Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Medicina (Buenos Aires)
versión impresa ISSN 0025-7680
Medicina (B. Aires) vol.71 no.1 Ciudad Autónoma de Buenos Aires ene./feb. 2011
ARTÍCULO ORIGINAL
Breast cancer and the stromal factor. The "prometastatic healing process" hypothesis
Mario Wernicke1, Pablo Roitman1, Daniela Manfré1, Robert Stern2
1Departmento de Patología, Hospital Italiano de Buenos Aires,
2Al-Quds University, Faculty of Medicine, Department of Pathology, Jerusalem, Israel
Postal Adress: Dr. Mario Wernicke, Departamento de Anatomía Patológica, Hospital Italiano de Buenos Aires, Gascón 450,1181 Buenos Aires, Argentina Fax (54-11) 4959-0352 e-mail: mariowernicke@hotmail.com
Abstract
The correlation between axillary status and several histological features of breast carcinomas has been well established, however stromal changes have rarely been analyzed. Detailed clinicopathological review of 1803 patients with infiltrating breast carcinoma was performed. Stromal myxoid changes (SMC), size (T2-T3: > 2 cm, T1c: 1-2 cm, T1 a-b: < 1cm), fibrotic focus, age, lymphovascular embolizations, tumor infiltrating lymphocytes (TIL), multifocality, histological grade (G), estrogen receptors (ER), progesterone receptors (PR) and HER2 were semi-quantitated in two or three grades and correlated to axillary status. SMC3 followed by T2-T3, G3, fibrotic focus, T1c, embolizations, SMC2, TIL2, G2 and multifocality were strongly associated with positive axillary nodes; an inverse association was found with ER+++ and PR+++. Our findings support a critical role of the peritumoral stroma in the development of metastases. These stromal alterations should be remarked in routine pathology reports as they can be easily assessed and provide important information about tumor biology and aggressiveness. They could also become, in a future, the target of novel therapeutics.
Key words: Breast cancer; Stroma; Metastases; Hyaluronan
Resumen
El factor estromal en el cáncer de mama. La hipótesis del "proceso cicatrizal prometastásico". La correlación entre estado axilar y varias características histológicas de los carcinomas de mama está bien establecida, sin embargo los cambios estromales rara vez fueron analizados. En el presente trabajo se realizó una revisión detallada de las características clínico-patológicas de 1803 pacientes con carcinoma infiltrante de mama. Los cambios mixoides estromales (SMC), el tamaño tumoral (T2-T3: > 2 cm, T1c: 1-2 cm, T1 a-b: < 1 cm), el foco fibroso, la edad, embolizaciones tumorales linfovasculares, infiltracion linfocitaria tumoral (TIL), multifocalidad, grado histológico (G), los receptores estrogénicos (RE) y los receptores progestacionales (RP) y HER2 fueron semicuantificados en dos o tres grados y correlacionados con el estado axilar. El estudio multivariante demostró la asociación entre SMC grado 3 seguido por el tamaño tumoral T2-T3, G3, foco fibroso, T1c, embolizaciones tumorales linfovasculares, SMC2, TIL2, G2, multifocalidad y presencia de ganglios axilares metastásicos (p < 0.0001). Asimismo pudo comprobarse una asociación inversa entre RE+++ y RP+++ (p < 0.0001) con la presencia de metástasis axilares. Nuestros hallazgos sugieren un rol crítico del estroma peritumoral en el desarrollo de metástasis. Estas alteraciones estromales deberían, en nuestra opinión, constar en los informes de patología quirúrgica dado que son de fácil evaluación y aportan importante información acerca de la biología y agresividad tumoral. Además podrían convertirse, en un futuro, en el blanco de nuevas terapéuticas.
Palabras clave: Cáncer de mama; Estroma; Metástasis; Ácido hialurónico
When only conventional histopathology is utilized for the assessment of breast cancer, clinicians rely on a few factors: histological grade (G), size and nodal status. They add as secondary predictive factors the hormone receptors expression and the amplification of the oncogene HER2. The most important of these, the presence of metastatic axillary lymph nodes (MALN), is a significant predictor of survival in patients with infiltrative breast carcinoma1.
Tumor size is a well recognized pathological feature that predicts MALN, however it is not uncommon to find small tumors with positive nodes and large tumors without metastases, a fact that suggests other factors besides tumor size are involved.
Carcinomas show two discrete but interdependent compartments: the malignant cells and the stroma they induce within which they are dispersed. The latter usually suffers three essential histological modifications that can be easily assessed by a surgical pathologist: stromal myxoid changes (SMC), tumor infiltrating lymphocytes (TIL) and desmoplasia.
SMC are a provisional myxoid-type reactive matrix composed of material that stains for Alcian Blue and is found interspersed between collagen fibers. Hyaluronan (HA) is one of the major components of this myxoid matrix and its synthesis is stimulated by the interaction between malignant cells and stromal fibroblasts2. Auvinen et al3 found a relationship between HA, poorly differentiated tumor cells and axillary nodes positivity. Other authors have reported that HA is a fundamental piece of the metastatic process4-6. Also, a myxoid stroma was associated with advanced rectal cancer7, microinvasive adenocarcinoma of the endometrium8 and aggressive tumor behavior in vulvar squamous cell carcinomas9.
At sites of tumor invasion, TIL follow the SMC and these inflammatory cells, traditionally considered a good histopathological prognostic factor, have a paradoxical effect, as reviewed recently10. In addition, pronounced desmoplasia, called by Hasebe fibrotic focus, was found to be associated with cancer aggressiveness11.
With emphasis on these components we have investigated several clinicopathological features of breast carcinomas, their interactions and their association with MALN to study the role of the peritumoral stroma in the development of metastases.
Materials and Methods
A total of 2787 consecutive cases of breast cancer surgical specimens, from the period between January, 1987 and July, 2006, were retrieved from the files of the Department of Pathology of the Italian Hospital of Buenos Aires. Cases without axillary sampling (axillary dissection or sentinel lymph node biopsy) or with either preoperatory radiotherapy or neoadjuvant chemotherapy were not included because of the epithelial and stromal distortion caused by treatments, leaving a total of 1803 cases for evaluation. All tumors studied had been formalin fixed. Four micron-thick hematoxylin and eosin-stained sections were examined in all cases.
The histological slides were approached with a double-headed microscope by two pathologists (MW, PDR); who were blind to axillary status. In case of disagreement, a final consensus was established.
The validity and reproducibility of most of the following variables are well established12. However it should be clarified that we did not consider histological tumor type as a workable variable. There are more than thirty tumor types described13 and association of patterns would add even more difficulty, hence, to provide homogeneous sets for statistical analysis, we only considered G.
Variable 1: SMC. Briefly, SMC can be defined as an amorphous stromal substance composed of an amphophilic or slightly basophilic vacuolated material found among the collagen fibers (Fig.1). Usually SMC are found intermingled with cells that compose the granulation tissue and within fibrous areas of an early wound healing process. HA is one of its major components as we demonstrated with immunohistochemistry utilizing a HA binding probe14.
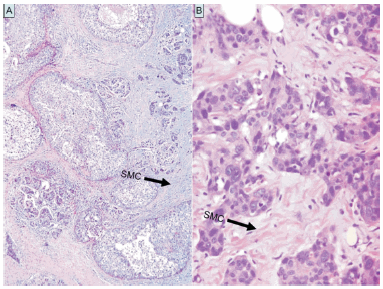
Fig. 1.- 1. A: SMC seen as a slightly basophilic material in the peritumoral stroma. 1. B: SMC found among the collagen fibers.
Cases were assessed with a low-power objective, examining both the periphery and center of the lesion. They were recorded as absent or scanty (SMC1), moderate, if at least three foci were found at the periphery (SMC2), and marked (SMC3) when large areas of SMC were observed at the periphery and center of the tumor. In the present work SMC were appraised with hematoxylin-eosin stained slides.
The level of agreement for this variable was evaluated with kappa statistics in a previous work14. There was substantial agreement among observers in SMC1 (kappa: 0.77). Greater variations were observed in SMC2 (kappa: 0.53) and SMC3 (kappa = 0.69). Overall kappa showed a substantial level of agreement (kappa: 0.67). There was also a strong correlation between this three grade classification and the staining intensity with the HA binding probe14.
Variable 2: Fibrotic focus. When there was a well-formed central sclerotic nodule, often associated with acellular hyalinized keloid-like tissue, it was called a fibrotic focus.
Variable 3: TIL. TIL1: Only occasional peritumoral lymphocytes were found, TIL 2: intermediate or dense cellular lymphocytic infiltration.
Variable 4: G. This was evaluated based on the Scarff Bloom Richardson grading system15. The grade was expressed as G1, G2 or G3.
Variable 5: Embolizations: This refers to peritumoral vascular invasion and was recorded as either positive or negative.
Variable 6: Size. Less than 1cm (T1a-T1b from the TNM Classification of the International Union Against Cancer); 1 to 2cm (T1c) and more than 2cm (T2-T3).
Variable 7: Age. In order to evaluate gradual changes related to aging we subdivided our population into four age groups: 45 years old or younger; 46-55 years; 56-65 years and older than 66.
Variable 8: Multifocality. Present or absent. A tumor was considered multifocal when two or more lesions were located in the same quadrant and distance from each other was less than 5 cm.
Variable 9: Axillary status. The presence of MALN was recorded as positive (including micrometastases) or negative (negative nodes in axillary dissection or negative sentinel lymph node when this technique was performed. We included isolated tumor cells in this group).
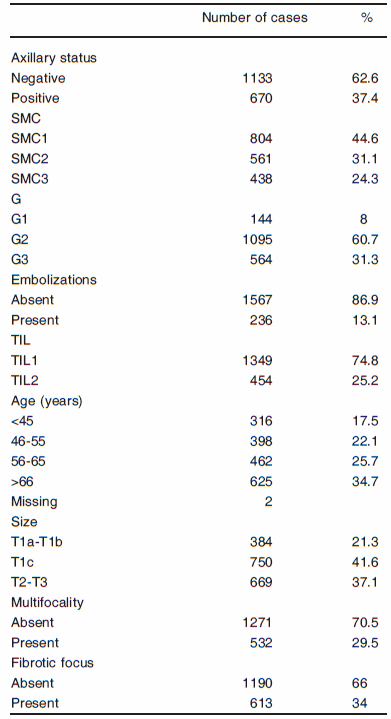
The clinicopathological characteristics of the patients are summarized in Table 1.
TABLE 1.- Clinicopathologic characteristics of the 1803 patients
Starting at our institution in 1999, immunohistochemical determination of hormone receptors and HER2 expression became gradually part of the routine examination, as a result, only 935 and 746 cases had hormone receptors and HER2 determinations performed respectively. Therefore from the 1803 cases, complete analysis was done on 746 patients.
Immunohistochemical studies were performed using antibodies against estrogen receptor (ER) (1D5; 1:35 dilution, DAKO Cytomation), progesterone receptor (PR) (PgR636; 1:40 dilution, DAKO Cytomation) and HER2 (CB11; 1:200 dilution, DAKO Cytomation). All immunostains were performed according to the avidin-biotin method, using tissue sections of 3 μm thickness. Briefly, sections were deparaffinized with xylene and rehydrated in graded ethanol. Antigen retrieval using citrate buffer (pH 6.0) in pressure cooker for ER and PR and in water bath for HER2 was performed. Endogenous peroxidase was blocked by 0.3% hydrogen peroxide for 10 minutes. Sections were incubated with primary antibody for 30 minutes at 37 °C, then with the biotinylated secondary antibody for 20 minutes at 37 °C, and then in avidin-biotin complex for a further 45 minutes. Diaminobenzidine tetrahydrochloride was used as the chromogen and the sections were counterstained with hematoxylin for 1 minute. All incubations were performed at room temperature; between each incubation step, sections were washed with tris-buffered saline buffer. Appropriate positive and negative controls were used throughout.
The staining was evaluated as follows: hormone receptors: +, ++, +++ and ++++ if nuclear staining was observed in 10-50%, 50-70%, 70-90% and more than 90% of tumor cells respectively (+++ and ++++ were merged). No staining or staining less than 10% was considered negative.
For HER2, cases were reevaluated according to the recently published recommendations16:
Score 0: no staining is observed in invasive tumor cells.
Score 1+: weak, incomplete membrane staining in any proportion of invasive tumor cells, or weak, complete membrane staining in less than 10% of cells.
Score 2+: complete membrane staining that is non-uniform or weak but with obvious circumferential distribution in at least 10% of invasive tumor cells, or intense complete membrane staining in 30% or less of invasive tumor cells.
Score 3+: intense complete membrane staining is observed in more than 30% of invasive tumor cells.
Frequency tables were employed for descriptive analysis. We used the chi-square test to estimate the association of axillary node status with categorical variables and the Mann Whitney U test for continuous variables. Statistically significant variables in the univariate studies (p < 0.05) were selected for multivariate analysis, which was performed using interactive stepwise logistic regression. Odds ratios (OR) were calculated together with 95% confidence intervals (CI95%).
Statistical analysis was performed by using the Epi Info 6.0 computer program package.
This study was designed and its results presented in accordance with reporting recommendations for tumor marker prognostic studies (REMARK) criteria17.
Results
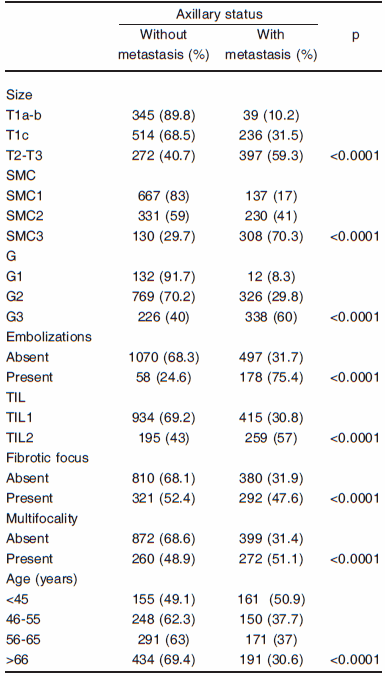
Association between the clinicopathological evaluated features and axillary status. Univariate analysis. All variables studied demonstrated statistically significant association with MALN. The figures are depicted in Table 2.
TABLE 2.- Association between the clinicopathological evaluated features and axillary status. Univariate analysis
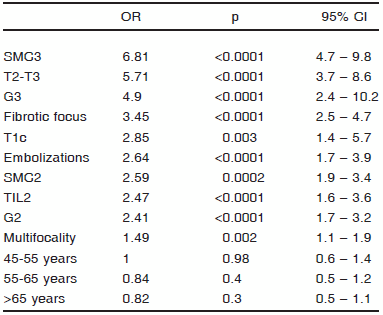
Association between the clinicopathological evaluated features and axillary status. Multivariate analysis. The variables are listed according to their OR (Table 3). Tumors with SMC3 (p < 0.0001) and T2-T3 (p < 0.0001) were strongly associated with MALN, followed by G3 (p < 0.0001), fibrotic focus (p < 0.0001), T1c (p = 0.003), embolizations (p < 0.0001), SMC2 (p = 0.0002), TIL2 (p < 0.0001), G2 (p < 0.0001) and multifocality (p = 0.002). SMC3 stood out as the predominant variable related to MALN. Age did not reach statistical significance.
TABLE 3.- Association between the clinicopathological evaluated features with MALN. Multivariate analysis. In two cases the age was not available leaving a total of 1801 cases
Multivariate analysis taking SMC3 as the dependent variable. It has been reported that an intrinsic relationship exists between lymphatic endothelial cells and functionally related molecules, particularly HA18, consequently we specifically studied the association between SMC3 and embolizations taking SMC3 as the dependent variable. A strong association with tumor embolizations (p < 0.0001) was observed and, as shown above, with MALN (p < 0.0001). A negative relationship with age (p < 0.0001) was also noted, suggesting a possible hormone influence in the development of SMC.
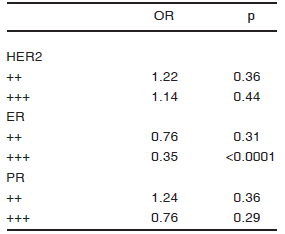
Evaluation of hormone receptors, HER2 expression and their association with MALN and SMC3. We first studied the association between HER2 and hormone receptors with MALN in a multivariate analysis (Table 4). Both ER +++ (p = 0.02) and PR +++ (p = 0.006) were inversely associated with MALN. In a second step, we investigated the association between SMC3 and hormone receptors. Previous research papers found a positive10 and inverse association20 of hormone receptors with cytosolic and stromal HA signal respectively. Our analysis, indicated that ER +++ was inversely associated with SMC3 (p < 0.0001) (Table 5).
TABLE 4.- Association between HER2 and hormone receptors with MALN. Multivariate analysis
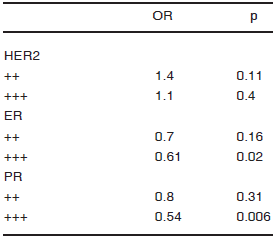
TABLE 5.- Association between HER2 and hormone receptors with SMC3. Multivariate analysis
Discussion
Several pieces of new information emerged from this study: SMC3 were, according to our figures, the most important factor related to MALN; TIL were related to MALN; fibrotic focus was related to MALN; SMC3 were related to tumoral embolizations and inversely associated with ER +++.
Summarizing, based solely on histological correlations, our findings encompass the entire process of wound healing, which appears to be associated with nodal metastases. This is supported by numerous basic and clinical investigations as it will be exposed in the following discussion.
The results of the first multivariate examination (Table 3) clearly demonstrated the importance of stromal variables in predicting the presence of MALN. SMC as the histological expression of the provisional matrix; fibrotic focus, a scar like lesion in late response to tumor invasion and TIL as the expression of an inflammatory response, were all related to MALN.
We also observed a close association between SMC3 and tumoral embolizations. Since HA is one of the major constituents of SMC14, our findings allow us to infer that this molecule is involved in the promotion of embolizations. Experimental confirmation of this phenomenon was stated in two recent reports21, 22.
Hence, if SMC3 mirror these aggressive lesions, an epithelial - SMC interaction might be responsible for such increased prometastatic activity. It appears that SMC are the histological expression of a major pathogenetic mechanism, providing a fertile soil for the cascade of changes that lead to metastases23. Yet, we do not have a clear idea of the true nature of the SMC and cannot unambiguously determine cause and effect. It is possible that they represent manifestations of another underlying process such as increased stromal degradation secondary to proteolytic enzymes. It should be stressed that SMC inevitability induce structural abnormalities in the tumor microvasculature, leading to a reduced oxygen transport capacity and hypoxia as occurs in early wound healing24. In turn, hypoxia can stimulate the production of HA and the activity of hyaluronidase, responsible for degrading HA, promoting angiogenesis as a compensation mechanism25. Moreover, it was recently reported with a tissue microarray technique, a positive correlation between intense stromal HA signal and high nitric oxide synthases expression in human breast cancer. This observation points to high concentrations of reactive oxygen species at the SMC, which also react with HA resulting in chain cleavage20. Therefore, in all likelihood, SMC are composed, besides putative growth promoting factors, of a mixture of high molecular weight hyaluronan (HMW-HA) and low molecular weight hyaluronan (LMW-HA) chains induced by HA degradation.
In our previous work, a correlation (not statistically significant) was observed between mortality and SMC2-314. However, Corte et al found decreased cytosolic HA in poorly differentiated tumors and high HA levels associated with significantly longer relapse-free survival19. Their measurements were done predominantly with an immunological radiometric assay selecting a HMW-HA population. In their data HA levels were significantly higher in well differentiated diploid ER positive tumors, while in our series SMC3 correlated with G3 and inversely with ER expression (Table 4). It seems that both methodologies select different micro-environments with also different cellular populations. What lies in our view behind these opposite findings, are evidences of a dual role of HA. HMW-HA contents may mean less HA turnover, with lesser tissue destruction and remodeling. Tissue destruction is the main cause of HA degradation25. Very likely the population selected by Corte are tumors with a reduction of HA degradation while SMC are the histological expression of such occurrence. There are several reports documenting a relationship between HA degradation and tumor invasion26, 27. Alternatively the HMW-HA maintains homeostasis and potentially down-regulates inflammation. The early pioneering work of Herrera-Gayol and Jothy demonstrated that intratumoral injection of HMW-HA does not induce stimulation but tumor regression in xenotransplanted breast cancer28, an observation supported by numerous literature data that prove that the effect of HA is closely dependent on its molecular mass.
This dichotomous behavior of HA has already been reported29. However this "Jekill and Hide" effect has not been clearly referred to neoplastic disease yet.
Harmonizing to these events is the observation that TIL, other actors of the stromal remodeling process behave as prometastatic (Table 3). The histological observations consistently demonstrate TIL interspersed within the spaces opened by SMC. It is known that LMW-HA enhances inflammation30. However, lymphocytes may become compromised once within the SMC31, 32 or may adversely adapt to the suppressive environment to promote growth and metastases10. This is probably due to the release of pro-inflammatory mediators. Chavey et al33 found in a population of 105 cases that elevated expression of chemokines in breast lesions correlated with marked leukocyte infiltration. They inferred that chemokines could be involved in tumor aggressiveness because ER negative cases exhibit high cytokine content. However they did not find a correlation between chemokine expression and MALN. Our data insinuate that such correlation does exist.
Lastly, our results show a strong relationship between fibrotic focus and MALN (Table 3). Reinforcing this, an association between fibrotic focus and intratumoral hypoxia was detected immunohistochemically34. We believe that fibrotic focus is itself an expression of the wasting product of the stromal process with the active course of action being carried on within the SMC.
All these pieces of information converge to two crucial works. The first is Dvorak's hypothesis35. He propose a never ending process of stromal remodeling, pointing that tumors are like "wounds that never heal." The second is the work of Chang et al36. They document that a gene expression program related to wounding is frequently activated in invasive carcinomas and confers increased risk of metastases.
Undoubtedly, behind these stromal changes there is an intricate balance between HA synthesis and degradation with a desmoplastic reaction following the active chronic inflammation. Even though a cause and effect relationship remains to be verified, these findings provide not only new pathological indicators of aggressiveness but a possible new, previously unappreciated, therapeutic target. HMW-HA, although associated with less aggressive carcinomas, appears to form a protective cellular coat. Cross-linked HA matrix was found to create a microenvironment favoring cells resistance to antimitotic agents37. HMW-HA induces antiapoptotic as well as drug resistance signals38, 39. Therefore, targeting HA, in order to dissolve the protective pericellular coat, might render malignant cells much more drug sensitive, more responsive to the effect of the stromal dendritic cells activated by LMW-HA fragments40 and to the oxidative stress of reactive oxygen species as well20. It is attractive to suggest that hyaluronidase targeted against SMC, may liquefy this protective pericellular coat cutting the vicious circle of the ongoing tissue destruction. Experimental and clinical evidence demonstrated an effect of hyaluronidase as a single agent41. It was also determined synergistic activity of hyaluronidase with other chemotherapeutic agents42-44.
In summary, this study underlines the importance of the stromal factor at the time of tumor evaluation. It demonstrates that the stroma suffers histological modifications that reflect a process, analogous to wound healing, where chemokines producing inflammatory cells, desmoplasia, hypoxia, oxygen reactive species and hyaluronan appear to confabulate in order to promote tumor spread and metastases. These changes, when carefully assessed, can predict the metastatic potential of a particular neoplasm and potentially avoid unnecessary surgical procedures.
Conflicts of interest: The authors declare that they have no conflict of interest.
1. Freund H, Grover NB, Durst AL. Factors affecting survival following radical mastectomy. J Surg Oncol 1978; 10: 191-6. [ Links ]
2. Knudson W, Biswas C, Toole BP. Interaction between human tumor cells and fibroblasts stimulate hyaluronate synthesis. Proc Natl Acad Sci USA 1984; 81: 6767-71. [ Links ]
3. Auvinen P, Tammi RH, Parkkinen J, et al. Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am J Pathol 2000; 156: 529-36. [ Links ]
4. Toole BP, Biswas C, Gross J. Hyaluronate and invasiveness of the rabbit V2 carcinoma. Proc Natl Acad Sci USA 1979; 76: 6299-303. [ Links ]
5. Bartolazzi A, Peach R, Aruffo A, et al. Interaction between CD44 and hyaluronate is directly implicated in the regulation of tumor development. J Exp Med 1994; 180: 53-66. [ Links ]
6. Zhang L, Underhill CB, Chen L. Hyaluronan on the surface of tumor cells is correlated with metastatic behavior. Can Res 1995; 55: 428-33. [ Links ]
7. Ueno H, Jones A, Jass JR, et al. Clinicopathological significance of the keloid like stroma and myxoid stroma in advanced rectal cancer. Histopathology 2002; 40: 327-34. [ Links ]
8. Murray SK, Young RH, Scully RE. Unusual epithelial and stromal changes in myoinvasive endometrioid adenocarcinoma: a study of their frequency, associated diagnostic problems, and prognostic significance. Int J Gynecol Pathol 2003; 22: 324-33. [ Links ]
9. Ambros RA, Malfetano JH, Mihm MC. Clinicopathological features of vulvar squamous cell carcinoma exhibiting prominent fibromyxoid stromal response. Int J Gynecol Pathol 1996; 15: 137-45. [ Links ]
10. Yu P, Fu YX. Tumor-infiltrating T lymphocytes: friends or foes. Lab Invest 2006; 86: 231-45. [ Links ]
11. Hasebe T, Sasaki S, Imoto S, et al. Highly proliferative fibroblast forming fibrotic focus govern metastasis of invasive ductal carcinoma of the breast. Mod Pathol 2001; 14: 325-37. [ Links ]
12. Association of Directors of Anatomy and Surgical Pathology. Recommendation for the reporting of breast cancer. Mod Pathol 1996; 9: 77-81. [ Links ]
13. Tavassoli FA, Devilee P. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Lyon: IARC Press, 2003. [ Links ]
14. Wernicke M, Piñeiro LC, Caramuti D, et al. Breast cancer stromal myxoid changes are associated with tumor invasion and metastasis: A central role for hyaluronan. Mod Pathol 2003; 16: 99-107. [ Links ]
15. Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer 1957; 11: 359-77. [ Links ]
16. Wolff AC, Hammond EH, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. Arch Pathol Lab Med 2007; 131: 18-43. [ Links ]
17. McShane LM, Altman DG, Sauerbrei W, et al; Statistics Subcommittee of NCI-EORTC Working Group on Cancer Diagnostics. REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 2006; 100: 229-35. [ Links ]
18. Jackson DG, Prevo R, Clasper S, et al. LYVE-1,The lymphatic system and tumor lymphangiogenesis. Trends Immunol 2001; 22: 317-21. [ Links ]
19. Corte MD, Gonzalez LO, Lamelas ML, et al. Expression and clinical signification of cytosolic hyaluronan levels in invasive breast cancer. Breast Cancer Res Treat 2006; 97: 329-37. [ Links ]
20. Karihtala P, Soini Y, Auvinen P, et al. Hyaluronan in breast cancer: Correlations with nitric oxide synthases and tyrosine nitrosylation. J Histochem Cytochem. 2007; DOI: 10.1369/jhc.7A7270. [ Links ]
21. Guo LX, Ju JH, Xif H. Hyaluronan promotes tumor lymphangiogenesis and intralymphatic tumor growth in xenografts. Acta Biochim Biophys Sin 2005; 37: 601-6. [ Links ]
22. Koyama H, Kobayashi N, Harada M, et al. Significance of tumor-associated stroma in promotion of intratumoral lymphangiogenesis. Pivotal role of a hyaluronan-rich tumor microenvironment. Am J Pathol 2008; 172: 179-93. [ Links ]
23. Stern R, Shuster S, Neudecker BA, et al. Lactate stimulates fibroblast expression of hyaluronan and CD44: The Warburg Effect Revisited. Experimental Cell Research Volume 2002; 276: 24-31. [ Links ]
24. Gou F, Okunieff P, Han Z, et al. Hypoxia-induced alteration in hyaluronan and hyaluronidase. Adv. Exp Med Biol 2005; 566: 249-56. [ Links ]
25. Jenkins RH, Thomas JG, Williams JG, et al. Myofibroblastic differentiation leads to hyaluronan accumulation trough reduced hyaluronan turnover. J Biol Chem 2004; 279: 44153-60. [ Links ]
26. Kosunen A, Ropponen K, Kellokoski J, et al. Reduced expression of hyaluronan is a strong indicator of poor survival in oral squamous cell carcinoma. Oral Oncol 2004; 40: 257-63. [ Links ]
27. Lokeshwar VB, Lokeshwar BL, Pham HT, et al. Association of elevated levels of hyaluronidase, a matrix-degraded enzyme with prostate cancer progression. Cancer Res 1996; 56: 651-7. [ Links ]
28. Herrera-Gayol A, Jothy S. Effect of hyaluronan on xenotransplanted breast cancer. Exp Mol Pathol 2002; 72: 179-85. [ Links ]
29. Cantor JO, Nadkarni PP. Hyaluronan: the Jekyll and Hyde molecule. Inflamm Allergy Drug Targets 2006; 5: 257-60. [ Links ]
30. Powel JD, Horton MR. Threat Matrix: low-molecular-weigth hyaluronan (HA) as a danger signal. Immunol Res 2005; 31: 207-18. [ Links ]
31. Delmage JM, Powards DM, Jaynes PK, et al. The selective suppression of immunogenicity by hyaluronic acid. Ann Clin Lab Scie 1986; 16: 303-10. [ Links ]
32. Del Fresno C, Otero K, Gomez-Garcia L, et al. Tumor cells deactivate human monocytes by up-regulating IL-1 receptor associated kinase-M expression via CD44 and TLR4. J Immunol 2005; 174: 3032-40. [ Links ]
33. Chavey C, Bibeau F, Gourgou-Bourgade S, et al. Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res 2007; 9: R15. [ Links ]
34. Colpaert CG, Vermeulen PB, Fox SB, et al. The presence of a fibrotic focus correlates with the expression of charbonic anhydrase IX and is a marker of hypoxia and poor prognosis. Breast Cancer Res Treat 2003; 81: 137-47. [ Links ]
35. Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. New Engl J Med 1986; 315: 1650-9. [ Links ]
36. Chang HY, Nuyten DMS, Snneddon JB, et al. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc Natl Acad Sci USA 2005; 102: 3738-43. [ Links ]
37. David L, Dulong V, Le Cerf D, et al. Hyaluronan hydrogel: an appropriate three-dimensional model for evaluation of anticancer drug sensitivity. Acta Biomater 2008; 4: 256-63. [ Links ]
38. Misra S, Ghatak S, Toole BP. Regulation of MDR1 expression and drug resistance by a positive feedback loop involving hyaluronan, phosphoinositide 3-kinase and ErbB2. J Biol Chem 2005; 280: 20310-5. [ Links ]
39. Wang SJ, Bourguignon LYW. Hyaluronan and the interaction between CD44 and epidermal growth factor receptor in oncogenic signaling and chemotherapy resistance in head and neck cancer. Arch Otolaringol Head Neck Surg 2006; 132: 771-8. [ Links ]
40. Termeer CC, Hennies J, Voith U, et al. Oligosaccharides of hyaluronan are potent activators of dendritic cells. J. Immunol 2000; 165: 1863-70. [ Links ]
41. Shuster S, Frost GI. Csoka AB, et al. Hyaluronidase reduces human breast cancer xenograft in SCID mice. Int J Cancer 2002; 102: 192-7. [ Links ]
42. Klocker J, Sabitzer H, Raunik W, et al. Hyaluronidase as additive to induction chemotherapy in advance squamous cell carcinoma of the head and neck. Cancer Lett 1998; 131: 113-5. [ Links ]
43. Baumgartner G, Gomar-Hoss C, Sakr L, et al. The impact of extracellular matrix on the chemoresistance of solid tumors. Experimental and clinical results of hyaluronidase as additive to cytostatic chemotherapy. Cancer Lett 1998; 131: 85-99. [ Links ]
44. St Croix B, Man S, Kerbel RS. Reversal of intrinsic and acquired forms of drug resistance by hyaluronidase treatment of solid tumors. Cancer Lett 1998; 131: 35-44. [ Links ]
Recibido: 22-7-2010
Aceptado: 18-11-2010














