Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Medicina (Buenos Aires)
versión impresa ISSN 0025-7680
Medicina (B. Aires) vol.71 no.5 Ciudad Autónoma de Buenos Aires oct. 2011
ARTÍCULO ESPECIAL
Melatonin in Chagas´ disease. Possible therapeutic value
Daniel P. Cardinali, Carlos B. Alvarez
Facultad de Ciencias Médicas, Pontificia Universidad Católica Argentina, Buenos Aires
Postal address: Dr. Daniel P. Cardinali, Departamento de Docencia e Investigación, Facultad de Ciencias Médicas, Pontificia Universidad Católica Argentina, Av. Alicia Moreau de Justo 1500, 4to piso, 1107 Buenos Aires, Argentina
Fax (54-11) 4338-0610 e-mail: danielcardinali@uca.edu.ar
Abstract
Chagas' disease is a severe health problem in Latin America, causing approximately 50 000 deaths a year, with approximately 18 million infected people. About 25-30% of the patients infected with Trypanosoma cruzi develop the chronic form of the disease. The protective response against T. cruzi depends on both innate and acquired immunity involving macrophages, natural killer cells, T and B lymphocytes, and the production of proinflammatory Th-1 cytokines. In addition, an increased nitric oxide (NO) production in macrophages leading to effective microbicidal action is needed to control parasitemia. Melatonin is detectable in T. cruzi and may play a role in promoting infection whereas, when administered in high doses during the acute phase of T. cruzi infection, it can decrease parasitemia while reducing NO production. During chronic disease progression, the sustained oxidative stress concomitant to myocardial damage could be reduced by administering melatonin. It is hypothesized that the coordinated administration of a melatonin agonist like the MT1/MT2 agonist ramelteon, that lacks antioxidant activity and may not affect NO production during the acute phase, and of melatonin in doses high enough to decrease oxidative damage, to preserve mitochondrial and to prevent cardiomyopathy during the chronic phase, could be a novel add-on treatment of Chagas´ disease.
Key words: Chagas´ disease; Melatonin; Melatonin receptors; Oxidative stress; Nitric oxide
Resumen
La melatonina en la enfermedad de Chagas. Su posible valor terapéutico. La enfermedad de Chagas es un problema grave de salud en América Latina, causando cerca de 50 000 muertes al año y unos 18 millones de infectados. Alrededor del 25-30% de los pacientes infectados con Trypanosoma cruzi desarrollan la forma crónica de la enfermedad. La respuesta de defensa ante el T. cruzi depende de la inmunidad innata y adquirida con la participación de macrófagos, células “natural killer”, linfocitos T y B, y la producción de citoquinas proinflamatorias de tipo Th-1. Además, el aumento en la producción de óxido nítrico (NO) en los macrófagos lleva a una acción microbicida eficaz necesaria para controlar la parasitemia. La melatonina es detectable en T. cruzi y podría desempeñar un papel en la promoción de la infección como lo hace en el paludismo, mientras que, cuando se administra en dosis farmacológicas altas durante la fase aguda de la infección por T. cruzi, disminuye la parasitemia, aun en presencia de una reducción de la producción de NO. Durante la progresión de la enfermedad de Chagas a la cronicidad, el estrés oxidativo aumentado con el concomitante daño miocárdico podría reducirse por la administración de melatonina, de reconocida acción antioxidante. Se propone como un nuevo enfoque complementario en el tratamiento de la enfermedad de Chagas la administración durante la fase aguda de un agonista MT1/MT2 de la melatonina como el ramelteon, que carece de actividad antioxidante y podría no afectar a la producción de NO, y de melatonina durante la fase crónica de en dosis suficientemente altas como para disminuir el daño oxidativo y prevenir la miocardiopatía.
Palabras clave: Enfermedad de Chagas; Melatonina; Receptores melatoninérgicos; Estrés oxidativo; Óxido nítrico
The ubiquitous methoxyindole melatonin is secreted by the pineal gland of most mammals, including man and additionally, its presence has been confirmed in many plants and unicellular organisms1 including Trypanosoma cruzi 2. Melatonin participates in diverse functions of the body including sleep and circadian rhythm regulation, immunoregulation and free radical scavenging, and may have anti-cancer actions (for ref. see3). Melatonin also protects organisms against bacterial, viral and parasitic infections by a variety of mechanisms and has been shown to be beneficial for reversing symptoms of septic shock4.
Chagas´ disease is a frequent anthropozoonosis in Latin America caused by T. cruzi, a parasitic protozoan of the ancient branch of eukaryotes. Chagas' disease affects 16-18 million people from Southern California to Argentina and Chile with nearly 100 million people at risk of infection5-7. It is a major public health concern in Latin America with around 50 000 deaths per year taking second place after malaria in prevalence and mortality due to vector associated diseases.
Transmission of T. cruzi occurs predominantly via insect vectors of the subfamily Triatoma that reside in the peridomestic habitat of mud-thatch houses in rural areas. Blood transfusion and organ transplantation represent further routes of T. cruzi transmission. Although the pathophysiology of Chagas' disease is not completely understood, it is widely accepted that the involvement of the immune response is critical in determining the outcome of the disease8,9. The protective response against T. cruzi depends both on innate and acquired immunity involving macrophages, natural killer (NK) cells, T and B lymphocytes, and the production of many different cytokines, which play key roles in regulating both parasite replication and the immune response10.
The innate immune response to T. cruzi involves production of cytokines that modulate cell activity, including interferons (IFN) and interleukin (IL)-12. IFN-γ augments nitric oxide (NO) production in macrophages leading to effective microbicidal action. In acute infection some parasite antigens can activate macrophages, and this may result in pro-inflammatory cytokine production, nitric oxide (NO) synthesis, and consequent control of parasitemia and mortality. Some trypanocidal drugs such as nifurtimox and benznidazole act by enhancing radical oxygen species (ROS) generation during their metabolism11,12. T. cruzi is very susceptible to the cell damage induced by these metabolites because enzymes scavenging free radicals are absent or have very low activities in the parasite. Cell-mediated immunity in T. cruzi infection is also modulated by cytokines and autoimmunity can be triggered8,9.
Despite this strong anti-parasite immune response, T. cruzi persists in a majority of hosts, the causes for immune evasion and persistence being largely unknown. Infection by T. cruzi elicits both humoral and cell-mediated immunity directed by a T-helper (Th) type 1 cytokine response. CD8+ T cells are essential for the control of the intracellular amastigote stages via IFN-γ secretion8,9.
Melatonin is detectable in T. cruzi where it seems to promote parasitemia2 but when administered during the acute phase of T. cruzi infection it reportedly reduces parasitemia13-15. The activity of melatonin during the acute phase may be partly related to the well-demonstrated immunoregulatory role the methoxyindole has3. However, melatonin decreases NO production by macrophages and may harm their effective trypanocidal action during the acute phase of the disease16. During the chronic phase, treatment with melatonin could be beneficial for combating the sustained oxidative stress concomitant to imyocardial damage17, therefore impairing disease progression.
Melatonin, the immune system and ROS generation
Melatonin is the major neurohormone secreted by the pineal gland during the dark hours at night. In addition, melatonin has been demonstrated to be produced by many organs other than the pineal gland, including the retina, the gastrointestinal tract, skin, leukocytes, the thymus and bone marrow cells3. The role of melatonin in the immune system and as far as ROS generation has been reviewed several times. The intention of this section is not to summarize in detail all of the knowledge in this field, but rather to highlight several facts which are of importance for the discussion of its potential relevance in Chagas´ disease.
Melatonin is formed by various leukocytes, including monocytes, eosinophiles, mast cells, T-lymphocytes, NK cells, bone marrow cells and several leukocyte-derived cell lines3. Melatonin biosynthesis has been also described in thymocytes and epithelial cells. The simultaneous biosynthesis of melatonin and expression of melatonergic MT1 receptors in many of these cells18 not only indicate that the methoxyindole has a role in communication within the immune system, but also possesses autocrine, paracrine and, perhaps, intracrine functions.
Besides binding to MT1 and MT2 membrane melatonin receptors19, melatonin also binds to transcription factors belonging to the retinoic acid receptor superfamily, in particular, splice variants of RORα and the product of another gene, RZRβ3. The simultaneous expression of membrane receptors and RORα subforms in various leukocytes seems to be of particular relevance for local actions, which might include a focal or intracellular accumulation of melatonin. The reported positive correlation between MT1 signaling and RORα expression in leukocytes18 indicates a concerted action of these two types of receptors. It has been concluded that melatonin through RORα binding, stimulates the secretion of IL-2, IL-6 and IFN-γ by T-helper cells type 1 and by monocytes20.
The functional spectrum of immunomodulation by melatonin is highly complex and involves various cytokines. The main findings on this point comprise stimulation of IL-2, Il-6 and IFN-γ formation in T-helper cells and monocytes, counteraction of the inhibitory effect of prostaglandin E2 (PGE2) on IL-2 production, and secretion of IL-1, IL-12, tumor necrosis factor (TNF)-α and macrophage-colony stimulating factor (M-CSF) in monocytes and/or monocyte-derived cells3. These effects are not only related to cytotoxicity, prooxidant and pro-inflammatory actions, but also to differentiation and interactions of T-lymphocytes with antigen-presenting (AP) cells. Melatonin promotes the expression of MHC class II molecules and transforming growth factor (TGF)-β in AP cells and influences, via IL-12, T-cell differentiation and growth in favor of Th-1. In addition to Th-1activation, melatonin has also been reported to promote Th-2 responses, findings that may require further substantiation and mechanistic explanation, since, under other conditions, such as trypanosome infection, melatonin was found to decrease Th-2 responses15.
The pro- and anti-inflammatory actions of melatonin deserve particular attention3. At first glance, these effects appear to be contradictory. However, these actions have to be interpreted in the context of different conditions. As far as it enhances the immune response via AP cells and T-lymphocytes, as well as the activation of monocytes and other ROS-generating cells by the methoxyindole alone, melatonin behaves in a prooxidant way. Under other conditions, however, melatonin can reduce oxidant formation, which has been repeatedly shown in numerous experiments using bacterial lipopolysaccharide (LPS)4. In particular, TNF-α was down-regulated. Moreover, melatonin inhibits the LPS-induced expression of various chemokines in peripheral blood mononuclear cells.
The presence of melatonin as well as MT1 and MT2 in a mast cell line was taken as evidence implicating the methoxyindole´s modulatory effects in these cells, too21, which might consist in an anti-inflammatory action via inhibition of TNF-α release. Other anti-inflammatory actions of melatonin, in addition to down-regulation of 5-lipoxygenase, concern to the antagonism to PGE2 and the inhibitory effects on PG synthesis22. Melatonin as well as its metabolites N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK) were shown to down-regulate cyclooxygenase expression in macrophages23.
One of the strongest anti-inflammatory effects of melatonin concerns the down regulation and inhibition of inducible and neuronal NO synthases (iNOS and nNOS)3. It should be stressed that melatonin does not substantially interfere with basal or moderately elevated NO levels and that melatonin and also AMK are highly effective in protecting from NO-mediated mitochondrial blockades and cell damage under severe inflammatory conditions such as sepsis4.
Because ROS generation is a continuous and physiological phenomenon, cells possess efficient antioxidant systems that protect them from oxidative damage. Data accumulated in the last decade strongly indicate that melatonin plays an important role in this defense24. Because of its amphiphilic properties melatonin passes through all biologic barriers with ease. Melatonin gets access freely to all compartments of the cell, and is especially concentrated in the nucleus and mitochondria25.
The discovery that melatonin was a remarkably potent scavenger of the particularly reactive, mutagenic and carcinogenic hydroxyl radical (•OH)26 was the finding that initiated numerous studies on melatonin´s role as a protector against free radicals. Melatonin reacts mainly with superoxide anion radical (O2•), hydrogen peroxide (H2O2), •OH and nitric oxide radical (•NO) and its derivative peroxynitrite (ONOO). Although direct radical scavenging has been effective under numerous experimental conditions at clearly supraphysiological melatonin concentrations, its relevance at physiological levels has been questioned for reasons of stoichiometry3. Even though a single melatonin molecule may generate products in a scavenger cascade which may collectively eliminate up to ten free radicals, such findings from chemical systems may not be fully applicable to physiological conditions.
In spite of this, melatonin was shown to protect from oxidotoxicity already at physiological concentrations27. A possible indirect action as mediated by up-regulation of antioxidant enzymes by melatonin was proposed. More recently, a number of mitochondrial effects of melatonin have come into focus, including safeguarding of respiratory electron flux, reduction of oxidant formation by lowering electron leakage) and inhibition of opening of the mitochondrial permeability transition pore3,28. These effects of melatonin and its metabolites are rather unique. For example, the MT1 / MT2 melatonergic agonist ramelteon displays no relevant antioxidant activity29.
Melatonin and Chagas´ disease
Infection by T. cruzi elicits acute non-specific symptoms e.g. fever, malaise, edema and/or enlarged liver or spleen to the characteristic swelling at the site of entry. In spite of the high blood parasitemia in the acute phase, clinical symptoms do not warranty hospital visit, and therefore, anti-parasitic treatment is often not initiated. In few (<5%) acute patients, sudden death due to congestive heart failure associated with myocarditis or meningoencephalitis may occur30.
Most individuals survive the acute infection and enter an “indeterminate” phase that is defined by the presence of T. cruzi specific antibodies but the absence of clinical signs of cardiac abnormalities31. Approximately 10-20 years after the initial infection, and depending on the region, about 30% of those infected manifest severe cardiomyopathy or gastrointestinal dysfunction. The clinical manifestations of chagasic cardiomyopathy comprise congestive heart failure, thromboembolism in brain, limbs or lungs, and ventricular fibrillation32-34.
T cruzi multiplies as flagellate epimastigote forms in the gut of the hematophagus insect with a subsequent transformation in the metacyclic trypomastigotic infective forms in the rectum of the insect host35. This last process exhibits a 24-h periodicity, mainly occurring under darkness. Endogenous melatonin in the parasite may play a promoting role in this phenomenon2. When exposing the epimastigote forms of T. cruzi to a LD cycle of 0:24 h, the parasite reached exponential growth by the 7th day. A pulse of 2 h of light (LD 2:22) was enough to block growth of the epimastigotes2, an effect that was correlated with the expression of heat-shock proteins and inhibition of parasitisation capacity. Epimastigotes cultured under continuous darkness produce melatonin over the 24 h period while under a LD cycle of 2:22 h showed a 55% decrease. The incubation of epimastigotes with melatonin (1 pM) did not affect parasite growth, but significantly reduced their transformation into metacyclic forms. The authors concluded that the light-dependent decrease in melatonin production by the unicell was responsible, at least partially, for the light-induced parasitisation inhibition2.
From the insect dejections, the trypomastigote forms of T. cruzi are able to enter the skin nucleate cells of the vertebrate host, where they become amastigotic forms. After multiplication in the cytoplasm, amastigotes become trypomastigotes that escape from the host and invade other cells. The vectors acquire the parasite after feeding on blood from an infected animal35.
It is clear that a T. cruzi acute infection like other parasitic diseases is a serious challenge for immune host defense mechanisms before, during and after entry into host´s cells. Multiple components of both the innate and the adaptive immune systems are simultaneously required for protection during the acute phase of infection, with IFN-γ induced NO production being an important mediator of resistance to T. cruzi10.
The first demonstration that melatonin treatment can control T. cruzi infection during the acute phase was given by Santello and co-workers in T. cruzi-infected male Wistar rats13. Rats received 5 mg of melatonin/kg p.o. daily either beginning 7 days prior to infection or concomitantly with the infection. Rats treated with melatonin showed a significant reduction in the number of blood trypomastigotes and an increased number of leucocytes as compared to untreated animals. This effect coincided with a significant increase in IL-2 levels. Melatonin administration also resulted in fewer and smaller amastigote burdens, and less inflammatory infiltrate and tissue disorganization in the heart13.
Other studies from the same group of investigators and using a similar experimental design ensued. They reported that 5 mg/kg of melatonin p.o. daily either beginning 7 days prior to T. cruzi infection or concomitant with the infection augmented the circulating levels of IL-12, IFN-γ and TNF-α14, and decreased the circulating levels of IL-4, IL-10 and tumor growth factor-β15, in particular after concomitant administration with the parasites. Melatonin treatment reduced the levels of NO, increased the number of peritoneal macrophages and impaired splenocyte proliferation after concanavalin A exposure. The authors concluded that the effect of melatonin on T. cruzi infection was exerted via enhanced Th-1 cytokine production14 and suppressed Th-2 response15 consequently promoting a reduction in blood and tissue parasites13.
Lately, the possible synergism between melatonin and the cyclooxygenase inhibitor meloxicam in up-regulating the immune response in male Wistar rats infected with T. cruzi was tested36. The combined treatment with melatonin and meloxicam significantly enhanced the release of IL-2 and IFN-γ into animals' serum, when compared with the infected control groups during the course of infection. The blockade of prostaglandin E2 synthesis and the increased release of NO by macrophage cells from T. cruzi-infected animals contributed to regulate the production of Th-1 subset cytokines significantly reducing the parasitemia in animals treated with the combination of both substances36.
ROS and oxidative stress have been hypothesized to play major roles in the development of systemic complications of Chagas´ disease and in particular Chagas´ cardiomyopathy17,37-40. Several studies in animals have shown that a cycle of mitochondrial functional decline and ROS generation, coupled with an inability to efficiently scavenge the mitochondrial ROS, predispose the chagasic hearts to sustained oxidative stress during infection and disease development. This correlates with clinical findings indicating a general increase of oxidative stress parallel to the progression of Chagas´ disease17,39.
As recently reviewed41,42, melatonin has a number of attributes predisposing it to the protection of the hypertrophied heart. In general, the effects of melatonin on the heart and blood vessels are beneficial and improve the physiology of the circulatory system. Melatonin is effective in protecting the cardiac musculature against ischemia-reperfusion43-45 and effect attributed to its action in inhibiting the mitochondrial permeability transition pore and in preserving the content and integrity of cardiolipin molecules3. Melatonin treatment resulted in significant reductions in infarct size46,47. Melatonin interferes with the central and peripheral vegetative nervous system and supports the dominance of the cholinergic over the adrenergic system. Therefore, the use of melatonin to delay the onset of oxidative insult and mitochondrial deficiency in the myocardium, would prove to be effective in preserving cardiac functions in Chagas´ disease. For example, in malarial infection the development of mitochondrial pathology and mitochondrial oxidative stress in hepatocytes was effectively prevented by melatonin48.
In conclusion the studies discussed above underline the complexity of melatonin's effects on Chagas´ disease (Fig. 1). During the acute phase, melatonin administration may have both enhancing and inhibitory effects on T. cruzi development. Through receptor-mediated promotion of Th-1 inflammatory response melatonin can be helpful to decrease parasitemia after T. cruzi infection. However, Inhibition of NO production, presumably a non-receptor mediated effect of melatonin, may harm the optimal phagocytic activity of macrophages, like that needed in the acute phase of Chagas´ disease. The use of MT1/MT2 melatonin agonists such as ramelteon during the acute phase could be appropriate since ramelteon displays no relevant antioxidant activity as compared to melatonin29. On the other hand, during the indeterminate phase, the administration of melatonin to inhibit free radical-mediated mitochondrial impairment could be useful to limit ROS production and ROS-induced cardiomyopathy (Fig. 1).
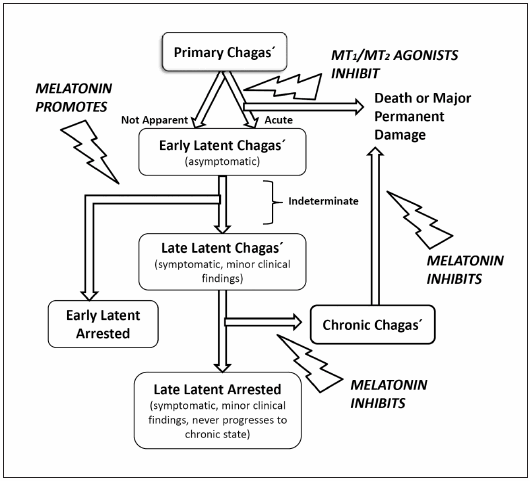
Fig. 1.Scheme summarizing the possible therapeutical validity of melatonin and melatonin analogs in Chagas´ disease. It is hypothesized that the administration of melatonin agonists could promote immunity without impairing NO production during the acute phase and that exogenous melatonin administration during the indeterminate phase could inhibit free radical-mediated mitochondrial-dependent apoptosis and cardiac damage to prevent chronic Chagas´ disease
It is therefore hypothesized that the coordinated administration of a melatonin agonist like the MT1/MT2 agonist ramelteon, that may promote immunity without impairing NO production during the acute phase, and of melatonin in doses high enough to decrease oxidative damage, to preserve mitochondrial and to prevent cardiomyopathy during the chronic phase, could be a novel add-on treatment of Chagas´ disease (Fig. 1). This can be explored in animals and eventually in humans in view of very low toxicity melatonin displays, as indicated by toxicological studies in rodents49 and clinical studies in children and adult humans50-53. Chronic treatment with melatonin has the additional advantage of its low cost, an ideal condition to treat an “orphan disease” like Chagas´ disease, which is far more prevalent in developing countries than in the developed world.
Acknowledgements: Studies in authors´ laboratory are supported by the Agencia Nacional de Promoción Científica y Tecnológica, Argentina (PICT 2007-01045). D.P.C. is a Research Career Awardee from the Argentine National Research Council (CONICET), Argentina and Professor Emeritus, University of Buenos Aires.
Conflicts of interest: None declared.
1. Paredes SD, Korkmaz A, Manchester LC, Tan DX, Reiter RJ. Phytomelatonin: a review. J Exp Bot 2009; 60: 57-69. [ Links ]
2. Macias M, Rodriguez-Cabezas MN, Reiter RJ, Osuna A, Acuna-Castroviejo D. Presence and effects of melatonin in Trypanosoma cruzi. J Pineal Res 1999; 27: 86-94. [ Links ]
3. Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR. Melatonin. A pleiotropic, orchestrating regulator molecule. Progr Neurobiol 2011; 93: 350-84. [ Links ]
4. Srinivasan V, Pandi-Perumal SR, Spence DW, Kato H, Cardinali DP. Melatonin in septic shock: Some recent concepts. J Crit Care 2010; 25: 656.e1-656.e6. [ Links ]
5. Coura JR, Dias JC. Epidemiology, control and surveillance of Chagas disease: 100 years after its discovery. Mem Inst Oswaldo Cruz 2009; 104 (Suppl 1): 31-40. [ Links ]
6. Bar ME, Oscherov EB, Pieri DM, Borda M. Epidemiología de la tripanosomiasis americana en el Norte de Corrientes. Medicina (B Aires) 2010; 70: 133-8. [ Links ]
7. de Rissio AM, Scollo K, Cardoni RL. Transmisión materno-fetal del Trypanosoma cruzi en Argentina. Medicina (B Aires) 2009; 69: 529-35. [ Links ]
8. Sathler-Avelar R, Vitelli-Avelar DM, Teixeira-Carvalho A, Martins-Filho OA. Innate immunity and regulatory T-cells in human Chagas disease: what must be understood? Mem Inst Oswaldo Cruz 2009; 104 (Suppl 1): 246-51. [ Links ]
9. Cunha-Neto E, Nogueira LG, Teixeira PC, et al. Immunological and non-immunological effects of cytokines and chemokines in the pathogenesis of chronic Chagas disease cardiomyopathy. Mem Inst Oswaldo Cruz 2009; 104 (Suppl 1): 252-8. [ Links ]
10. Gonzalez-Cappa S. Inmunopatología en enfermedad de Chagas experimental. Medicina (B Aires) 2000; 60: 681-4. [ Links ]
11. Roldan EJ. Tratamiento de la enfermedad de Chagas con benznidazol y ácido tióctico. Medicina (B Aires) 2005; 65: 85-6. [ Links ]
12. Sosa-Estani S, Armenti A, Araujo G, et al. Tratamiento de la enfermedad de Chagas con benznidazol y ácido tióctico. Medicina (B Aires) 2004; 64: 1-6. [ Links ]
13. Santello FH, Frare EO, Dos Santos CD, et al. Melatonin treatment reduces the severity of experimental Trypanosoma cruzi infection. J Pineal Res 2007; 42: 359-63. [ Links ]
14. Santello FH, Frare EO, Caetano LC, AlonsoToldo MP, do Prado JC Jr. Melatonin enhances pro-inflammatory cytokine levels and protects against Chagas disease. J Pineal Res 2008; 45: 79-85. [ Links ]
15. Santello FH, Frare EO, Dos Santos CD, Caetano LC, Alonso Toldo MP, do Prado JC Jr. Suppressive action of melatonin on the TH-2 immune response in rats infected with Trypanosoma cruzi. J Pineal Res 2008; 45: 291-6. [ Links ]
16. Zhang S, Li W, Gao Q, Wei T. Effect of melatonin on the generation of nitric oxide in murine macrophages. Eur J Pharmacol 2004; 501: 25-30. [ Links ]
17. Gupta S, Wen JJ, Garg NJ. Oxidative stress in Chagas disease. Interdiscip Perspect Infect Dis 2009; 2009: 190354. [ Links ]
18. Lardone PJ, Carrillo-Vico A, Molinero P, Rubio A, Guerrero JM. A novel interplay between membrane and nuclear melatonin receptors in human lymphocytes: significance in IL-2 production. Cell Mol Life Sci 2009; 66: 516-25. [ Links ]
19. Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev 2010; 62: 343-80. [ Links ]
20. Guerrero JM, Pozo D, Garcia-Maurino S, Osuna C, Molinero P, Calvo JR. Involvement of nuclear receptors in the enhanced IL-2 production by melatonin in Jurkat cells. Ann N Y Acad Sci 2000; 917: 397-403. [ Links ]
21. Maldonado MD, Mora-Santos M, Naji L, Carrascosa-Salmoral MP, Naranjo MC, Calvo JR. Evidence of melatonin synthesis and release by mast cells. Possible modulatory role on inflammation. Pharmacol Res 2010; 62: 282-87. [ Links ]
22. Cardinali DP, Ritta MN. The role of prostaglandins in neuroendocrine junctions: studies in the pineal gland and the hypothalamus. Neuroendocrinology 1983; 36: 152-60. [ Links ]
23. Deng WG, Tang ST, Tseng HP, Wu KK. Melatonin suppresses macrophage cyclooxygenase-2 and inducible nitric oxide synthase expression by inhibiting p52 acetylation and binding. Blood 2006; 108: 518-24. [ Links ]
24. Reiter RJ, Paredes SD, Manchester LC, Tan DX. Reducing oxidative/nitrosative stress: a newly-discovered genre for melatonin. Crit Rev Biochem Mol Biol 2009; 44: 175-200. [ Links ]
25. Acuña Castroviejo D, Lopez LC, Escames G, Lopez A, Garcia JA, Reiter RJ. Melatonin-mitochondria Interplay in Health and Disease. Curr Top Med Chem 2011;11:221-40. [ Links ]
26. Tan DX, Poeggeler B, Reiter RJ, et al. The pineal hormone melatonin inhibits DNA-adduct formation induced by the chemical carcinogen safrole in vivo. Cancer Lett 1993; 70: 65-71. [ Links ]
27. Tan D, Reiter RJ, Chen LD, Poeggeler B, Manchester LC, Barlow-Walden LR. Both physiological and pharmacological levels of melatonin reduce DNA adduct formation induced by the carcinogen safrole. Carcinogenesis 1994; 15: 215-8. [ Links ]
28. Acuña-Castroviejo D, Escames G, Rodriguez MI, Lopez LC. Melatonin role in the mitochondrial function. Front Biosci 2007; 12: 947-63. [ Links ]
29. Mathes A, Kubuls D, Waibel L, et al. Selective activation of melatonin receptors with ramelteon improves liver function and hepatic perfusion after hemorrhagic shock in rat. Crit Care Med 2008; 36: 2863-70. [ Links ]
30. Tanowitz HB, Machado FS, Jelicks LA, et al. Perspectives on Trypanosoma cruzi-induced heart disease (Chagas disease). Prog Cardiovasc Dis 2009; 51: 524-39. [ Links ]
31. Sosa ES, Segura EL. Tratamiento de la infección por Trypanosoma cruzi en fase indeterminada. Experiencia y normatización actual en la Argentina. Medicina (B Aires) 1999; 59 (Suppl 2): 166-70. [ Links ]
32. Elizari MV. Miocardiopatía chagásica: perspective histórica. Medicina (B Aires) 1999; 59 Suppl 2: 25-40. [ Links ]
33. Rigou DG, Gullone N, Carnevali L, De Rosa AF. Enfermedad de Chagas asintomática. Hallazgos electrocardiográficos y ecocardiográficos. Medicina (B Aires) 2001; 61: 541-4. [ Links ]
34. Cunha-Neto E. Repensando la patogenia de la cardiopatía crónica chagásica en el fin del milenio. Medicina (B Aires) 1999; 59: 496-500. [ Links ]
35. Noireau F, Diosque P, Jansen AM. Trypanosoma cruzi: adaptation to its vectors and its hosts. Vet Res 2009; 40: 26. [ Links ]
36. Oliveira LG, Kuehn CC, Santos CD, Toldo MP, do PJ, Jr. Enhanced protection by melatonin and meloxicam combination in experimental infection by Trypanosoma cruzi. Parasite Immunol 2010; 32: 245-51. [ Links ]
37. Ribeiro CM, Budni P, Pedrosa RC, et al. Antioxidant therapy attenuates oxidative insult caused by benzonidazole in chronic Chagas' heart disease. Int J Cardiol 2010; 145:27-33. [ Links ]
38. Kittleson MM, Lowenstein CJ, Hare JM. Novel pathogenetic mechanisms in myocarditis: nitric oxide signaling. Heart Fail Clin 2005; 1: 345-61. [ Links ]
39. de Oliveira TB, Pedrosa RC, Filho DW. Oxidative stress in chronic cardiopathy associated with Chagas disease. Int J Cardiol 2007; 116: 357-63. [ Links ]
40. Wen JJ, Vyatkina G, Garg N. Oxidative damage during chagasic cardiomyopathy development: role of mitochondrial oxidant release and inefficient antioxidant defense. Free Radic Biol Med 2004; 37: 1821-33. [ Links ]
41. Reiter RJ, Tan DX, Paredes SD, Fuentes-Broto L. Beneficial effects of melatonin in cardiovascular disease. Ann Med 2010; 42: 276-85. [ Links ]
42. Reiter RJ, Manchester LC, Fuentes-Broto L, Tan DX. Cardiac hypertrophy and remodelling: pathophysiological consequences and protective effects of melatonin. J Hypertens 2010; 28 Suppl 1: S7-12. [ Links ]
43. Tan DX, Manchester LC, Reiter RJ, Qi W, Kim SJ, El Sokkary GH. Ischemia/reperfusion-induced arrhythmias in the isolated rat heart: prevention by melatonin. J Pineal Res 1998; 25: 184-91. [ Links ]
44. Kaneko S, Okumura K, Numaguchi Y, et al. Melatonin scavenges hydroxyl radical and protects isolated rat hearts from ischemic reperfusion injury. Life Sci 2000; 67: 101-12. [ Links ]
45. Salie R, Harper I, Cillie C, et al. Melatonin protects against ischaemic-reperfusion myocardial damage. J Mol Cell Cardiol 2001; 33: 343-57. [ Links ]
46. Sahna E, Parlakpinar H, Turkoz Y, Acet A. Protective effects of melatonin on myocardial ischemia/reperfusion induced infarct size and oxidative changes. Physiol Res 2005; 54: 491-5. [ Links ]
47. Castagnino HE, Lago N, Centrella JM, et al. Cytoprotection by melatonin and growth hormone in early rat myocardial infarction as revealed by Feulgen DNA staining. Neuro Endocrinol Lett 2002; 23: 391-5. [ Links ]
48. Srinivasan V, Spence DW, Moscovitch A, et al. Malaria: therapeutic implications of melatonin. J Pineal Res 2010; 48: 1-8. [ Links ]
49. Jahnke G, Marr M, Myers C, Wilson R, Travlos G, Price C. Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague-Dawley rats. Toxicol Sci 1999; 50: 271-9. [ Links ]
50. Voordouw BC, Euser R, Verdonk RE, et al. Melatonin and melatonin-progestin combinations alter pituitary-ovarian function in women and can inhibit ovulation. J Clin Endocrinol Metab 1992; 74: 108-17. [ Links ]
51. Molina-Carballo A, Munoz-Hoyos A, Reiter RJ, et al. Utility of high doses of melatonin as adjunctive anticonvulsant therapy in a child with severe myoclonic epilepsy: two years' experience. J Pineal Res 1997; 23: 97-105. [ Links ]
52. Jan JE, Wasdell MB, Freeman RD, Bax M. Evidence supporting the use of melatonin in short gestation infants. J Pineal Res 2007; 42: 22-7. [ Links ]
53. Seabra ML, Bignotto M, Pinto LR, Jr, Tufik S. Randomized, double-blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. J Pineal Res 2000; 29: 193-200. [ Links ]
Recibido: 2-12-2010
Aceptado: 13-7-2011














