Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Medicina (Buenos Aires)
Print version ISSN 0025-7680
Medicina (B. Aires) vol.71 no.2 Ciudad Autónoma de Buenos Aires March/Apr. 2011
ARTÍCULO ORIGINAL
In vivo effects of adenosine 5´-triphosphate on rat preneoplastic liver
Ana V. Frontini1*, Carlos D. De La Vega Elena1*, María V. Nicolorich1, Ariel Naves1, Pablo Schwarzbaum2, Graciela D. Venera1,2
1Instituto Universitario Italiano de Rosario, Virasoro 1249, Rosario,
2Instituto de Química y Fisicoquímica Biológicas, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires
*Both authors contributed equally to the study
Postal address: Dra. Graciela D. Venera, IQUIFIB, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, CONICET, Junín 956, 1113 Buenos Aires, Argentina Fax: (54-11) 496-25457 e-mail: venera@qb.ffyb.uba.ar
Abstract
The utilization of adenosine 5´-triphosphate (ATP ) infusions to inhibit the growth of some human and animals tumors was based on the anticancer activity observed in in vitro and in vivo experiments, but contradictory results make the use of ATP in clinical practice rather controversial. Moreover, there is no literature regarding the use of ATP infusions to treat hepatocarcinomas. The purpose of this study was to investigate whether ATP prevents in vivo oncogenesis in very-early-stage cancer cells in a well characterized two-stage model of hepatocarcinogenesis in the rat. As we could not preclude the possible effect due to the intrinsic properties of adenosine, a known tumorigenic product of ATP hydrolysis, the effect of the administration of adenosine was also studied. Animals were divided in groups: rats submitted to the two stage preneoplasia initiation/promotion model of hepatocarcinogenesis, rats treated with intraperitoneal ATP or adenosine during the two phases of the model and appropriate control groups. The number and volume of preneoplastic foci per liver identified by the expression of glutathione S-transferase placental type and the number of proliferating nuclear antigen positive cells significantly increased in ATP and adenosine treated groups. Taken together, these results indicate that in this preneoplastic liver model, ATP as well as adenosine disturb the balance between apoptosis and proliferation contributing to malignant transformation.
Key words: Hepatocarcinoma; Preneoplastic model; ATP; Adenosine; Preneoplastic foci; Altered hepatocytes foci; Proliferation; Apoptosis
Resumen
Efecto in vivo de adenosina 5´-trifosfato sobre el hígado preneoplásico de la rata. La utilización de adenosina 5´-trifosfato (ATP ) para inhibir el crecimiento de algunos tumores en humanos y en animales se basa en la actividad anticancerígena observada en experimentos in vitro e in vivo. El uso del ATP en la práctica clínica es discutido debido a resultados contradictorios. Por otra parte, no existen antecedentes del uso de ATP en el tratamiento de hepatocarcinomas. El objetivo del presente estudio fue determinar si el ATP previene la oncogénesis in vivo en un modelo de preneoplasia hepática murina de dos etapas. Para determinar la probable contribución de la adenosina, producto de la hidrólisis de ATP y descrita como tumorigénica, se estudió también el efecto del nucleósido exógeno sobre los focos preneoplásicos. Los animales se dividieron en grupos: ratas sometidas al modelo de preneoplasia de iniciación/promoción, ratas tratadas con ATP o adenosina intraperitonealmente durante las dos fases del modelo y los correspondientes grupos controles. El número y el volumen de focos preneoplásicos por hígado, identificados por la expresión de la forma placentaria de la glutation S- transferasa de rata y el número de células positivas para el antígeno nuclear proliferante, aumentaron significativamente en los grupos tratados con ATP y adenosina. Los resultados en su conjunto indican que en este modelo preneoplásico, el ATP y la adenosina alteran el balance entre apoptosis y proliferación, contribuyendo a la transformación maligna.
Palabras clave: Hepatocarcinoma; Modelo preneoplásico; ATP; Adenosina; Foco preneoplásico; Proliferación; Apoptosis
Hepatocellular carcinoma (HCC) is the fifth most common malignancy in the world. Its incidence is increasing worldwide and is estimated to cause approximately half a million deaths annually with 2-5 new cases per 100 000 inhabitants/year in Western countries and more than 20 per 100 000 inhabitants/year in Asia. The major risk factors are chronic hepatitis B and cirrhosis related to chronic hepatitis C. Alcoholic cirrhosis is associated with smaller risk for HCC1, 2. The foci of altered hepatocytes (AHF) would emerge during the long preneoplastic stage, in which the liver is often the site of chronic hepatitis, cirrhosis, or both3, 4.
Accordingly, an in vivo system was used to study early stages of neoplasia in rat liver5. The initiation-promotion or 2-stage model of cancer mimics the early events of the latent period of human carcinogenesis6-8. The system consists of an initiation phase involving two intraperitoneal necrogenic doses of the carcinogen diethylnitrosamine (DEN) and a promotion phase that enhances the initiated cell populations by oral administration of 2-acetylaminofluorene (2-AAF).
Experimental evidence in animals and humans indicates that the administration of ATP , ATP hydrolysis products and other nucleotides can inhibit tumor growth by acting alone or synergistically with chemotherapeutic drugs9-11. However, the participation of extracellular adenosine 5´-triphosphate on the cell fate has paradoxical aspects since it can direct the cell towards proliferation, differentiation or apoptosis possibly taking part in promoting or preventing malignant transformation12.
Once ATP is in the extracellular space the nucleotide is subjected to sequential dephosphorylation by ectonucleotidases into ADP, AMP and adenosine (ADO)13. Besides, adenosine produced by hypoxic tumors, stimulates tumor growth by immunosuppressive effect and angiogenic actions14.
The objective of this work is to contribute to the understanding of the controversial ATP and cancer relationship by the evaluation of exogenous ATP treatment on: a) the number of foci per liver, b) the volume of liver occupied by AHF, c) proliferation and d) apoptosis in the aforementioned preneoplastic rat liver model. As ATP can be completely dephosphorylated in the extracellular compartment, we also evaluated the effect of exogenous adenosine on the parameters described above.
Materials and Methods
DEN, 2-AAF, ATP , ADO, dimethyl sulfoxide (DMSO ) and tricaprylin were obtained from Sigma Chemical Co; rabbit polyclonal antibody glutathione S-transferase placental form (rGST -p) was provided by Assay Designs Inc., Ann. Arbor, MI, USA; mouse monoclonal anti-proliferating cell nuclear antigen (PCNA) antibody sc-56 was from Santa Cruz Biotechnology, Santa Cruz, CA, USA; the TdT-mediated dUTP Nick-End Labeling (TUNEL) kit was from Promega, Madison, Wi; Link and Label detection kit was from Cell Marque Corp., Hot Springs, AR, USA.
Adult male Wistar rats weighing between 330 and 380 g were housed 3-4 per cage and maintained in a room at a constant temperature with a 12-hour light-dark cycle. Tap water and pelleted rat chow (Cargill SACI, Argentina) were available ad libitum. All experiments were performed under protocols approved by the Ethical Committee of the Instituto Universitario Italiano de Rosario (IUNIR).
Rats were divided into 6 groups of 6 to 7 rats each. The animals of the initiation-promotion group (IP) were subjected to the two-phase hepatocarcinogenic model as described previously5. Briefly, initiation was induced by the administration of two DEN intraperitoneal necrogenic doses of 150 mg/kg body weight dissolved in phosphate buffer saline (PBS), at the beginning of the 1st and 3rd weeks. The promotion phase was carried out using 2-AAF dissolved in DMSO then suspended in tricaprylin to a final concentration of 8 mg/ml. The rats received 20 mg/kg body weight of 2-AAF/tricaprylin suspension by gavage during 3 weeks for 4 consecutive days per week, starting 1 week after the last injection of DEN. The animals were randomly assigned to the experimental groups after the first DEN injection.
The IP-ATP and IP-ADO groups were subjected to the same 2-phase protocol and received adenosine 5´-triphosphate and adenosine intraperitoneal injections of 100 mg/kg body weight (4 times a week for 6 weeks) dissolved in PBS pH 7.4 and PBS-8% Tween 20, respectively15-17. A scheme of the experimental protocol is shown in Fig. 1.
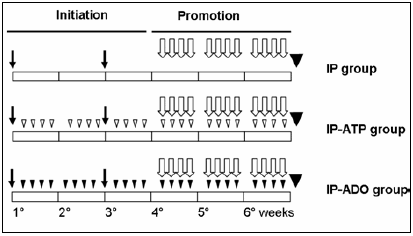
Fig. 1.- Scheme of the initiation/promotion carcinogenic model. All rats were subjected to DEN intraperitoneal injections at the beginning of the 1th and 3rd weeks (black arrow), followed by 1 week of rest. Then, 2-AAF (open arrow) was given by gavage 4 times per week for 3 weeks. The rats were killed at the end of the 6th week (black triangle). Rats were randomly assigned to no treatment (IP group), treatment with ATP (IP-ATP group) or adenosine (IP-ADO group). ATP (white head) and adenosine (black head) were injected intraperitoneally in IP-ATP and IP-ADO groups, 100 mg/kg body weight, 4 days a week during 6 weeks starting the day after the first DEN injection.
Animals without treatment and rats subjected to adenosine 5´-triphosphate or adenosine treatment were the normal control, ATP control, and ADO control groups. The volume injected was between 0.2 - 0.3 ml. The animals were killed at the end of the 6th week after bleeding by cardiac punction under pentobarbital anesthesia (50 mg/kg body weight). Thereafter, the livers were removed and weighed, and pieces of tissue from three different lobes were processed for histological and immunohistochemical studies.
Liver tissues were fixed in 10% formalin, embedded in paraffin, serially sectioned and processed for hematoxylin-eosin (HE) staining or immunohistochemistry. In the immunohistochemical studies, rat liver with preneoplastic foci was used as a positive control. Negative controls were performed by replacing the primary antibody. At least 1 cm2 of three slices (6 μm) of each lobe was studied for each technique. The histology and immunohistochemistry were evaluated independently by two investigators.
Quantification of preneoplastic foci. As the rGST-p isozyme is specifically expressed during rat hepatocarcinogenesis18, the immunohistochemical detection of rGST -p is the most widely used method for identification and quantification of rat AHF 19. The location of rGST -p in the liver was determined by the streptavidin-biotin-peroxidase method using an anti-rat GST -p polyclonal antibody. Sections were counterstained with hematoxylin. rGST -p-positive foci were measured with a computer-assisted image processor. Two parameters were considered and calculated by the Saltykov method20 using digitized images: 1) the number of foci per liver. This is an estimate of the total number of initiated cells capable of developing AHF clones based on liver weight; 2) the percentage of liver tissue occupied by AHF . This is the volume occupied by altered foci relative to total liver volume and reflects the growth and total cellular population of the foci21, 22.
Determination of Proliferative Index and Cell-Cycle Phases. Proliferating cell nuclear antigen (PCNA) is a component of the DNA replication process involved in growth regulation synthesized in late G1 and S phases of the cell-cycle and its reactivity may be detectable in any situation where cell proliferation takes place. The nuclear pattern of expression of PCNA differs in the stages of the cell cycle. PCNA expression increases in the nucleus during the late G1 phase, reaches its maximum during the S phase and declines again during the G2 phase. Mitotic cells have a very low PCNA signal23. The proliferative activity amongst groups was compared. Serially sectioned slides were examined by immunohistochemical staining with anti-rGST -p and anti-PCNA antibodies. The number of PCNA-labeled nuclei was determined within randomly selected rGST - p-positive foci. Counting was undertaken by using an optical microscope with 400x magnification. All PCNA-positive cells in G1, S, G2 and M phases were used to calculate the total number of proliferating cells. The proliferative index (PI) was defined as the number of PCNA-positive nuclei per focus in the preneoplastic tissue and as the number of PCNA-positive nuclei per 100 hepatocytes in the surrounding tissue24. The number of proliferating cells within rGST -p positive foci and surrounding tissue was determined by examining at least 1,000 to 10,000 hepatocytes, respectively.
Determination of Apoptotic Index (AI). Apoptotic cells were quantified by TUNEL in serial sections stained for the rat placental form of glutathione S-transferase (rGST p) scoring 1,000 to 5,000 hepatocytes in the foci and 5,000 to 10,000 cells in the surrounding tissue. The data was confirmed by light microscopic examination (Zeiss Axiolab microscope, Germany) at 400x magnification by counting the same number of cells on HE-serial stained slides. Apoptotic cells were recognized by patterns of morphological changes such as loss of cell surface structures, cell shrinkage and shape change, condensation of cytoplasm and nuclei, nuclear envelope changes, nuclear fragmentation and apoptotic body formation25. Apoptotic index was defined as the total number of apoptotic cells per focus in the preneoplastic tissue and as the number of apoptotic cells per 100 hepatocytes in the surrounding tissue.
Values are expressed as mean ± SE. The statistical evaluation was performed using the program SPSS version 10.0. Data were compared using 1-way ANOV A; in the case of significance, a Tukey test was applied. Differences were considered significant when p<0.05.
Results
Histological changes were observed due to adenosine 5´-triphosphate and adenosine. In IP and IP-ADO groups hydropic degeneration was observed in HE-stained liver sections in the foci and in the surrounding tissue (Fig. 2A); in IP-ATP group, hydropic vacuoles were observed in most of the foci but not in the surrounding tissue (Fig. 2D). Portal inflammation, portal and periportal cholestasis and bile pigment deposits in the portal space and its proximal cells were predominantly observed in IP-ATP group. In control groups alteration of the liver tissue was not observed.
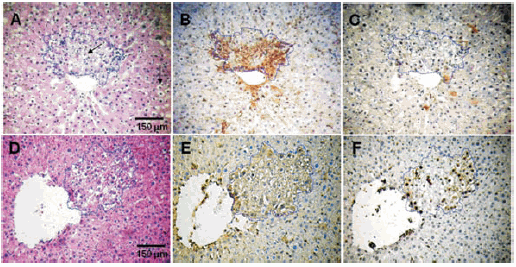
Fig. 2.- Histological serial sections of rat liver subjected to the IP preneoplastic model: A. Hydropic degeneration (black arrow) in the focus and the surrounding tissue HE-stained. B. Immunohistochemical rGST -p positive cells. C. Immunohistochemical PCNA-positive cells. Histological serial sections of rat liver subjected to the IP preneoplastic model plus ATP : D. HE-stained. E. rGST-p positive cells. F. PCNA positive cells. Foci areas are highlighted by dashed lines. (This figure is presented in colour, in www.medicinabuenosaires.com)
The number of foci per liver and the percentage of liver as altered hepatocytes significantly increased in the rats given ATP or ADO during the two-phase protocol compared with the animals subjected to the model without further treatment. Moreover, ATP treatment significantly enhanced the above parameters when compared with adenosine treatment. Panels 2B and 2E show rGST p positive foci from IP and IP-ATP groups, respectively. Quantitative data and statistics are shown in Figs. 3A and 3B. Neither ATP nor ADO treatments produced changes in normal livers since foci were absent in the control groups.

Fig. 3.- Bars representing the number of AHF per liver (A) and percentage of volume occupied by AHF (B). Altered hepatocytes foci were visualized by rGST p staining. Groups are indicated under the bars. Each bar represents mean ± SE of livers from seven rats. The number of AHF per liver (A) and the volume percentages of AHF (B) in IP-ATP group was significantly different compared with IP-ADO (*p < 0.001; **p < 0.05)
In the foci, a significant difference in proliferation and apoptosis was observed among the three groups. IP-ATP group showed the highest proliferation index and apoptotic index, followed by IP-ADO and IP groups. The PI/AI ratio for IP-ATP group increased about twofold compared with IP-group ratio. The PI/AI ratio for IP-ADO group was between the ratios of IP-ATP and IP groups. Data are summarized in Table 1. Fig. 2F shows PCNA positive nuclei in the foci of IP-ATP group of rGST p-positive tissue (Fig. 2E). In the surrounding tissue, both proliferation and apoptosis were similar in IP, IP-ATP and IP-ADO groups. Compared with control groups, they showed normal proliferative activity but slightly higher rates of apoptosis (data not shown). Table 2 shows the percentages of PCNA positive nuclei in each phase of the cycle in the foci. The IP-ATP group showed a significant higher percentage of cells in the S phase (p < 0.001).
TABLE 1.- Effect of ATP and adenosine on proliferation and apoptosis
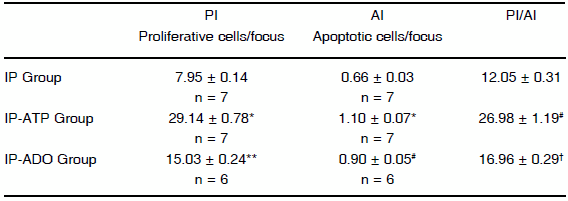
NOTE. Proliferating cells and apoptotic cells per focus were scored from at least 1,000 hepatocytes. All values represent mean ± SE
*Different from IP and IP-ADO (p < 0.001); **different from IP (p < 0.01); #different from IP (p < 0.001);†different from IP and IP-ATP (p < 0.05
TABLE 2.- Cell-cycle distribution of hepatocytes in AHF. Determined from PCNA immunostaining

NOTE. Data were expressed as percentage of proliferating cells (PCN A-positive cells) ± SE
*Different from IP and IP-ADO (p<0.001)
**Different from IP (p<0.001)
#Different from IP (p<0.05); different from ADO (p<0.01)
Discussion
Antitumor effects of ATP were first observed in cell culture26, 27 and carcinoma treatment in animal models15, 16, 28, 29. In clinical practice the use of ATP infusions in the treatment of a variety of human tumors is rather controversial due to the contradictory results of clinical trials carried out up to now. Most of these trials deal with non-small-cell cancer patients and there are few results concerning other tumors10, 11, 17, 30.
In the present study, we analyzed the effect of ATP on AHF of rats subjected to a liver preneoplastic protocol widely used to assess the effect of anticancer drugs5, 31, 32.
In contrast with previous results11, 17, 30 we found that ATP treatment enhanced initiation and promotion of altered hepatocytes. We suggest that the activity of ATP on cell proliferation and altered hepatocytes foci number could involve adenosine generation as a consequence of the rapid ATP breakdown, as it has been described that the nucleoside takes part in tumorigenesis via A2A receptor14.
Exogenous adenosine increased significantly the number of focus per liver and the volume of liver occupied by AHF respect to the untreated group but both parameters were significantly lower than that observed in ATP treated group. Therefore, although adenosine plays a role in the progression of the foci, our study provides evidence that ATP has an intrinsic effect on altered hepatocytes proliferation.
In agreement with several reports describing that adenosine 5´-triphosphate and adenosine can trigger apoptosis in tumor cells26, 27, 33 we observed that the apoptotic index increased significantly in the foci of IP-ATP and IP-ADO groups respect to IP-group. Although apoptosis increased in the treated animals, the largest preneoplastic lesions were observed in these two groups because of the elevated hepatocyte proliferation. The highest PI/AI ratio was attained by IP-ATP group followed by IP-ADO group, indicating that apoptosis could not counterbalance increased proliferation.
When investigating the cell cycle, we noted that ATP led to an increased rate of cell entry to the S phase as detected by PCNA expression and nuclei morphology. Fast growth and deregulation of the cell cycle has been described as a characteristic of preneoplastic and neoplastic lesions34.
A potential mechanism mediating the effects of extracellular ATP is the activation of purinergic receptors. Administration of ATP can activate P2 receptors, while exogenous adenosine triggers the activation of P1 receptors. Extracellular nucleotides might regulate proliferation, differentiation and apoptosis of cancer cells through P2 receptors subtype. Activation of the metabotropic P2Y2 receptors would increase proliferation in most cancers while metabotropic P2Y1, and ionotrophic P2X5 and P2X7 receptor subtypes might change the cell cycle from proliferation to differentiation and apoptosis35.
Hepatocytes express several purinergic P2Y receptors including the metabotropic P2Y1, P2Y2, P2Y4 and P2Y6. Recently, ionotrophic receptors have been recognized in the liver. P2X3, P2X4 and P2X7 receptors are dominant in isolated hepatocytes and P2X4 and P2X7 are also present in rat hepatocarcinoma36.
The conflict-ridden results about pro-proliferative and anti-proliferative or pro-apoptotic and anti-apoptotic effect could be a consequence of the different affinities to nucleotides displayed by specific purinergic receptors expressed on the surface of HCC or an enhanced or decreased expression of some of these receptors in different tissues12. On the other hand, different downstream mechanisms of signal transduction could modulate the different tissue-specific responses making the role and mechanisms of purinergic signaling as anticancer drug target unclear12, 37. We await further experiments to confirm a change in the expression of purinergic receptors as a result of endogenous ATP treatment in the IP-model.
In regard to the peri-preneoplastic tissue, the IP, IPATP and IP-ADO groups showed normal proliferative activity but higher rates of apoptosis than control groups. Initiated cells in the surrounding tissue may undergo apoptosis and never develop in preneoplastic foci38. It also has been demonstrated that hepatocytes adjacent to HCC have the ability to induce apoptosis in an autocrine or paracrine way39.
Both P2Y and P2X receptors have been implicated not only in the formation of tumors, but also in chronic inflammation40, 41. In agreement with the latter finding, we observed a severe portal inflammation and portal and periportal cholestasis in the IP-ATP group41. However, in the surrounding tissue of IP and IP-ADO groups we observed hydropic degeneration while a normal appearance was observed in IP-ATP and control groups. It is noteworthy that in hepatic and hepatoma cell models, extracellular ATP has been reported to downregulate cell volume of swollen hepatic cells42-44.
Our results suggest that in this preneoplastic model, ATP -directly or indirectly- increases both the number and volume of preneoplastic foci. Although apoptosis increases, we observed a pro-tumoral effect of adenosine 5'-triphosphate. As ATP significantly augmented the proliferative activity respect to adenosine its effect might not be ascribed exclusively to extracellular adenosine generation from ATP.
Acknowledgements: This work was supported by funds from the Instituto Universitario Italiano de Rosario (IUNIR) and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). We aknowledge the consultant assistance of Dr. María Cristina Carrillo, Dr. María del Luján Alvarez, Dr. María Paula Faillace and M.Sc. Gerardo Pisani. We thank the technical assistance of the medicine students Melisa Secchi, Ana Roca, Ingrid Rechiman, Pablo Presentado Larrey and Marisol Cuffia.
Conflict of interest: The authors have nothing to declare regarding conflict of interest.
1. El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol 2002; 35: S72-8. [ Links ]
2. Borbath I, Leclercq IA, Abarca-Quinones J, et al. Inhibition of early preneoplastic events in the rat liver by the somatostatin analog lanreotide. Cancer Sci 2007; 98: 1831-9. [ Links ]
3. Altmann HW. Hepatic neoformations. Pathol Res Pract 1994; 190: 513-77. [ Links ]
4. Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet 2002; 31: 339-46. [ Links ]
5. Alvarez ML, Cerliani JP, Monti JA, et al. [In vivo apoptotic effect of alpha-2b interferon (IFN) on rat preneoplastic liver ]. Medicina (Buenos Aires) 2001; 61: 666-9. [ Links ]
6. Rao PM, Nagamine Y, Roomi MW, et al. Orotic acid, a new promoter for experimental liver carcinogenesis. Toxicol Pathol 1984; 12: 173-8. [ Links ]
7. Ito N, Tsuda H, Tatematsu M, et al. Enhancing effect of various hepatocarcinogens on induction of preneoplastic glutathione S-transferase placental form positive foci in rats-an approach for a new medium-term bioassay system. Carcinogenesis 1988; 9: 387-94. [ Links ]
8. Solt D, Farber E. New principle for the analysis of chemical carcinogenesis. Nature 1976; 263: 701-3. [ Links ]
9. Rapaport E. Anticancer activities of adenine nucleotides in tumor bearing hosts. Drug Dev Res 1993; 28: 428-31. [ Links ]
10. Abraham EH, Salikhova AY, Rapaport E. ATP in the treatment of Advanced Cancer. Curr Top Membr 2003; 54: 415-52. [ Links ]
11. Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev 2006; 58: 58-86. [ Links ]
12. Deli T, Csernoch L. Extracellular ATP and cancer: an overview with special reference to P2 purinergic receptors. Pathol Oncol Res 2008; 14: 219-31. [ Links ]
13. Zimmermann H. Ectonucleotidases: Some recent developments and a note on nomenclature. Drug Dev Res 2001; 52: 44-56. [ Links ]
14. Ohta A, Gorelik E, Prasad SJ, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A 2006; 103: 13132-7. [ Links ]
15. Lasso de la Vega MC, Terradez P, Obrador E, et al. Inhibition of cancer growth and selective glutathione depletion in Ehrlich tumour cells in vivo by extracellular ATP . Biochem J 1994; 298: 99-105. [ Links ]
16. Obrador E, Navarro J, Mompo J, et al. Glutathione and the rate of cellular proliferation determine tumour cell sensitivity to tumour necrosis factor in vivo. Biochem J 1997; 325 ( Pt 1): 183-9. [ Links ]
17. Beijer S, Gielisse EA, Hupperets PS , et al. Intravenous ATP infusions can be safely administered in the home setting: a study in pre-terminal cancer patients. Invest New Drugs 2007; 25: 571-9. [ Links ]
18. Imai T, Masui T, Ichinose M, et al. Reduction of glutathione S-transferase P-form mRNA expression in remodeling nodules in rat liver revealed by in situ hybridization. Carcinogenesis 1997; 18: 545-51. [ Links ]
19. Pitot HC. Altered hepatic foci: their role in murine hepatocarcinogenesis. Annu Rev Pharmacol Toxicol 1990; 30: 465-500. [ Links ]
20. Saltykov SA. The determination of the size distribution of particles in an opaque material from a measurement of the size distribution of their sections. In: H E, (ed.) Proceedings of the Second International Congress on Stereology. Berlin: Springer-Verlag, 1967:153-73. [ Links ]
21. Xu YH, Campbell HA, Sattler GL, et al. Quantitative stereological analysis of the effects of age and sex on multistage hepatocarcinogenesis in the rat by use of four cytochemical markers. Cancer Res 1990; 50: 472-9. [ Links ]
22. Dragan YP, Hully JR, Nakamura J, et al. Biochemical events during initiation of rat hepatocarcinogenesis. Carcinogenesis 1994; 15: 1451-8. [ Links ]
23. Robbins BA, de la Vega D, Ogata K, et al. Immunohistochemical detection of proliferating cell nuclear antigen in solid human malignancies. Arch Pathol Lab Med 1987; 111: 841-5. [ Links ]
24. Greenwell A, Foley JF, Maronpot RR. An enhancement method for immunohistochemical staining of proliferating cell nuclear antigen in archival rodent tissues. Cancer Lett 1991; 59: 251-6. [ Links ]
25. Hacker G. The morphology of apoptosis. Cell Tissue Res 2000; 301: 5-17. [ Links ]
26. Wen LT , Knowles AF. Extracellular ATP and adenosine induce cell apoptosis of human hepatoma Li-7A cells via the A3 adenosine receptor. Br J Pharmacol 2003; 140: 1009-18. [ Links ]
27. Zheng LM, Zychlinsky A, Liu CC, et al. Extracellular ATP as a trigger for apoptosis or programmed cell death. J Cell Biol 1991; 112: 279-88. [ Links ]
28. Rapaport E, Fontaine J. Anticancer activities of adenine nucleotides in mice are mediated through expansion of erythrocyte ATP pools. Proc Natl Acad Sci U S A 1989; 86: 1662-6. [ Links ]
29. Becker EP, Sun D, Minuk GY. Exogenous adenosine 5'- triphosphate does not improve survival in rats with acute liver failure. Dig Dis Sci 2008; 53: 794-8. [ Links ]
30. Agteresch HJ, Dagnelie PC, van den Berg JW, Wilson JH. Adenosine triphosphate: established and potential clinical applications. Drugs 1999; 58: 211-32. [ Links ]
31. Nakaji M, Yano Y, Ninomiya T, et al. IFN-alpha prevents the growth of pre-neoplastic lesions and inhibits the development of hepatocellular carcinoma in the rat. Carcinogenesis 2004; 25: 389-97. [ Links ]
32. Sakakima Y, Hayakawa A, Nagasaka T, Nakao A. Prevention of hepatocarcinogenesis with phosphatidylcholine and menaquinone-4: in vitro and in vivo experiments. J Hepatol 2007; 47: 83-92. [ Links ]
33. Eguchi Y, Srinivasan A, Tomaselli KJ, et al. ATP-dependent steps in apoptotic signal transduction. Cancer Res 1999; 59: 2174-81. [ Links ]
34. Calvisi DF, Pinna F, Pellegrino R, et al. Ras-driven proliferation and apoptosis signaling during rat liver carcinogenesis is under genetic control. Int J Cancer 2008; 123: 2057-64. [ Links ]
35. White N, Burnstock G. P2 receptors and cancer. Trends Pharmacol Sci 2006; 27: 211-7. [ Links ]
36. Emmett DS, Feranchak A, Kilic G, et al. Characterization of ionotrophic purinergic receptors in hepatocytes. Hepatology 2008; 47: 698-705. [ Links ]
37. Burnstock G. Unresolved issues and controversies in purinergic signalling. J Physiol 2008; 586: 3307-12. [ Links ]
38. Schulte-Hermann R, Bursch W, Kraupp-Grasl B, et al. Cell proliferation and apoptosis in normal liver and preneoplastic foci. Environ Health Perspect 1993; 101 Suppl 5: 87-90. [ Links ]
39. Roskams T, Libbrecht L, Van Damme B, Desmet V. Fas and Fas ligand: strong co-expression in human hepatocytes surrounding hepatocellular carcinoma; can cancer induce suicide in peritumoural cells? J Pathol 2000; 191: 150-3. [ Links ]
40. Kunzli BM, Berberat PO , Giese T, et al. Upregulation of CD39/NTPDases and P2 receptors in human pancreatic disease. Am J Physiol Gastrointest Liver Physiol 2007; 292: G223-30. [ Links ]
41. Idzko M, Hammad H, van Nimwegen M, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med 2007; 13: 913-9. [ Links ]
42. Wang Y, Roman R, Lidofsky SD, Fitz JG. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc Natl Acad Sci U S A 1996; 93: 12020-5. [ Links ]
43. Pafundo DE, Mut P, Perez Recalde M, et al. Effects of extracellular nucleotides and their hydrolysis products on regulatory volume decrease of trout hepatocytes. Am J Physiol Regul Integr Comp Physiol 2004; 287: R833-43. [ Links ]
44. Pafundo DE, Chara O, Faillace MP, et al. Kinetics of ATP release and cell volume regulation of hyposmotically challenged goldfish hepatocytes. Am J Physiol Regul Integr Comp Physiol 2008; 294: R220-33. [ Links ]
Recibido: 28-6-2010
Aceptado: 17-12-2010














