Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Medicina (Buenos Aires)
Print version ISSN 0025-7680
Medicina (B. Aires) vol.71 no.6 Ciudad Autónoma de Buenos Aires Nov./Dec. 2011
COMUNICACIÓN BREVE
On the immunostimulatory hypothesis of cancer
Juan Bruzzo, Paula Chiarella, Raúl A. Ruggiero
División Medicina Experimental, Instituto de Investigaciones Hematológicas, IMEX-CONICET, Academia Nacional de Medicina, Buenos Aires
Postal address: Dr. Raúl A. Ruggiero, División Medicina Experimental, Instituto de Investigaciones Hematológicas, IMEX-CONICET, Academia Nacional de Medicina, Pacheco de Melo 3081, 1425 Buenos Aires, Argentina
Fax (54-11) 4803-9475 e-mail: ruloruggiero@yahoo.com.ar
Abstract
There is a rather generalized belief that the worst possible outcome for the application of immunological therapies against cancer is a null effect on tumor growth. However, a significant body of evidence summarized in the immunostimulatory hypothesis of cancer suggests that, upon certain circumstances, the growth of incipient and established tumors can be accelerated rather than inhibited by the immune response supposedly mounted to limit tumor growth. In order to provide more compelling evidence of this proposition, we have explored the growth behavior characteristics of twelve murine tumors -most of them of spontaneous origin- arisen in the colony of our laboratory, in putatively immunized and control mice. Using classical immunization procedures, 8 out of 12 tumors were actually stimulated in “immunized” mice while the remaining 4 were neither inhibited nor stimulated. Further, even these apparently non-antigenic tumors could reveal some antigenicity if more stringent than classical immunization procedures were used. This possibility was suggested by the results obtained with one of these four apparently non-antigenic tumors: the LB lymphoma. In effect, upon these stringent immunization pretreatments, LB was slightly inhibited or stimulated, depending on the titer of the immune reaction mounted against the tumor, with higher titers rendering inhibition and lower titers rendering tumor stimulation. All the above results are consistent with the immunostimulatory hypothesis that entails the important therapeutic implications -contrary to the orthodoxy- that, anti-tumor vaccines may run a real risk of doing harm if the vaccine-induced immunity is too weak to move the reaction into the inhibitory part of the immune response curve and that, a slight and prolonged immunodepression -rather than an immunostimulation- might interfere with the progression of some tumors and thus be an aid to cytotoxic therapies.
Key words: Immunostimulatory hypothesis; Murine tumors; Immunization assays
Resumen
Sobre la hipótesis inmunoestimulatoria del cáncer. Existe la creencia de que el peor resultado posible de la aplicación de terapias inmunológicas contra el cáncer es un efecto nulo sobre el crecimiento tumoral. Sin embargo, existe evidencia, resumida en la hipótesis inmuno-estimulatoria del cáncer que sugiere que, bajo ciertas circunstancias, el crecimiento tumoral puede ser acelerado, en lugar de inhibido, por la respuesta inmune supuestamente inducida para atacar el tumor. Para obtener una mayor confirmación de esta proposición, estudiamos el crecimiento de doce tumores murinos -la mayoría de origen espontáneo- surgidos en el criadero de nuestro laboratorio, tanto en ratones presumiblemente inmunizados como en controles. Usando procedimientos clásicos de inmunización, 8 de los 12 tumores fueron estimulados en ratones “inmunizados” mientras que los 4 restantes no fueron ni inhibidos ni estimulados. Más aún, incluso estos 4 tumores aparentemente no-antigénicos podrían revelar algún grado de antigenicidad si procedimientos más drásticos de inmunización fueran usados en lugar de los clásicos. Esta posibilidad fue sugerida por los resultados obtenidos con el linfoma aparentemente no-antigénico denominado LB. En efecto, en ratones que recibieron estos procedimientos más drásticos de inmunización, LB fue ligeramente inhibido o estimulado, dependiendo del grado de la reacción inmune anti-tumoral inducida, con altos títulos produciendo inhibición y bajos títulos, estimulación tumoral. Los resultados arriba descriptos son consistentes con la hipótesis inmuno-estimulatoria del cáncer. Esta hipótesis tiene dos importantes consecuencias terapéuticas que son contrarias a la interpretación convencional. En primer lugar, sugiere la posibilidad cierta de que una vacuna “anti-tumoral” puede producir un efecto estimulador del crecimiento neoplásico si la respuesta inmune producida no es lo suficientemente robusta como para mover la reacción más allá de la parte estimulatoria de la curva. En segundo lugar, que una suave y prolongada inmunosupresión -en lugar de una inmuno-estimulación- podría interferir con la progresión de algunos tumores y ayudar de este modo a las terapias citotóxicas.
Palabras clave: Hipótesis inmuno-estimulatoria; Tumores de ratón; Ensayos de inmunización
Conventional therapies against cancer, such as surgery, radiation and chemotherapy are limited either by their application restricted to localized tumors (surgery) or by their lack of tumor-specificity and their toxic consequences (radiation and chemotherapy)1. On the other hand, an immunological therapy would, at least theoretically, circumvent such limitations, because it could discriminate between the cells of the tumor and their normal cell counterparts wherever they are in the body2.
Since the pioneer work of Prehn and Main3 demonstrating that vaccination against some experimental murine tumors was feasible, there have been numerous attempts to treat human tumors using immunological schedules. Although most of those former attempts were disappointing, a more profound understanding of the cellular and molecular aspects of the immune response achieved in the last few years, prompted the development of new schedules of immunotherapy against cancer such as the use of vaccines based on tumor cells made putatively more immunogenic by the addition of new adjuvants or by transfection with genes encoding co-stimulatory molecules or cytokines that enhance T-cell immune responses or by pulsing dendritic cells with tumor-cell extracts or tumor antigens4, 5.
Presumably, the implicit assumption for these numerous and new attempts is the belief that, as far as immunotherapy is concerned, the worst possible outcome for the application of immunological therapies, is a null effect on tumor growth. However, a significant body of experimental evidence suggests that, upon certain circumstances, the growth of incipient and established tumors might be accelerated rather than inhibited by the immune response supposedly mounted to attack the tumor or to limit its growth.
This body of evidence can be summarized in four groups of observations:
1) In both in vivo and in vitro settings, very weak immune reactions tend to be stimulatory to tumor growth while quantitatively larger amounts of the same immune reactants may be inhibitory6-8; in consequence, the shape of the anti-tumor immune response curve (IRC) can be considered as a particular case of hormesis9, 10, a biological phenomenon that is characterized by a non-monotonic dose-response, that is biphasic, exhibiting opposite effects at low and high doses. In Fig. 1, we adapted the curve from Prehn (quotations 6 and 7) in which the phenomenon of hormesis is represented for anti-tumor immune responses. This curve was originally demonstrated using 3-methylcholanthrene-induced tumors and Winn tests by which different quantities of anti-tumor immune spleen cells were mixed in vitro with tumor cells and then inoculated into syngeneic mice which had been previously immune depressed by radiation and thymectomy6; this curve has been corroborated by a large number of subsequent studies7. 2) Even the most highly immunogenic of mouse tumors, produced for example, by relatively large doses of methylcholanthrene, exhibit little or no detectable immunity if tested in those mice in which the tumor had originated7. 3) There is much evidence that immunity influences progression in both mouse and human tumors4,7. 4) In contrast to virally or chemically induced rodent tumors, those tumors that occur spontaneously without known cause, and that have been considered the best models for human cancer, exhibit, in general, not only an undetectable immunogenicity judged by their incapacity to be inhibited after putative immunization, but in fact a tendency to grow in an accelerated way in pre-immunized hosts7, 11. Given the existence of the hormetically shaped IRC (see observation 1) this is the result that would be anticipated if the spontaneous murine tumors had been, not non-antigenic as it has been proclaimed by some authors11, but actually, weakly antigenic producing a tumor-stimulating immune response.
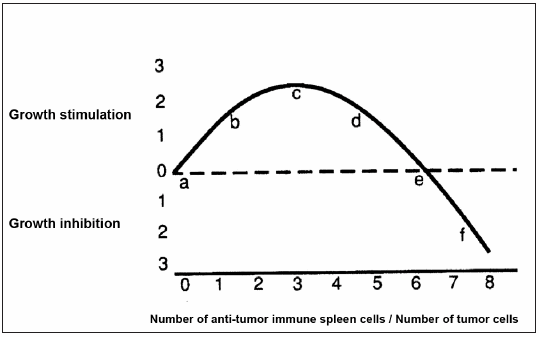
Fig 1.– Idealized anti-tumor immune reaction curve (IRC) relating the quantity of anti-tumor immune spleen cells to the quantity of target tumor cells (adapted from Prehn [quotations 6 and 7] with permission of the author).
If the above four propositions are correct, the hypothesis that any cancer, whether in man or in mouse is usually growing and progressing because it is being continuously immunonologically stimulated, by a relatively weak anti-tumor immune response, follows naturally. This immunostimulatory hypothesis of cancer was originally proposed by Prehn some years ago6 and entails the very important therapeutic implication that, at least in some cases, a slight and prolonged immunodepression -rather than an immunostimulation- might interfere with tumor progression and thus be an aid to cytotoxic therapies. This conclusion –opposite to the present orthodoxy– is the logical therapeutic corollary of the above four mentioned propositions. However, although the first three propositions seem to be founded on a solid experimental ground, the fourth proposition that the growth of the spontaneous murine tumors are stimulated upon “immunization”, needs, in our opinion, to be further confirmed before it can be considered as a general rule.
In order to provide more compelling evidence of that fourth proposition, we have explored, in this work, the growth behavior characteristics of twelve murine tumors arisen in our mouse colony. Ten out of 12 of them arose spontaneously (9 of BALB/c mice origin and 1 of AKR mice origin) and the remaining two were induced, either by medroxyprogesterone acetate (MPA) [C7-HI tumor] or by a novel exogenous murine mammary tumor virus (MMTV) [T2880 tumor]. All tumors were kept biofrozen and were thawed and maintained by serial subcutaneous (s.c.) passages in syngeneic mice. As shown in Table 1, in not a single case was the TD50 (defined as the number of tumor cells able to grow in 50% of the mice) higher in treated - putatively immunized - than in control mice; that is, in not a single case the “immunization” procedure produced an inhibitory effect on tumor growth. However, 8 out of 12 tumors were actually stimulated in the “immunized” mice, which was reflected in the significantly lower TD50 observed in “immunized” as compared with controls, while the remaining four tumors grew in a similar fashion in both “immunized” and control mice. To test the effectiveness of the immunization procedures (that is, if these procedures can induce an anti-tumor Th1 immune response) we evaluated their effect on the growth of a methylcholanthrene-induced fibrosarcoma called MC-C that has been previously described5 and that we used herein as a positive control of immunization. As shown in Table 1, and confirming previous reports5, 8, 12, MC-C proved to be strongly immunogenic as evidenced by the fact that its TD50 was more than 100 times higher in immunized (using the same immunization procedures utilized against the other tumors) as compared with control non-immunized mice.
TABLE 1.– Effect of putative immunization procedures on the growth of apparently non-immunogenic murine tumors
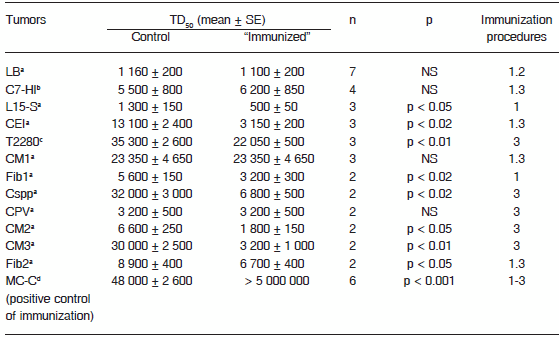
a: Spontaneous tumor. b. Induced by in a BALB/c female mouse treated with 40 mg of medroxyprogesterone acetate (MPA) every three months for one year and thereafter maintained as an MPA-independent line. c. Induced by a novel exogenous MMTV in a BALB/c female mouse. d. Induced after implantation of a methylcholanthene pellet.
LB and L15-S are lymphomas. C7-HI, CEI, CM1, CM2, CM3, Cspp, T2280 and CPV are carcinomas. Fib1, Fib2 and MC-C are fibrosarcomas.
TD50: number of tumor cells able to grow in 50% of mice.
n: number of experiments. For each tumor and in each experiment, 11-18 controls and 11-19 experimental (putatively immunized) mice were used.
p: statistical significance, Student's t-test.
Immunization procedures:
1: Pretreatment with 2 doses of irradiated tumor cells 14 and 7 days before tumor challenge. 2: Pretreatment with dendritic cells (DC) pulsed in vitro with tumor extracts and inoculated in mice 14 and 7 days before tumor challenge. 3: Tumor implantation and extirpation 7-14 days before tumor challenge.
This suggested that the 8 stimulated tumors bear weak antigens that are only able to induce a weak (stimulatory) immune response. Furthermore, even the four apparently non-antigenic tumors, could reveal some degree of antigenicity if different or more stringent immunization procedures were carried out. This possibility was suggested by the results obtained by studying the most recalcitrant apparently non-antigenic tumor we have worked with: the murine LB lymphoma of spontaneous origin. Upon in vivo treatments using two putative immunization assays [pretreatment with irradiated tumor cells and pretreatment with dendritic cells (DC) pulsed in vitro with LB tumor extracts], LB tumor implants were neither inhibited nor stimulated, apparently suggesting that LB would not have any specific tumor antigen. On the other hand, when DC were pulsed in vitro with a tumor extract of the strongly immunogenic MC-C tumor, in vitro maturation of DC (measured by enhanced expression of markers such as CD40, CD80 and CD86) was observed along with a strong in vivo protection against MC-C implants. Finally, when DC were pulsed in vitro with both LB and MC-C extracts (proportion 50%-50%), maturation of DC and a weak but significant in vivo protection against LB tumor implants was achieved in five independent experiments (TD50 in immunized was 3,000 ± 500 versus 1,000 ± 200 in controls, p < 0.01). Since no immune cross-reaction between MC-C and LB tumors was ever observed, the most likely interpretation was that LB actually bears specific tumor antigens but lacks other signals to achieve DC maturation; these signals would be provided by the extract of MC-C which would enable DC to mature and to initiate an anti-LB immune response12. Furthermore, when new experiments were carried out and DC were pulsed in vitro with both LB and MC-C extracts but in a proportion 75%-25%, respectively, a lower maturation of DC was observed and, when these DC were inoculated in vivo, a slight but significant stimulation of LB tumor growth was observed in two experiments (TD50 in “immunized” was 550 ± 50 versus 1,100 ± 100 in controls, p < 0.05) A preliminary conclusion of these experiments would be that a weak tumor antigen, present in an up to date apparently non-antigenic spontaneous tumor (LB), can promote both inhibitory or stimulatory signals for tumor growth depending on the concentration of maturative signals for DC present in, or near, the tumor cells.
All the above results are in agreement with the hormetically-shaped anti-tumor immune response curve depicted in Fig. 1. In effect, in the case of the 8 tumors that were stimulated upon immunization, their pattern of growth would have moved from near “a” - in non-immunized- up to “b”, “c” or “d” in immunized mice (Fig. 1) because their antigens would be weak and in consequence would be able to produce a slight (stimulatory) immune response only. On the other hand, in the case of the remaining 4 tumors that neither were stimulated nor inhibited upon immunization, the immunization procedures used were not able to move their pattern of growth from near “a” to another position in the Fig. 1. This could occur, at least in some cases, not necessarily because they lack specific tumor antigens but because these tumors are unable to produce additional maturative signals for DC. In effect, at least in the case of LB tumor, when a high concentration of those maturative signals was provided for another tumor (as it occurred when DC were pulsed with both LB and MC-C extracts in the proportion 50%-50%, and then inoculated in mice), the growth of LB seemed to move from the position near “a” to the position “f” in Fig. 1, while when the concentration of maturative signals was lower (as it occurred when DC were pulsed with both LB and MC-C extracts in the proportion 75%-25%, respectively), the growth of LB moved back to positions “d”, “c” or “b”. In the limit, when the proportion of LB and MC-C extracts was 100%-0%, that is when DC were only pulsed with the LB extract, the growth of LB remained near the position “a”.
The immunostimulatory hypothesis that we have explored in this work has usually been neglected on the basis of two main arguments. First, this hypothesis questions some aspects of the immune surveillance theory –that is considered by many as an established fact-, and second, it entails the undesired suggestion that anti-tumor vaccines may run a real risk of doing harm if the vaccine-induced immunity is too weak to move the reaction beyond the tumor-stimulatory part of the IRC.
However, the immune surveillance theory is not an established fact. For example, in the human, immunodepression produced in order to enable allografting, increases the incidence of, most notably, skin and lymphoreticular tumors but lowers the incidence of rectal and mammary malignancies7, 13, 14. In addition, the incidence of most remaining human tumors is not affected by immunosuppression7. These observations do not fall in easily with the immune surveillance theory which assumes that the anti-tumor immune response is linear. In contrast, the immunostimulatory hypothesis of cancer, that assumes that the anti-tumor immune response is biphasic, can offer a relatively simple interpretation for the above mentioned observations. In effect, the increased incidence of skin and lymphoreticular tumors upon immunodepression could be caused by the movement of the reaction from near “d” or “e” toward “c” on the IRC (Fig. 1). On the other hand, the reduced incidence of rectal and breast cancers in immunodepressed humans, probably results from the decreased stimulation associated with movement from near “b” or “c” toward “a”. Finally, most of the remaining tumors that are not affected by immunosuppression, probably lie, before the immunosuppression, near “a” on the immune-response curve. In consequence, no significant movement could be anticipated upon immunosuppression.
If immunodepression could reduce the incidence of some human tumors, then, immunostimulation could, upon certain circumstances, enhance tumor growth. However, these enhancing effects, that have been reported in experimental settings8, 11 are very difficult to find in human studies, because in these cases, only “positive responses” are informed. For example, Cranmer et al2 reported the effects of dendritic cell-based vaccines on tumor growth in 615 tumor-bearing patients. A small percentage (21%) exhibited a “significant” (5%) or a “weak” (16%) “positive response”, while the remaining 79% displayed “no positive response”. However, it was not possible to know, by analyzing the data presented in their work, if some of these patients exhibited an enhancement of their tumors or merely tumor growth was similar to controls.
Perhaps the most convincing evidence that human cancers may induce a biphasic (hormetic) anti-tumor immune response derives from the behavior of Kaposi's sarcoma, a lesion that is common in AIDS patients. The tumor commonly “flares” during the period of immune recovery while the AIDS is being treated. This suggests that the tumor grows best when the immune capacity of the patients is still impaired, but not too impaired15.
In conclusion, the above commented clinical observations and the results of the experiments reported herein seem consistent with the immunostimulatory hypothesis of cancer. However, more experiments carried out in vivo and in vitro aimed to demonstrate more accurately that the stimulating effects on tumor growth reported in this work, were actually immunologically-mediated, will be necessary to confirm the validity of this hypothesis and its important therapeutic consequences.
Acknowledgements: This work was supported by grants from CONICET and Fundación Röemmers. The authors are grateful to Drs. Richmond T. Prehn and Christiane Dosne Pasqualini for critical discussion of this manuscript.
Conflicts of interest: No conflicts of interest were disclosed.
1. Bailar JC, Gornik HL. Cancer undefeated. N Engl J Med 1997; 306: 1569-74. [ Links ]
2. Cranmer LD, Trevol KT, Hersh EM. Clinical implications of dendritic cell vaccination in the treatment of cancer. Cancer Immunol Immunother 2004; 53: 275-306. [ Links ]
3. Prehn RT, Main JM. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst 1957; 18: 769-78. [ Links ]
4. Janeway CA, Travers P, Walport M, Shlomckik MJ. Manipulation of the immune response. In: Immunobiology; Garland, New York 2001; p 566-77. [ Links ]
5. Chiarella P, Vulcano M, Bruzzo J, et al. Anti-inflammatory pretreatment enables an efficient dendritic cell-based immunotherapy against established tumors. Cancer Immunol Immunother 2008; 57: 701-18. [ Links ]
6. Prehn RT. The immune reaction as a stimulator of tumor growth. Science 1972; 176: 170-1. [ Links ]
7. Prehn RT, Prehn LM. The flip side of immune surveillance: immune dependency. Immunol Rev 2008; 222: 341-56. [ Links ]
8. Chiarella P, Reffo V, Bruzzo J, Bustuoabad OD, Ruggiero RA. Therapeutic anti-tumor vaccines: from tumor inhibition to enhancement. Clin Med Oncol 2008; 2: 237-45. [ Links ]
9. Calabrese EJ. Hormesis and medicine. Br J Clin Pharmacol 2008; 66: 594-617. [ Links ]
10. Bruzzo J, Chiarella P, Meiss RP, Ruggiero RA. Biphasic effect of a primary tumor on the growth of secondary tumor implants. J Cancer Res Clin Oncol 2010; 136: 1605-15. [ Links ]
11. Hewitt HB, Blake ER, Walder AS. A critique of the evidence for active host defence against cancer based on personal studies of 27 murine tumors of spontaneous origin. Br J Cancer 1976; 33: 241-59. [ Links ]
12. Reffo VL, Chiarella P, Bruzzo J, Bustuoabad OD, Ruggiero RA. Hallazgo de antígenos en un tumor murino espontáneo no inmunogénico mediante el uso de una vacuna basada en células dendríticas. Medicina (B Aires) 2008; 68: 301-4. [ Links ]
13. Stewart THM, Henderson R, Grayson H, Opetz G. The incidence of gastric and rectal cancer in a population of 73,076 men and women chronically immunosuppressed. Clin Cancer Res. 1997; 3: 51-5. [ Links ]
14. Stewart THM. Immunological enhancement of breast cancer. Parasitology 1997; 115: 141-53. [ Links ]
15. Leidner RS, Aboulafia DM. Recrudescent Kaposi's sarcoma after initiation of HAART: a manifestation of immune reconstitution syndrome. AIDS Patient Care STDS 2005; 19: 635-44. [ Links ]
Submitted: 1-6-2011
Accepted: 26-10-2011














