Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Medicina (Buenos Aires)
Print version ISSN 0025-7680
Medicina (B. Aires) vol.71 no.5 Ciudad Autónoma de Buenos Aires Oct. 2011
ARTÍCULO ORIGINAL
HIV and pregnancy: Maternal and neonatal evolution
Diego Cecchini, Analía Urueña, Patricia Trinidad, Fernando Vesperoni, Débora Mecikovsky, Rosa Bologna
Helios Salud, Buenos Aires
Dirección postal: Dr. Diego Cecchini, Helios Salud, Perú 1515, 1141 Buenos Aires, Argentina
Fax: (54-11) 4300-5021 e-mail: diegocec@gmail.com
Abstract
Data regarding epidemiological aspects, antiretroviral drug safety, and outcomes of HIV-infected pregnant women and their newborns are limited in Argentina. We underwent a retrospective analysis of registries of HIV-infected pregnant women assisted at Helios Salud, Buenos Aires, Argentina (1997-2006). Variables associated with preterm delivery and neonatal complications were analyzed by univariate and logistic regression analyses. A total of 204 mother-child binomium were included. Maternal age (median): 29 years; 32.5% without prior diagnosis of HIV-infection. Baseline median CD4 T-cell count: 417 cell/μl; 98% received antiretroviral drugs during pregnancy [2 nucleoside analogs plus either nevirapine (55%) or a protease inhibitor (32%)]. Overall incidence of toxicity was 12.5%: rash (8%), anemia (3.5%) and hepatotoxicity (1%). Rash was associated with exposure to nevirapine. Eighty one percent and 50% reached HIV-viral loads <1000 and <50 copies/ml at the end of pregnancy, respectively. Twenty six percent had obstetric complications and 16% had preterm delivery. Of the newborns, 1.6% had congenital defects and 9% had neonatal complications. Overall neonatal mortality was 1% and perinatal transmission was 0.7%. Protease inhibitor use and obstetric complications were associated to preterm delivery while obstetric complications were associated with neonatal complications. In our population, hepatotoxicity was low despite frequent use of nevirapine. Protease inhibitor use was associated to preterm delivery. A favorable virological response and a low rate of perinatal transmission was observed, what supports the consensus that antiretroviral therapy benefits during pregnancy outweigh risks of maternal and neonatal adverse events.
Key words: HIV; Pregnancy; Toxicity; Preterm delivery; Vertical transmission; Nevirapine
Resumen
HIV y embarazo: Evolución materna y neonatal. La información sobre aspectos epidemiológicos, seguridad de drogas antirretrovirales y evolución de mujeres embarazadas HIV positivas y sus hijos es limitada en la Argentina. Realizamos un análisis retrospectivo de registros de embarazadas HIV positivas asistidas en Helios Salud, Buenos Aires, Argentina (1997-2006). Las variables asociadas con parto prematuro y complicaciones neonatales se estudiaron mediante análisis univariado y regresión logística. Estudiamos 204 binomios madre-hijo. Edad materna (mediana): 29 años, 32.5% sin diagnóstico previo de HIV. Recuento de linfocitos T CD4+ (mediana): 417 células/μl. El 98% recibió tratamiento antirretroviral durante el embarazo [dos análogos de nucleósidos más nevirapina (55%) o un inhibidor de proteasa (32%)]. La incidencia global de toxicidad fue 12.5%: erupción cutánea (8%), anemia (3.5%) y hepatotoxicidad (1%). La exposición a nevirapina se asoció con rash. El 81% y 50% alcanzaron cargas virales <1000 y <50 copias/ml preparto, respectivamente. Cesárea programada: 68%; complicaciones obstétricas: 26%; parto prematuro: 16%. De los neonatos, 1.6% presentaron defectos congénitos y el 9% complicaciones neonatales. La mortalidad neonatal fue 1% y la transmisión vertical: 0.7%. Las complicaciones obstétricas y el uso de inhibidores de proteasa se asociaron a parto prematuro; las complicaciones obstétricas se asociaron con complicaciones neonatales. La tasa de hepatotoxicidad fue baja a pesar de la utilización frecuente de nevirapina; el uso de inhibidores de proteasa se asoció a parto prematuro. Se observó una respuesta virológica favorable y una baja tasa de transmisión vertical, lo que apoya el consenso de que el beneficio de las drogas antirretrovirales durante el embarazo supera el riesgo de efectos adversos maternos y neonatales.
Palabras clave: HIV; Embarazo; Toxicidad; Parto pretérmino; Transmisión vertical; Nevirapina
Since the beginning of the epidemic, millions of women have become infected with Human Immunodeficiency Virus (HIV)1. In the last two decades, major progress has been made in the medical management of the HIV-infected pregnant woman, and a dramatic decline in perinatal transmission was observed2, 3. In 1994, the PACTG 076-ANRS 024 trial showed that zidovudine reduced the rate of mother-to-child transmission from 22.6% to 7.6%4. Subsequent clinical trials and observational studies demonstrated that combination of antiretroviral prophylaxis (initially dual5 and then triple combination therapy6) given to the mother antenatally was associated with further declines in transmission to <2% when associated with adequate intrapartum and postpartum neonatal prophylaxis7, 8.
Therefore, antiretroviral therapy is strongly recommended for all HIV-infected pregnant women in order to reduce vertical transmission7, 8. However in recent years, antiretroviral drug toxicity in pregnant women has been a cause of concern for clinicians and a major debate has been generated regarding the safety of these drugs in pregnancy, considering issues such as maternal hepatotoxicity, increased risk of preterm delivery and congenital defects9-15. The development of local information considering maternal and fetal safety of antiretroviral drugs is a priority in order to improve strategies for prevention of perinatal transmission of HIV. In this context, data from HIV-infected pregnant women from Latin American countries are limited, particularly in the population assisted through the Health Maintenance Organizations-like systems (HMOs).
Helios Salud is an outpatient clinic for HIV treatment, research and prevention, which assists patients from the HMO-like system in Buenos Aires city, Argentina. The purpose of this study was to assess epidemiological aspects, maternal antiretroviral drug safety, obstetric and neonatal outcomes in this population.
Materials and Methods
We analyzed the medical records of all HIV-infected pregnant women assisted at Helios Salud, Buenos Aires, Argentina from 1997 to 2006, and their newborns until they had the Enzyme-linked immunosorbent assay done at 18 months of age. For each mother the following variables were studied: epidemiological profile, CD4 T-cell count, HIV viral load, hepatitis C (HCV) and hepatitis B (HBV) status, CDC category, antiretroviral therapy during pregnancy, toxicity events, obstetric complications, mode of delivery, frequency of preterm delivery (defined as delivery < 37 weeks of gestational age), use of intrapartum zidovudine and breastfeeding. Drug related toxicities were classified according to National Institute of Allergy and Infectious Diseases Division of AIDS Toxicity grading scale. For each newborn: sex, birth weight, antiretroviral prophylaxis, neonatal complications, overall mortality and final HIV status were studied (an infant was consider non-infected with two negative HIV proviral DNA PCRs before 12 months plus a negative Enzyme-linked immunosorbent assay at 18 months). Variables associated with preterm delivery and neonatal complications were analyzed. For preterm delivery, the following variables were evaluated: obstetric complications, maternal antiretroviral therapy, CD4 T-cell count, CDC category and early antiretroviral exposure during pregnancy (defined as initiation in the first or second trimester). For neonatal complications, the following variables were considered: birth weight <2500 g, obstetric complications, mode of delivery, preterm delivery and maternal antiretroviral therapy.
Data were processed using Statistix software 7.0 (Analytical Software, Tallahassee, FL, USA). Dichotomous variables were analyzed with chi2 and Fisher´s exact test. Variables associated with preterm delivery and neonatal complications were analyzed by logistic regression models.
The study was approved by the Helios Salud-FUNCEI Institutional Ethical Review Board.
Results
During the period of the study, a total of 204 mother-child binomium (including two twin pregnancies) were assisted at our institution; 18 were lost to follow-up before delivery. Of 181 live births, 138 completed diagnosis of HIV-1 infection. Regarding maternal characteristics, the median of age was 29 years (interquartile range [(IQR)]= 26-33). Sixty six (32%) had HIV diagnosis during pregnancy and thirty seven (18%) had positive HCV serology. Eigthteen (9%) had serological evidence of prior HBV exposure. Most of the pregnancies were unintended (82%). Median time between HIV diagnosis and pregnancy was 12 months. Ten patients (5%) were intravenous drug users and the overall predominant way of HIV transmission was previous unprotected sexual intercourse (94%). Median CD4 T-cell count was 417/μl (332-617). Eighty one patients (40%) were exposed to antiretroviral therapy at the time of conception. Baseline median HIV-1 viral load in naïve patients was 11300 copies/ml (range: 2305-46075).
Two hundred (98.5%) patients received antiretroviral therapy during pregnancy; 47% of them exclusively for mother-to-child transmission prophylaxis. Nevirapine plus two nucleoside reverse transcriptase inhibitors (NRTIs) was the most frequent regimen used (55%), followed by the combination of two NRTIs and a protease inhibitor (PI) [31%]. The most commonly NRTIs used were zidovudine (91%) and lamivudine (86%) and the most frequently PI used was nelfinavir (26%). Viral load was available for 170 patients in the third trimester. Of these, 139 (81%) and 86 (50%) reached viral loads < 1000 and < 50 copies/ml, respectively.
The overall incidence of antiretroviral drug toxicity was 12.5%. The toxicity events were: rash 16 (8%); anemia 7 (3.5%) and hepatotoxicity 2 (1%). Six rash episodes were severe and one was considered potentially life threatening. Nevirapine exposure was associated to rash development [odds ratio(OR) = 15.95% confidence interval (CI) = 1.94-115.80]. Both hepatotoxicity events were severe and occurred in patients with nevirapine-based HAART. Anemia was always mild or moderate, and attributed to zidovudine in all cases. All patients fully recovered from toxicity events.
Considering the mode of delivery, 29 (16%) patients had vaginal delivery, 29 (16%) had an emergency cesarean section and 124 (68%) had a scheduled cesarean section. Ninety-seven percent received intravenous zidovudine during labour. Twenty six percent of patients had obstetric complications, being the most frequent hypertension (5%), threatened preterm labour (3.5%) and first trimester bleeding (2%). Overall prevalence of preterm delivery was 16% and PI use and obstetric complications were the only variables associated to this event (OR: 3.21, 95% CI: 1.33-7.75 and OR: 3.51, 95% CI: 1.42-8.64, respectively). Univariate analyses to identify variables associated with preterm delivery are shown in Table 1. No woman breastfed and formula feeding was provided in all cases.
TABLE 1.Univariate analysis to identify variables associated with preterm delivery in a population of HIV-infected pregnant women in Buenos Aires, Argentina
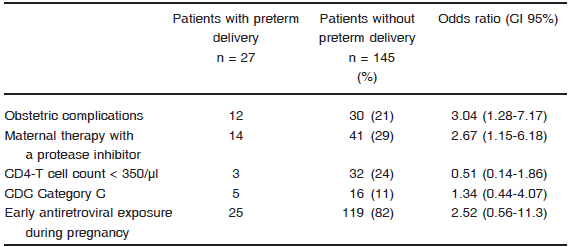
Of 183 newborns with available data, all except two received antiretroviral prophylaxis: 96.7% of them received zidovudine alone, 1.6% received a combination of zidovudine, lamivudine and nevirapine and 0.5% received zidovudine plus nevirapine.
Seventeen (10%) newborns had birth weight < 2500 g and 16 (9%) of them had neonatal complications, which were only associated to obstetric complications in logistic regression analyses (OR: 3.63; 95% CI: 1.27-10.34). Most frequent neonatal complications included: sepsis/distress 5, bronchiolitis 2 and diarrhoea 2. Univariate analyses to identify variables associated with these complications are shown in Table 2. Overall prevalence of congenital defects and neonatal mortality were 1.6% and 1% respectively.
TABLE 2.Univariate analysis to identify variables associated with neonatal complications in a population of mother-child binomium in Buenos Aires, Argentina

Of 138 newborns whose final PCR and 18-month ELISA tests results were available, only one had documented HIV-1 infection (0.7%).
Discussion
The present study describes clinical, epidemiological and therapeutic aspects of mother-child binomium assisted through the HMO-like system in Buenos Aires, Argentina. Several aspects of this study should be highlighted.
A high proportion of women had their HIV diagnosis during pregnancy. Considering this information, HMO´s administrators should strongly consider the development of prevention programs for HIV and other sexually transmitted diseases tailored to women of childbearing age and their partners. In this scenery, the importance of early screening of HIV infection in pregnancy must be emphasized. In patients with negative HIV screening tests in the first or second trimester of pregnancy, HIV screening tests for their partners should be offered as well as counseling regarding HIV and other sexually transmitted diseases prevention must be warranted. In addition, HIV test should be repeated in third trimester in order to diagnose seroconversion during pregnancy.
Other aspect of interest is that most of the pregnancies were unwanted, as women reported that current pregnancy was not planned. Considering this information, priority should be given to education in family planning strategies to HIV-infected women and their partners.
In our study, cutaneous toxicity occurred in 13.7% of nevirapine-exposed women and 7 (6.4%) presented severe forms. This is similar to previous communications16, 21.
Most of our patients initiated antiretroviral therapy before the nevirapine hepatotoxicity warning disclosure in different guidelines. However, despite more than 85% of patients who initiated nevirapine during pregnancy had baseline CD4 T-cell count > 250/μl, the overall hepatotoxicity rate was low in our population. The Pediatric AIDS Clinical Trials Group (PACTG) 1022 team has questioned whether pregnancy per se constitutes an additional risk factor for nevirapine-related hepatic toxicity beyond the baseline risk associated with female gender13. In our study, a lower than expected incidence of hepatotoxicity was observed (1.84%). This has also been reported by other investigators10-12, 16-17. These studies and our own data contrast with the very high rate (29.4%) reported by PACTG 102213. Considering both nevirapine and non-nevirapine based therapy, recent investigations suggest also that pregnancy itself constitutes a risk factor for hepatotoxicity, with a considerable increase of this event in pregnant versus non-pregnant women19, 20. In a retrospective analysis of all 879 nevirapine-exposed patients assisted in our institution, hepatotoxicity occurred in 4.9% of women and 3.5% of men. In a multivariate model only female sex was associated with nevirapine toxicity and no association was found with either pregnancy status or the CD4 T-cell count18.
Our cohort had a higher overall prevalence of preterm delivery when compared to the general Argentinean population which is approximately 8%22, and PI use and obstetric complications were associated with this event in logistic regression analyses. The association of PI-based antiretroviral therapy and preterm delivery is controversial in current literature. Cotter et al, described that, after adjustment for possible confounders, only combination therapy with a PI was associated with an increased risk of preterm birth, compared with any other combination in a north-american cohort15. The European Collaborative Study and the Swiss Cohort Study also described that infants in utero exposed to a PI containing regimen were 2.6 times more likely to be born prematurely23. In contrast, Kourtis et al showed in a meta-analysis that antiretroviral therapy during pregnancy is not associated with an overall increased risk of preterm birth, but the use of combination regimens before or early in pregnancy may slightly increase the risk of prematurity24. Data regarding Latin America and the Caribbean are limited. In contrast to our findings, Szyld et al described in a 681 women cohort that maternal receipt of PI-containing regimens during pregnancy was not associated with a statistically significant increase in risk of low birth weight or PD25. However, in spite of the association to preterm delivery in our cohort, PI use was not associated with NC, so the clinical impact of this association is uncertain.
Among mother-child binomium assisted through Buenos Aires city public health system, overall vertical transmission was 6% in 1623 deliveries from 2003 to 2008, a considerably higher rate than the one observed in our cohort26. Inadequate antenatal care, delay in the diagnosis of HIV infection and lack of access to antiretroviral therapy during pregnancy, were factors significantly associated to transmission in the public health system26. In the only documented perinatal HIV-1 infection in our population, transmission occurred despite adequate antenatal, intrapartum and postpartum prophylaxis. The low overall mother-to-child transmission rate observed in our institution, which is closer to the one reported in developed countries, prevented the identification of risk factors for perinatal transmission in our setting.
Our investigation has some limitations to be addressed. First we had an important number of mother-child binomium lost to follow up. This may be attributable to changes in the health insurance coverage, which is frequent in our country. We had a high proportion of HCV seroprevalence in our cohort, but we couldn´t study the vertical transmission of this virus as this is not included in the health insurance coverage at our institution. Another important limitation is that our results may not be representative of other private institutions, other provinces and/or the public health system. This issue should be particularly emphasized as an association between preterm delivery and PI-use was not previously described in a Latin American cohort.
In conclusion, our results support the consensus that antiretroviral therapy, in addition to intrapartum and neonatal components of PACTG 076, provides a considerably safe and highly effective strategy for prevention perinatal transmission of HIV-1. Further studies are needed to assess if pregnancy increases the risk of nevirapine and non-nevirapine related liver toxicity in HIV-infected women in our region. However, considering its low cost and good placental transfer, nevirapine, with careful management, remains a useful antiretroviral drug to use in pregnancy, especially in low or middle income countries, with limited access to PI regimens. Moreover, clinicians should consider all potential causes of rash in pregnancy to avoid unnecessarily eliminating nevirapine from the patient's therapeutic options and must also try to avoid simultaneous initiation of therapies with overlapping toxicities. In relation to the association between PI use and preterm delivery further research is needed in order to better define if our outcomes persist over the time or reproduce in other settings.
Acknowledgements: The authors would like to thank María del Carmen Iannella, Universidad de Buenos Aires, for the statistical supervision.
Conflict of interests: The authors declare that they have no conflict of interests.
1. UNAIDS. 2009 AIDS Epidemic Update. http://www.unaids.org/en/dataanalysis/epidemiology/2009aidsepidemicupdate/; consultado el 03/01/2011. [ Links ]
2. Centers for Disease Control and Prevention. Progress towards elimination of perinatal HIV infection-Michigan, 1993-2000. MMWR 2002; 51: 93-7. [ Links ]
3. Wade NA, Zielinski MA, Butsashvili M, et al. Decline in perinatal HIV transmission in New York State (1997-2000). J Acquir Immune Defic Syndr 2002; 30: 429-39. [ Links ]
4. Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N Engl J Med 1994; 331: 1173-80. [ Links ]
5. Petra Study Team. Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda (Petra study): a randomized, double-blind, placebo-controlled trial. Lancet 2002; 359: 1178-86. [ Links ]
6. Dabis F, Bequet L, Ekouevi DK, et al. Field efficacy of zidovudine, lamivudine and single-dose nevirapine to prevent peripartum HIV transmission. AIDS 2005; 19: 309-18. [ Links ]
7. Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. May 24, 2010; pp 1-117. [ Links ]
8. Read JS, Cahn P, Loss M, et al. Management of Human Immunodeficiency Virus-Infected Pregnant Women at Latin American and Caribbean Sites. Obstet Gynecol 2007; 109: 1358-67. [ Links ]
9. Stek AM. Antiretroviral medications during pregnancy for therapy or prophylaxis. Curr HIV/AIDS Rep 2009; 6: 68-76. [ Links ]
10. Thomas T, Amornkul P, Mwidau J, et al. Preliminary findings: incidence of serious adverse events attributed to nevirapine among women enrolled in an ongoing trial using HAART for prevent Mother-to-child HIV transmission [abstract 809]. In: 12th Conference on Retroviruses and Opportunistic Infections. Boston, 22-25 February 2005. [ Links ]
11. Hanlon M, O'Dea S, Clarke S, Mulcahy F. Maternal hepatotoxicity with boosted saquinavir as part of combination ART in pregnancy [abstract 753]. In: 14th Conference on Retroviruses and Opportunistic Infections. Los Angeles 25-28 February 2007.
12. Natarajan U, Pym A, McDonald C, et al. Safety of nevirapine in pregnancy. HIV Medicine 2007; 8: 64-9. [ Links ]
13. Hitti J, Frenkel LM, Stek AM, et al. Maternal toxicity with continuous nevirapine in pregnancy. J Acquir Immune Defic Syndr 2004; 36: 772-6. [ Links ]
14. Myers S, Torrente S, Hinthorn D, Clark P. Life-threatening maternal and fetal macrocytic anemia from antiretroviral therapy. Obstet Gynecol 2005; 106: 1189-91. [ Links ]
15. Cotter A, Garcia A, Duthely M, Luke B, O'Sullivan M. Is antiretroviral therapy during pregnancy associated with an increased risk of preterm delivery, low birth weight, or stillbirth? J Infect Dis 2006; 193: 1195-1201 [ Links ]
16. Kondo W, Carraro A, Prandel E, et al. Nevirapine-induced side effects in pregnant women - Experience of a Brazilian university hospital. The Brazilian Journal of Infectious Diseases 2007; 11: 544-8. [ Links ]
17. Joao EC, Calvet GA, Menezes JA, et al. Nevirapine toxicity in a cohort of HIV-1 infected pregnant women. Am J Obstet Gynecol 2006; 194: 199-202. [ Links ]
18. Bottaro E, Huberman M, Iannella M, et al. Nevirapine-associated toxicity in clinical practice in Buenos Aires, Argentina. J Int Assoc Physicians AIDS Care (Chic) 2010; 9: 306-12. [ Links ]
19. Ouyang D, Shapiro D, Lu M, et al. Increased risk of hepatotoxicity in HIV-infected pregnant women receiving antiretroviral therapy independent of nevirapine exposure. AIDS 2009; 23: 2425-30. [ Links ]
20. Snijdewind I, Smit C, Godfried M, et al. Side effects of HAART in HIV-1 infected pregnant and non-pregnant women: pregnancy is associated with hepatotoxicity, while ethnicity is associated with rash [abstract 948]. In: 16th Conference on Retroviruses and Opportunistic Infections. Montreal 8-11 February 2009. [ Links ]
21. Jamisse L, Balkus J, Hitti J, et al. Antiretroviral-associated toxicity among HIV-1-seropositive pregnant women in Mozambique receiving nevirapine-based regimens. J Aquir Immune Defic Syndr 2007; 44: 371-6. [ Links ]
22. Ministerio de Salud de la Nación. Dirección de Estadística e Información de la Salud. Nacidos vivos registrados según tiempo de gestación, por jurisdicción de residencia de la madre. República Argentina- Años 2006, 2007 y 2008. [ Links ]
23. The European Collaborative Study and the Swiss Mother + Child HIV Cohort Study. Combination antiretroviral therapy and duration of pregnancy. AIDS 2000; 14: 2913-20. [ Links ]
24. Kourtis A, Schmid C, Jamieson D, Lau J. Use of antiretroviral therapy in pregnant HIV-infected women and the risk of premature delivery: a meta-analysis. AIDS 2007; 21: 607-15. [ Links ]
25. Szyld E, Warley E, Freimanis L, et al. Maternal antiretroviral drugs during pregnancy and infant low birth weight and preterm birth. AIDS 2006; 20: 2345-53. [ Links ]
26. Portnoy F, Basombrío A, Durán A, et al. ¿Por qué no sigue bajando la tasa de transmisión vertical (TV) en la ciudad de Buenos Aires? Estudio sobre variables asociadas a la TV del VIH. Actualizaciones en SIDA 2009; supl 1: 56. [ Links ]
Recibido: 18-4-2011
Aceptado: 18-8-2011














