Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Medicina (Buenos Aires)
Print version ISSN 0025-7680
Medicina (B. Aires) vol.75 no.1 Ciudad Autónoma de Buenos Aires Feb. 2015
COMUNICACIÓN BREVE
Meta-tyrosine. A powerful anti-metastatic factor with undetectable toxic-side effects
Damián Machuca1, Paula Chiarella1, Daniela Montagna1, Graciela Dran1, Roberto P. Meiss2, Raúl A. Ruggiero1
1Laboratorio de Oncología Experimental, IMEX-CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas), Academia Nacional de Medicina de Buenos Aires,
2Laboratorio de Patología, IEO (Instituto de Estudios Oncológicos), Academia Nacional de Medicina de Buenos Aires, Argentina
Postal address: Dr. Raúl A. Ruggiero, Academia Nacional de Medicina de Buenos Aires, Pacheco de Melo 3081, 1425 Buenos Aires, Argentina Fax: (54-11) 4806-6638 e-mail: ruloruggiero@yahoo.com.ar
Recibido: 27-VIII-2014
Aceptado: 12-XI-2014
Abstract
Concomitant tumor resistance (CR) is a phenomenon in which a tumor-bearing host is resistant to the growth of secondary tumor implants and metastasis. While former studies have indicated that T-cell dependent processes mediate CR in hosts bearing immunogenic small tumors, the most universal manifestation of CR induced by immunogenic and non-immunogenic large tumors had been associated with an antitumor serum factor that remained an enigma for many years. In a recent paper, we identified that elusive factor(s) as an equi-molar mixture of meta-tyrosine and ortho-tyrosine, two isomers of tyrosine that are not present in normal proteins and that proved to be responsible for 90% and 10%, respectively, of the total serum anti-tumor activity. In this work, we have extended our previous findings demonstrating that a periodic intravenous administration of meta-tyrosine induced a dramatic reduction of lung and hepatic metastases generated in mice bearing two different metastatic murine tumors and decreased the rate of death from 100% up to 25% in tumor-excised mice that already exhibited established metastases at the time of surgery. These anti-metastatic effects were achieved even at very low concentrations and without displaying any detectable toxic-side effects, suggesting that the use of meta-tyrosine may help to develop new and less harmful means of managing malignant diseases, especially those aimed to control the growth of metastases that is the most serious problem in cancer pathology.
Key words: Meta-tyrosine; Concomitant tumor resistance; Anti-metastatic effects.
Resumen
Meta-tirosina. Un poderoso agente anti-metastásico sin efectos tóxicos colaterales detectables. La resistencia concomitante antitumoral (RC) es el fenómeno según el cual un individuo portador de tumor inhibe el crecimiento de implantes tumorales secundarios y metástasis. Si bien desde hace tiempo se sabe que la RC inducida por tumores inmunogénicos de pequeño tamaño es generada por mecanismos inmunológicos dependientes de células T, por otro lado, la manifestación más universal de la RC, generada tanto por tumores inmunogénicos como no-inmunogénicos de gran tamaño, había sido asociada con un (unos) factor sérico antitumoral cuya naturaleza permaneció elusiva por años. En un trabajo reciente, nuestro grupo de trabajo identificó este factor como la mezcla equi-molar de meta-tirosina y orto-tirosina, dos isómeros de tirosina que no están presentes en proteínas normales y que demostraron ser responsables del 90% y 10%, respectivamente, de la actividad antitumoral total del suero. En este trabajo, continuamos nuestras investigaciones demostrando que la administración periódica de meta-tirosina reducía drásticamente el número de metástasis pulmonares y hepáticas en ratones portadores de dos tumores murinos altamente metastásicos y disminuía dramáticamente la mortandad (de 100% a 25%) de ratones con metástasis ya establecidas al momento de la extirpación quirúrgica del tumor. Estos efectos anti-metastásicos se lograron aun con muy bajas concentraciones de meta-tirosina y sin efectos tóxicos perceptibles, lo que sugiere que su uso puede ayudar a diseñar nuevas y menos nocivas estrategias para el tratamiento del cáncer, especialmente aquellas destinadas a controlar el crecimiento metastásico, que es el problema más grave en la enfermedad oncológica.
Palabras clave: Meta-tirosina; Resistencia concomitante antitumoral; Efectos anti-metastásicos.
Concomitant tumor resistance (CR) is the phenomenon according to which a tumor-bearing host inhibits or retards the growth of secondary tumor implants. It was first described by Ehrlich in 19061 but, apart from a few isolated papers2, 3 this phenomenon remained virtually forgotten for about 60 years4, 5. After its renascence, some groups have studied this phenomenon demonstrating that both immunogenic and non-immunogenic tumors can induce CR in different animal models such as mice, rats and hamsters6-8. However, CR has not received much attention as compared with other areas of cancer research despite the fact that it has been detected in association with human cancer and despite its relevance to the mechanisms of metastases control, taking into account that metastases could be considered as secondary tumor implants developed spontaneously during the primary tumor growth. In this sense, clinical and experimental evidence accumulated throughout the years has suggested that the removal of human and murine tumors may, upon certain circumstances, be followed by an abrupt increase in metastatic growth9, suggesting that a primary tumor could exert a controlling action on its metastases. Metastatic growth is considered a far more serious problem than the original tumor because, for most cases, they ultimately prove to be fatal for the patient. In effect, prior to metastases, most cancers can be cured surgically and 5-year survival rates are about 90%. However, when a tumor has spread to different sites, those rates, even using some forms of systemic therapy such as chemotherapy, often fall below 15%10. Therefore, the understanding of the mechanisms underlying the phenomenon of CR could have a significant impact in the management of the malignant diseases.
Different hypothesis have been proposed to explain the phenomenon of CR. According to the immunological hypothesis, the growth of a tumor generates a specific antitumor immune response which even though it is not strong enough to inhibit the primary tumor growth, is still capable of preventing the development of a relatively small secondary tumor inoculum. The immunological hypothesis was originally proposed by Bashford in 19082 which, in turn, coined the term "concomitant immunity" by which this phenomenon has been known in the past. This interpretation is supported by solid evidence mainly based on experiments with strongly immunogenic murine tumors induced by chemical agents or viruses8. However, it does not provide a satisfactory explanation for the fact that CR has also been observed in association with spontaneous murine tumors of non-detectable immunogenicity7, 11. As for non-immunological explanations, basically two hypotheses have been formulated. Ehrlich1 believed that nutrients essential for tumor growth are consumed by the primary tumor, making it difficult for a second implant to develop (atrepsis theory). Others7, 12 have postulated that the primary tumor produce -or induce the production of- anti-proliferative nonspecific substances or anti-angiogenic molecules which limit the replication of tumor cells of the second inoculum. These non-immunological hypotheses can offer a putative explanation for the phenomenon of CR induced by non-immunogenic tumors but not for the specific inhibition of secondary tumor implants observed during the growth of immunogenic tumors.
For the last 30 years, we have studied, in our laboratory, the phenomenon of CR associated with the growth of 20 murine tumors displaying widely different degrees of immunogenicity, in an attempt to integrate the different hypotheses into a coherent picture. Our results7, 8, 11 demonstrated that, two temporally separate peaks of CR are generated during primary tumor growth. The first peak was only induced by immunogenic tumors of small size (≤ 500 mm3); it was tumor-specific and thymus-dependent and a typical immunological rejection was observed histologically at the site of the second tumor implant undergoing CR. On the other hand, the second peak of CR was induced by both immunogenic and non-immunogenic large tumors (≥ 2000 mm3); it was tumor-non-specific and thymus-independent and the inhibition of the secondary tumor was not associated with an immunological cell infiltration, but with the presence of viable but non-infiltrating tumor cells (dormant tumor) located at the inoculation site between the skin and the muscular layer. Some years ago, an intermediate peak of CR was reported to be associated with a particular type of mild-sized tumors (1000-1500 mm3) that restrain secondary tumors indirectly, by limiting tumor neovascularization12. Although the mechanisms associated with the first and intermediate peaks of CR have been elucidated as T cell-dependent and angiostatin-dependent, respectively, the molecular basis of the most universal manifestation of CR, that is, the second peak, remained an enigma for many years. In former studies, we demonstrated that the second peak of CR correlated with the activity of a serum factor(s) that inhibited the in vitro and in vivo proliferation of tumor cells and that was unrelated to other well characterized growth-inhibitory molecules such as interferons, TNF-α, TGF-β, angiostatin, endostatin, etc., taking into account the larger molecular weight of the latter and other physical and biological properties7, 8. In a recently published work13, starting from mice bearing a non-immunogenic lymphoma (called LB), that produces the strongest second peak of CR among all our tumor models, we identified such serum factor(s) as meta-tyrosine and ortho-tyrosine, two isomers of tyrosine that it is thought to be absent from normal proteins. Meta-tyrosine and ortho-tyrosine proved to be responsible for 90% and 10%, respectively, of the total serum anti-tumor activity, as demonstrated by in vitro and in vivo experiments on the growth of three different murine tumors that induce CR and on the growth of established spontaneous metastases generated by a fourth tumor that does not induce CR but is sensitive to the CR induced by other tumors.
In this work, in order to extend our previous observations concerning the anti-metastatic role of meta-tyrosine (herein we used only meta-tyrosine because its anti-tumor power proved to be 10 times more robust than that of ortho-tyrosine), we have carried out the following series of experiments:
a) Study of the dose-response of meta-tyrosine administration on the growth of spontaneous metastases generated by the presence of two highly metastatic murine tumors: the mammary carcinomas C7HI and LMM3, kindly provided by Dra. C. Lanari (Instituto de Biología y Medicina Experimental, Buenos Aires, Argentina) and Dr. L. Colombo (Instituto Ángel Roffo, Buenos Aires, Argentina), respectively.
b) Evaluation of the effect of meta-tyrosine on the survival of LMM3-excised mice exhibiting established lung metastases at the time of surgery.
c) Analysis of putative cytotoxic side-effects associated with a periodic administration of meta-tyrosine.
a) BALB/c mice bearing a subcutaneous (s.c.) C7HI tumor for 40 days were divided in four groups that received, between days 40 and 60, a daily intravenous (i.v.) injection of three different doses of meta-tyrosine (3.3; 33 and 67 mg/kg) or saline (control). A fifth group was sacrificed at day 40 to evaluate the number of metastases at the onset of treatment. At day 60, all treated and control mice were sacrificed and macroscopic (diameter ≥ 0.1 mm, x 10) and microscopic (diameter < 0.1 mm, H&E x 100) lung metastases were counted. As shown in Table 1, all the doses of meta-tyrosine reduced drastically the number of macroscopic lung metastases as compared with controls. In fact, the higher dose decreased the number of metastases that had been observed at the onset of treatment (Table 1). Microscopic lung metastases (data not shown) as well as macroscopic hepatic metastases were also significantly reduced at the end of treatment; hepatic metastases (median [range]) in control: 8 [3-18] vs. treated (67 mg/kg/day): 0 (p < 0.001, Mann-WhitneyU-test). Similarly, BALB/c mice bearing a s.c. LMM3 tumor for 25 days were divided in three groups that received, between 25 and 45 days, a daily i.v. injection of meta-tyrosine (3.3 and 67 mg/kg) or saline. A fourth group was sacrificed at day 25 to evaluate the number of metastases at the onset of treatment. At day 45, all treated and control mice were sacrificed and metastases counted. As shown in Table 1 all the doses of meta-tyrosine reduced drastically the number of macroscopic lung metastases as compared with controls (Table 1). Microscopic lung metastases (data not shown) as well as macroscopic hepatic metastases were also significantly reduced at the end of treatment; hepatic metastases in control: 10 [5-16] vs. treated (67 mg/kg/day): 0 (p < 0.001, Mann-Whitney U-test). It is worth to note that the administration of meta-tyrosine by the intra-peritoneal (i,p.) route was not effective to restrict metastatic growth.
Table 1. Effect of periodic i.v. administration of meta-tyrosine on the number of macroscopic lung metastases generated by C7HI and LMM3 tumor-bearing mice

Number of macroscopic lung metastases at the onset of treatment: 17.3 [9-32] for C7HI (n = 17, day 40) and 25.0 [22-28] for LMM3 (n = 10, day 25).
n = number of mice.
ND: not done.
a: p < 0.05 vs. control, Mann Whitney U-test
b: p < 0.01
c: p < 0.001
b) Twenty five BALB/c mice were inoculated sc with 2 x 105 LMM3 tumor cells. Eighteen days later, 19 tumor-bearing mice were surgically operated to remove the tumor and the remaining 6 were sacrificed to evaluate the number of lung metastases at the time of surgery (mean [range] = 6 [3-10]). Then, the tumor-excised mice were divided into two groups. One group (n = 8) received, for the following consecutive 35 days, a daily i.v. injection of meta-tyrosine (67 mg/kg). The other group (n = 9) received saline. The surgical excision was very satisfactory since no tumor relapsed. As shown in Fig. 1, all controls died exhibiting high number of lung and hepatic metastases at 43 ± 8 days (mean ± standard error) after surgery. In contrast only two treated-mice died, while the other 6 mice remained alive without exhibiting signs of local or metastatic disease for, at least, eight months after surgery (p < 0.001 Log Rank test).
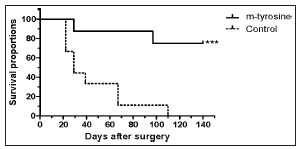
Fig. 1. Percent survival of mice after surgical excision of LMM3 tumors. The figure shows the percentage of the survivors of meta-tyrosine-treated and control mice (ordinate) as a function of the days after surgery (abscissa). No local recurrence at the place of surgery was observed in any case. Death of mice was associated with lung and hepatic metastases. The difference between both groups was highly significant (p < 0.001).
c) The highest dose of meta-tyrosine (67 mg/kg) used in our previous experiments was administered daily by the i.v. route for 42 days in BALB/c mice. At day 42, a sample of mice was sacrificed and the following organs - skin, liver, kidney, spleen, lung, bone marrow, small and large intestine - were investigated histopathologically. Neither histologic nor cytologic alterations were detected in any case, even when organs with high rate of renewal such as skin, bone marrow or small intestine were studied. Hematologic cell populations in blood and lymphoid populations in lymph nodes and spleen were not altered either, as evaluated by direct microscopic observation and flow cytometry. In the same way, meta-tyrosine-treated mice did not display a lower humoral (titer of antibodies against sheep red blood cells) or cellular (delayed hypersensitivity) immune response (data not shown). More experiments measuring different physiologic variables not only in mice but also in other species such as rats and rabbits, in acute, sub-acute and chronic schedules of meta-tyrosine administration, will be necessary to demonstrate more accurately its lack of toxic-side effects.
Surgical extirpation is the mainstay treatment of solid tumors and may be curative when metastatic cells have not already disseminated from the primary tumor. However, although it is recommended in most clinical cases, tumor removal may entail an undesired side effect: the acceleration of regional and distant (metastases) residual neoplastic disease. This effect may account for the disappointingly modest survival benefits observed when surgery is used as a single strategy of treatment. Some investigators have proposed some therapeutic options to limit metastatic growth after tumor removal, including the use of perioperative (instead of postoperative) chemotherapy, antioxidant agents, immunotherapy, and bio-modulation15, but to date, the results have not been as promissory as expected. An understanding of the phenomenon of CR, in particular the recent finding that meta-tyrosine and ortho-tyrosine would be the responsible for the most universal manifestation of CR13, 14, could help to overcome this problem. Data presented in this communication, suggest that, for therapeutic purposes, meta-tyrosine has many attractive features. For example, it exerts its anti-metastatic effect even at very low concentrations and does not exhibit any detectable toxic-side effects even at the highest therapeutic dose. In a former paper13 we have demonstrated that the anti-tumor effect mediated by meta- and ortho-tyrosine was associated -at least in part- with a very early inhibition of MAP/ERK signaling pathway and a subsequent decay of p-STAT3. We have also suggested that down-regulation of the expression of several genes engaged with cell proliferation and survival that are targets of pSTAT3, such as BCL-XL, cyclin D1, surviving and myc, among others, might induce tumor cells to enter into a state of dormancy in G0. Other mechanisms associated with an S-phase arrest, presumably related to the activation of an intra-S phase checkpoint, might also be involved14. A more profound understanding of the molecular basis of the antitumor effects mediated by meta-tyrosine and ortho-tyrosine, might help to develop new and less harmful means of managing malignant diseases, especially by controlling the growth of metastases after the removal of a primary tumor, or after other surgical injuries or stressors that might promote the escape of metastases from dormancy.
Acknowledgements: This work was supported by grants from CONICET, Fundación Roemmers and Fundación F. Fiorini.
Conflicts of interest: None to declare
1. Ehrlich P. Experimentelle Carcinomstudien an mausen. In: Ehrlich P (ed). Arbeiten aus dem Koiglichen Institut fur Experimentelle Therapie zu Frankfurt/AM. Jena, Germany: Gustav Fischer, 1906, p 77-103.
2. Bashford E, Murray J, Haaland M. General results of propagation of malignant new growths. In: Bashford E (ed). Third scientific report on the investigation of the Imperial Cancer Research Fund London, Vol.3, Taylor and Francis, 1908, p 262-8.
3. Woglom WH. Immunity to transplantable tumors. Cancer Rev 1929; 4: 129-209. [ Links ]
4. Brunschwig A, Southam CM, Levin A. Host resistance to cancer. Clinical experiments by homotransplants, autotransplants and admixture of autologous leucocytes. Ann Surg 1965; 162: 416-25. [ Links ]
5. Gershon RK, Carter RL, Kondo K. On concomitant immunity in tumor-bearing hamsters. Nature (London) 1967; 213: 674-6. [ Links ]
6. Gorelik E. Concomitant tumor immunity and the resistance to a second tumor challenge. Adv Cancer Res 1983; 39: 71-120. [ Links ]
7. Ruggiero RA, Bustuoabad OD, Cramer P, Bonfil RD, Pasqualini CD. Correlation between seric antitumor activity and concomitant resistance in mice bearing non-immunogenic tumors. Cancer Res 1990; 50: 7159-65. [ Links ]
8. Franco M, Bustuoabad OD, di Gianni PD, Goldman A, Pasqualini CD, Ruggiero RA. A serum-mediated mechanism for concomitant resistance shared by immunogenic and non-immunogenic tumours. Br J Cancer 1996; 74: 178-86. [ Links ]
9. Demicheli R, Retsky MW, Hrushesky WJ, Baum M, Gukas ID. The effects of surgery on tumor growth: a century of investigations. Ann Oncol 2008; 19: 1821-8. [ Links ]
10. Etzioni R, Urban N, Ramsey S, et al. The case for early detection. Nat Rev Cancer 2003; 3: 243-52. [ Links ]
11. Meiss RP, Bonfil RD, Ruggiero RA, Pasqualini CD. Histologic aspects of concomitant resistance induced by nonimmunogenic tumors. J Natl Cancer Inst 1986; 76: 1163-75. [ Links ]
12. O'Reilly MS, Holmgren L, Shing Y, et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 1994; 79: 315-28. [ Links ]
13. Ruggiero RA, Bruzzo J, Chiarella P, et al. Tyrosine isomers mediate the classical phenomenon of concomitant tumor resistance. Cancer Res 2011; 71: 7113-24. [ Links ]
14. Ruggiero RA, Bruzzo J, Chiarella P, Bustuoabad OD, Meiss RP, Pasqualini CD. Concomitant tumor resistance: the role of tyrosine isomers in the mechanisms of metastases control. Cancer Res 2012; 72: 1043-50. [ Links ]
15. Coffey JC, Wang JH, Smith MJ, Bouchier-Hayes D, Cotter TG, Redmond HP. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol 2003; 4: 760-8. [ Links ]














