopic receptors like gastric inhibitory polypeptide (GIP), beta adrenergic, vasopressin V2-V3, serotonin (5-HT7) receptors, and abnormally active eutopic receptors like vasopressin V1, luteinizing hormone/human gonadotrophin (LH/hCGR) and serotonin (5-HT4) receptors have being described2. We present the study of a patient evaluated for Cushing's syndrome. A 64-year old man reported a history of obesity, severe arterial hypertension, diabetes mellitus, congestive heart failure, mild chronic renal insufficiency, weakness and depression. Physical examination showed clinical signs of Cushing's syndrome including central abdominal obesity, thin skin, dorsocervical fat pad, reddish purple striae, proximal muscle weakness, and ecchymoses. Biochemical studies revealed: 24-hour urinary free cortisol (UFC) 250 μg (normal up to 100), late- night free salivary cortisol: 24 nmol/l (0.7- 5) and serum cortisol after 1 mg overnight dexamethasone suppression test: 32 μg/dl (up to 5). Morning plasma ACTH was undetectable: less than 10 pg/ml (10 - 46), confirmed in three assays. An abdominal computed tomography (CT) scan showed bilateral macronodular adrenal hyperplasia. The right gland measured 11 x 4 cm, and the left, 8 x 5 cm. To screen for aberrant adrenal expression of GPCRs we performed some of the stimulation tests, according to the protocol suggested by Lacroix et al3, 4. The strategy is based on stimulation tests with different hormones (endogenous or exogenous) for potential aberrant receptors, while monitoring serum levels of cortisol. First, a mixed meal was given to assess the presence of GIP, also 250μg of ACTH was administrated intravenously. During the second day, 100 μg of gonadotrophin-releasing hormone (GnRH) was administrated intravenously and on the last day, 1 mg of glucagon was administrated intramuscularly. Serial measurement of cortisol levels were performed every 30 minutes intervals during 3 hours following the intervention. ACTH was not measured during the tests3. The results showed an increased cortisol response to stimulation with glucagon (Table 1). Cortisol response to ACTH is always present in AIMAH patients5. As previously described, more than 50% increase from the baseline of cortisol levels is considered as a positive response3. After 12-months of successful treatment with ketoconazole, the patient was submitted to bilateral adrenal surgery. Right adrenal excision had to be postponed because of harmful bleeding. Based on biochemical studies that showed cortisol response after exogenous glucagon administration, we decided to treat the patient with octreotide (20 mg/month) in order to control cortisol secretion6 as well as to slow down adrenal hyperplasia7. After four months of octreotide treatment cortisol secretion could be partially controlled (UFC: 140-200 μg/24 h) and the surgical excision of the right adrenal could be easily made without abnormal bleeding. Interestingly, exogenous glucagon stimulus during octreotide treatment did not elicit significant cortisol response. We have no argument to explain this finding.
TABLE 1.- Stimuli tests for cortisol

Pathological studies revealed adrenal nodular hyperplasia. The patient is under hydrocortisone (30 mg) and fludrocortisone (0.015 mg) treatment.
We studied adrenal glucagon receptor (GCGR) mRNA expression in normal adrenal glands and in the patient adrenal gland by Real Time PCR (RT-PCR) assays. In order to do this, total RNA was extracted from the patient right adrenal gland and purified with Trizol RNA purification kit (Invitrogen, USA). The corresponding cDNA was obtained using RT polymerase Improm II (Promega) and used as template for RT-PCR assay. cDNA obtained from an hepatocarcinoma cell line was used as a positive control for GCGR expression, and cDNA from normal adrenal gland as a negative control. Beta-actin was used as an amplifications control. RT- PCR was performed in a Light-Cycler 2.0 System (Roche Applied Science, USA) with the specific GCGR primers forward (5´- CGCTGACCCTCATCCCTCCTG-3´) and reverse (5´- TAGAGGACAGCCACCAGCAG-3´) using the Taq Platinum polymerase (Invitrogen) and SYBR Green I (Invitrogen) to follow the kinetic reaction. Cycling conditions were: one cycle at 94 °C for 10 min and 45 cycles at 94 °C for 10 s, 57 °C for 10 s and 72 °C for 10 s. As shown in Fig. 1, the 148 bp PCR product corresponding to GCGR mRNA was present in the patient´s adrenal gland but absent in the normal control gland.
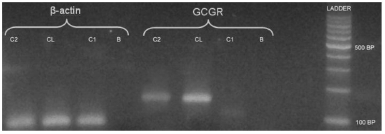
Fig. 1.- Electrophoresis in agarose gel stained by ethydium bromide. Control beta actin, a PCR product of 122 bp; line 1: patient (C2); line 2: hepatocarcinoma cell line(CL); line 3: normal adrenal gland(C1); line 4: negative control assay (B). PCR product of 148 bp RNAm GCGR is present in line 5/C2, line 6/CL, and absent in line 7/C; line 8/B.
In conclusion, we suggest that cortisol hypersecretion was related to ectopic expression of glucagon receptor in the adrenal gland of this patient. The cortisol response to exogenous glucagon stimulation and the finding of glucagon receptor mRNA strongly support this hypothesis.
This is the first case reported of AIMAH associated with ectopic glucagon receptors.
Conflict of interest: None to declare.
1. Nieman, L K. Cushing´s syndrome. In: De Groot L J, Jamerson, J L (eds). Endocrinology. Philadelphia: W.B. Sanders Company, 2001, p 1691-720. [ Links ]
2. Lacroix A, Baldacchino V, Bourdeau I, Hamet P, Tremblay J. Cushing´s syndrome variants secondary to aberrant hormone receptors. Trends in Endocrinol Metab 2004; 15: 375-82. [ Links ]
3. Lacroix A, N´Diaye N, Tremblay J, et al.. Ectopic and abnormal hormone receptors in adrenal Cushing´s syndrome. Endocr Rev 2001; 22: 45-110. [ Links ]
4. Lacroix A., Mirescu H, Hamlet P. Clinical evaluation of the presence of abnormal hormone receptors in adrenal Cushing´s syndrome. The Endocrinologist 1999; 9: 9-15. [ Links ]
5. Mirescu H, Jilwan, J D'Diaye, N, et al. Are ectopic or abnormal membrane receptors frequently present in adrenal Cushing's syndrome? J Clin Endocrinol Metab 2000; 85: 3531-6. [ Links ]
6. Strowski MZ, Parmar RM, Blake AD, Schaffer JM. Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: an in vitro of pancreatic islets from somatostatin receptor 2 knock out mice. Endocrinology 2000; 141: 111-7. [ Links ]
7. Zou Y, Xiao X, Li Y, Zhou T. Somatostatin analogues inhibit cancer cell proliferation in an SSTR2- dependent manner via both cytostatic and cytotoxic pathways. Oncol Rep 2009; 21: 379-86. [ Links ]
Recibido: 19-1-2010
Aceptado: 30-3-2010














