Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Medicina (Buenos Aires)
Print version ISSN 0025-7680
Medicina (B. Aires) vol.75 no.4 Ciudad Autónoma de Buenos Aires Aug. 2015
ARTÍCULO ORIGINAL
Allogeneic hematopoietic stem cell transplantation in the elderly. Predicting the risk for non relapse mortality
Mariano Berro1, Ana L. Basquiera2, 10, María M. Rivas1, María Cecilia Foncuberta3, Rubén Burgos3, Gregorio Jaimovich4, Vera Milovic5, Juliana Martínez Rolón6, Guillermina Remaggi6, Jorge Arbelbide7, Jorge Milone8, Virginia Prates8, María L. Rizzi9, Gustavo Jarchum9, Juan José García2, 10, Gustavo Kusminsky1
1Hospital Universitario Austral,
2Hospital Privado Centro Médico de Córdoba,
3Instituto Alexander Fleming,
4Fundación Favaloro,
5Hospital Alemán,
6FUNDALEU,
7Hospital Italiano de Buenos Aires,
8Hospital Italiano de La Plata,
9Sanatorio Allende de Córdoba,
10Instituto Universitario de Ciencias Biomédicas de Córdoba (IUCBC) on behalf of GATMO (Grupo Argentino de Trasplante de Médula Ósea), Argentina
Postal address: Mariano Berro, Hospital Universitario Austral, Av. Juan Domingo Perón 1500, 1629 Derqui, Buenos Aires, Argentina
e-mail: mberro@cas.austral.edu.ar
Recibido: 29-XII-2014
Aceptado: 17-VI-2015
Abstract
We have retrospectively reviewed 137 medical records of patients older than 50 years receiving an allogeneic hematopoietic stem cell transplantation (HSCT) between January 1997 and July 2013. Median follow up was 1.3 years. Sex, age, diagnosis, disease stage, comorbidities (according to HCT-CI score), type of donor, histocompatibility, conditioning regimen and graft-versus-host disease (GVHD) prophylaxis were evaluated. The incidence and severity of acute and chronic GVHD, overall survival (OS), disease free survival (DFS), non-relapse mortality (NRM) and relapse were investigated according those variables. Acute GVHD incidence was 41% (7.3% GIII-IV). Patients with acute myeloid leukemia had lesser aGVH GII-IV (14% vs. 35%, p < 0.01) comparing to the entire population. Extensive cGVHD incidence was 9.4%. Global OS 1-3 years was 44-20%, DFS 33-20%, relapse 35-41% and NRM 36-43% respectively. The presence of comorbidities showed a significant increase in NRM (CT-CI 0 vs. 1 vs ≥ 2: 1-3 years 17-24% vs. 40-46% vs. 45-67%, p = 0.001, MA HR 2.03, CI 95% 1.02-5.29), as well as cyclosporine vs. tacrolimus (1-3 years 47-53% vs. 25-36%, p = 0.01). Tacrolimus patients had higher 1-3 years OS (49-25% vs. 31-13%, p = 0.01) and DFS (41-26% vs. 20-11%, p < 0.01). Age, type of donor and myeloablative conditioning showed no significant differences in any outcome.
Allogeneic HSCT is a valid therapeutic option for older patients in Argentina. The main risk factor for a significantly increased NRM and a trend to inferior OS was the number of comorbidities. Age was not a factor for a worse result. The other factor having a significant effect in better outcome was tacrolimus administration.
Key words: Elderly; Hematopoietic stem cells transplantation; Co-morbidities.
Resumen
Trasplante alogénico de precursores hematopoyéticos en pacientes mayores de 50 años. Riesgo de mortalidad libre de enfermedad
Se efectuó un análisis retrospectivo de 137 historias clínicas de pacientes mayores de 50 años que recibieron un trasplante alogénico de precursores hematopoyéticos (TAPH). Se evaluaron las siguientes características: sexo, edad, enfermedad, estadio, comorbilidades (según el HCT-CI), donante, acondicionamiento e inmunosupresión. Se analizó la incidencia de enfermedad injerto vs. huésped aguda (aEICH) y crónica (cEICH), supervivencia global (SG), supervivencia libre de enfermedad (SLE), recaída y mortalidad libre de enfermedad (MLE). Los trasplantes fueron realizados entre 1997-2013, mediana de seguimiento 1.3 años. La incidencia de aEICH fue de 41% (7.3% GIII-IV). Los pacientes con leucemia mieloide aguda presentaron menor incidencia de EICHa GII-IV (14% vs. 34%, p < 0.01). La incidencia de EICHc extenso fue de 9.4%. La SG a 1-3 años fue 44-20%, SLE 33-20%, recaída 35-41% y la MLE 36-43%. Los pacientes con comorbilidades tuvieron un aumento significativo de la MLE (HCT-CI 0 vs. 1 vs. ≥2: 1-3 años 17-24% vs. 40-46% vs. 45-67%, p = 0.001, AMV HR 2.03, IC 95% 1.02-5.29), al igual que el uso de ciclosporina vs. tacrolimus (1-3 años 47-53% vs. 25-36%, p = 0.01). Los pacientes que recibieron tacrolimus tuvieron una mayor SG (1-3 años 49-25% vs. 31-13%, p = 0.01) y SLE (1-3 años 41-26% vs. 20-11%, p < 0.01). La edad, tipo de donante y acondicionamiento no resultaron significativos para ningún evento.
El TAPH es una herramienta terapéutica válida en pacientes mayores. Los factores pronósticos que inciden mayormente en el trasplante son las comorbilidades y no la edad. El otro factor que demostró un efecto significativo fue el uso de tacrolimus.
Palabras clave: Pacientes mayores; Trasplante hematopoyético de células madre; Comorbilidades.
Glossary
HSCT: Hematopoietic Stem Cell Transplant
HCT-CI: Hematopoietic cell transplant-Comorbidity Index
GVHD: graft-vs-host disease
aGVHD: acute GVHD
cGVHD: chronic GVHD
OS: overall survival
DFS: disease free survival
NRM: non relapse mortality
MA: multivariate analysis
FK: tacrolimus
Allogeneic hematopoietic stem cell transplantation (HSCT) is an effective therapy for several hematological malignancies. HSCT has the potential to cure some diseases otherwise incurable with conventional treatments1-5. Initially, allogeneic HSCT was offered to young patients due to the high morbidity and mortality associated with the procedure6-8; however the majority of the hematological malignancies have an incidence peak at advanced age9-14. This group of patients is especially vulnerable for the allogeneic HSCT toxicities, not only because of age, but also because of comorbidities present in this population. During the last decade less toxic conditioning were introduced in the transplant setting. Reduced intensity conditioning has allowed older patients to undergo HSCT1, 15-20.
Based on comorbidities, Sorror et al validated the hematopoietic co-morbidity index (HCT-CI) predicting the risk for non-relapse mortality and overall survival before HSCT21. This score is used in the majority of HSCT publications at present22-24. We described the experience with allo-HSCT in 137 patients older than 50 years in nine institutions in Argentina. We aimed to study the value of the HCT-CI score in our population, as well as evaluate other risk factors associated with morbidity and mortality. Preliminary data were presented at the Sociedad Argentina de Hematología meeting in 2013.
Materials and Methods
We retrospectively reviewed 137 consecutive medical records of patients older than 50 years receiving an allogeneic HSCT in our institutions. The following characteristics were evaluated: sex, age, diagnosis, stage at transplant, co-morbidities (according to the HCT-CI score), type of donor, histocompatibility, conditioning and immunosuppressive agents. Early stage at transplant was considered for acute myeloid leukemia or acute lymphoblastic leukemia if patients were in first complete remission, for chronic myeloid leukemia in chronic phase, and myelodisplastic syndrome in complete remission, otherwise was considered late stage.
For the statistical analysis SPSS (17.0) and R (2.9.1) were used. The incidence and severity of acute Graft-vs.-Host disease (aGVHD) was compared with Chi Square test. Overall survival (OS) and disease free survival (DFS) were analyzed with Kaplan Meier method. For chronic GVHD (cGVHD), relapse and non-relapse mortality (NRM) it was employed a cumulative incidence analysis (NRM was the competing event for relapse, for NRM the competing event was relapse and for cGVHD were death/relapse). For multivariate analysis variables that in univariate analysis had a p ≤ 0.2 were included. Cox regression model was utilized for time dependant outcomes and logistic regression for dichotomic variables, considering significant a p < 0.05.
Results
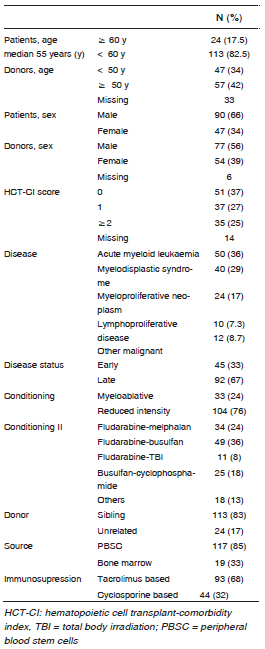
Transplants were performed between January 1997 and July 2013, and the median follow up was 1.3 years. Patients’ characteristics are listed in Table 1. Eighty two percent of the patients (113) were younger than 60 years, 66% (90) were male. Nearly two third of the patients (63%) had HCT-CI score 1 or more. Acute myeloid leukaemia was diagnosed in 36%, myelodisplastic syndrome in 29%, of these, 67% were in late stage. Seventy six percent received a reduced intensity conditioning regimen, mainly fludarabine-based, 82% received a transplant from a sibling donor and 68% received tacrolimus-containing GVHD prophylaxis.
Table 1. Cohorts characteristics
Acute GVHD incidence was 41% (19% were grades II and 7.3% grades III-IV). The only variable associated with aGVHD clinically significant (grades II-IV) was the diagnosis of acute myeloid leukemia, being protective (14% vs. 34%, p < 0.01) and also significant in multivariate analysis (HR 0.29; 95% CI 0.12-0.72). Chronic GVHD incidence was 25%, extensive in 9.4% and the only risk factor associated with this outcome was myeloproliferative neoplasm (1-3 years 40-40% vs. 12-20%, p = < 0.01).
One and three years relapse incidence was 35% and 41%, and NRM was 36 and 43% respectively. Patients with comorbidities showed a significant increase in NRM (HCT-CI 0 vs. 1 vs. ≥2, 1-3 years 17-24%, 40-46% and 45-67%, p < 0.01, figure 1.A; significant in multivariate analysis, for HCT-CI 0 vs. ≥ 1, HR 2.3, 95% CI 1.02-5.29, Table 2); as well as male patients (1-3 years 36-47% vs. 23-27%, p = 0.01), diagnosis of myeloproliferative neoplasm (1-3 years 43-65% vs. 29-34%, p = 0.02) and cyclosporine containing GVHD prophylaxis vs. tacrolimus (1-3 years 47-53% vs. 25-36%, p < 0.01) (figure 1.B). Regarding relapse incidence, acute myeloid leukemia patients experienced a higher rate (1-3 years 53-56% vs. 27-29%, p < 0.01) compared to other diagnosis.
Table 2. Multivariate analysis of disease free survival
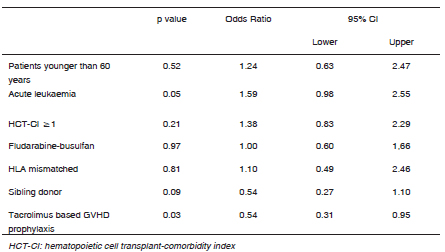
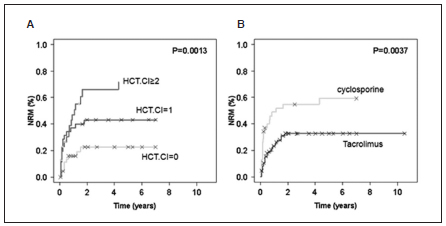
Fig. 1. Non relapse mortality (NRM) curves. A: according to Hematopoietic Cell Transplant-Comorbidity Index score (HCT-CI), B: according to graft-vs-host disease (GVHD) prophylaxis: cyclosporine (CSA) vs. tacrolimus.
Global OS at 1 and 3 years was 44 and 20% and DFS was 33 and 20%. Patients receiving tacrolimus vs. cyclosporine based prophylaxis had higher OS (1-3 years 50-25% vs. 31-13%, p = 0.01) (figure 2.A) and DFS (1-3 year 41-27% vs. 17-8%, p < 0.01, figure 2.B); significant in multivariate analysis (HR 0.54, 95% CI 0.31-0.95 (Table 2). Similarly, patients without comorbidities (HCT-CI 0 vs. ≥1) had a trend towards a higher OS (1-3 years 54-30% vs. 38-17%, p=0.05) as well as a higher DFS (1-3 years 42-30% vs. 30-16%, p =0.06).
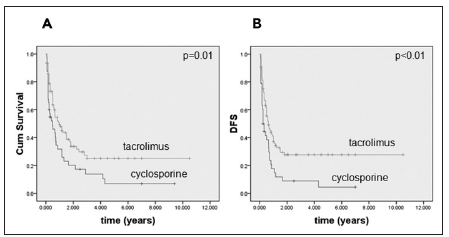
Fig. 2. Overall survival (OS). A: and disease free survival (DFS), B: according to GVHD prophylaxis: cyclosporine vs. tacrolimus
Patients older than 60 years showed no significant differences in terms of NRM (1-3 years 17-23 vs. 34-43%, p = 0.08) and DFS (1-3 years 46-35% vs. 31-18%, p = 0.1). Interestingly, regarding OS, this group experienced higher OS (1-3 years 59-45% vs. 41-18%, p = 0.02), not significant in multivariate analysis. Similarly, type and age of donor, use of myeloablative conditioning regimen, use of in vivo T cell depletion and stem cell source showed no significant difference in any outcome analyzed.
Discussion
In this analysis we described our experience with HSCT in patients older than 50 years, showing outcomes comparable to other centres18, 25-27. Forty percent of the patients had aGVHD, 26% had clinical significant forms and only 7% had severe forms. NRM incidence was around 36% at 1 year and less than 45% at 3 years; and long term DFS and OS were around 20%. In a similar experience, Alyea et al, describe an incidence of clinically significant aGVHD of 27-29% and 17-20% of severe forms depending on conditioning and type of donors. In terms of time dependant variables, two years OS was 39-29%, DFS 27-25% and a NRM cumulative incidence of 32-50% for non-myeloablative and myeloablative regimes respectively17.
For aGVHD, the only factor associated with significant differences was the diagnosis of acute myeloid leukemia, showing a protective effect. Alousi et al described an increase of incidence and severity of aGVHD in unrelated donors, peripheral blood stem cell source and cyclosporine based prophylaxis8. Probably due to the low numbers of unrelated donor and HLA mismatch donors in our cohort, these variables did not show a significant increase in aGVHD incidence. For cGVHD the only factor associated was the diagnosis of myeloproliferative neoplasm. As for aGVHD, Alousi et al. described similar risk factors (unrelated donor, peripheral blood stem cell source and T-cell deplete graft) associated with cGVHD.
Comorbidities assessed by HCT-CI score were the most important predicting factor for NRM. Similarly to Sorror et al. experience, validated by Takasaki in a population similar to ours28, intermediate HCT-CI score patients (1 or 2) have a non-relapse mortality (NRM) rate of 21% at 2 years and high-risk patients (HCT-CI 3 or more) have a NRM rate of 40% at 2 years21. In our population, transplant related toxicities increase with the number of comorbidities. Patients with HCT-CI 0 had 20% NRM rate at 1 year, compared to 40 and 45% with 1 or ≥ 2 HCT-CI score respectively. These variables had a direct influence on OS and DFS, although not significant probably due a low number of patients.
We observed that tacrolimus-containing regimens compared to those with cyclosporine had a better outcome. This approach showed a significantly higher long term (3 years) OS (25% vs. 13%) and DFS (27% vs. 8%) based on a lower 3-year NRM (36% vs. 53%). Several authors evaluated this comparison with no conclusive results with the exception of a Japanese study describing a better OS due to low TRM for the tacrolimus-based approach in unrelated donor HSCT cohort, but not in the sibling donor cohort29-35. The same problem was addressed by Ram et al in a meta-analysis comparing both treatments, observing no differences in terms of survival outcomes, aGVHD incidence and severity was the only variable influenced by the type of prophylaxis employed36.
Interestingly, patients older than 60 years had higher OS, although not significant in multivariate analysis, compared to younger patients. This was probably due to a better patient selection: only 13% of the patients had HCT-CI higher than 1, 12% received a transplant from an unrelated donor as well as a myeloablative conditioning regimen and 96% received tacrolimus-based GVHD prophylaxis.
In conclusion, in this experience we demonstrate that allogeneic HSCT is an option for older patients in Argentina, even beyond the age of 60 years. The best candidates would be patients with one or no comorbidities, receiving tacrolimus-based GVHD prophylaxis and a reduce intensity conditioning regimen.
Acknowledgements: To the transplant centers unit members who collaborated in the care of the patients
Conflict of interests: None to declare
1. Baron F, Storb R. Hematopoietic cell transplantation after reduced-intensity conditioning for older adults with acute myeloid leukemia in complete remission. Curr Opin Hematol 2007; 14: 145-51. [ Links ]
2. Cassileth PA, Harrington, DP, Appelbaum FR, et al. Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission. N Engl J Med 1998; 339: 1649-56. [ Links ]
3. Keating S, De Witte T, Suciu S, et al. The influence of HLA-matched sibling donor availability on treatment outcome for patients with AML: an analysis of the AML 8A study of the EORTC Leukaemia Cooperative Group and GIMEMA. European Organization for Research and Treatment of Cancer. Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto. Br J Haematol 1998; 102: 1344-53.
4. Slovak ML, Kopecky KJ, Cassileth P, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood 2000; 96: 4075-83. [ Links ]
5. Oliansky DM, Antin JH, Bennett JM, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of myelodysplastic syndromes: an evidence-based review. Biol Blood Marrow Transplant 2009; 15: 137-72. [ Links ]
6. Burnett AK, Wheatley K, Goldstone A, et al. The value of allogeneic bone marrow transplant in patients with acute myeloid leukaemia at differing risk of relapse: results of the UK MRC AML 10 trial. Br J Haematol 2002; 118: 385-400. [ Links ]
7. Suciu S, Mandelli F, de Witte T, et al. Allogeneic compared with autologous stem cell transplantation in the treatment of patients younger than 46 years with acute myeloid leukemia (AML) in first complete remission (CR1): an intention-to-treat analysis of the EORTC/GIMEMAAML-10 trial. Blood 2003; 102: 1232-40. [ Links ]
8. Alousi AM, Le-Rademacher J, Saliba RM, et al. Who is the better donor for older hematopoietic transplant recipients: an older-aged sibling or a young, matched unrelated volunteer? Blood 2013; 121: 2567-73. [ Links ]
9. Wingard R, Majhail N, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J C Oncol 2011; 29: 2230-2239. [ Links ]
10. Cutler CS, Lee SJ, Greenberq P, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood 2004; 104: 579-85. [ Links ]
11. Kindwall-Keller T, Isola LM. The evolution of hematopoietic SCT in myelodysplastic syndrome. Bone Marrow Transplant 2009; 43: 597-609. [ Links ]
12. Craig CM, Schiller GJ. Acute myeloid leukemia in the elderly: conventional and novel treatment approaches. Blood Rev 2008; 22: 221-34. [ Links ]
13. Laport GG, Sandmaier BM, Storer BE, et al. Reduced-intensity conditioning followed by allogeneic hematopoietic cell transplantation for adult patients with myelodysplastic syndrome and myeloproliferative disorders. Biol Blood Marrow Transplant 2008; 14: 246-55. [ Links ]
14. Bermudez A, Perez-Vazquez G, Insunza A, et al. FLAGIDA-lite is an effective regimen for patients between 70 and 80 years with acute myeloid leukemia or rafractory anemia with excess blasts-2 and is feasible as outpatient treatment. Am J Hematol 2012; 87: 42-4. [ Links ]
15. Baron F, Maris M, Sandmaier B, et al. Graft-vs-tumor effects after allogeneic hematopoietic cell transplantation wit nonmyeloablative conditioning. J C Oncol 2005; 23: 1993-2003. [ Links ]
16. Estey E, de Lima M, Tibes R, et al. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS). Blood 2007; 109: 1395-400. [ Links ]
17. Alyea EP, Kim HT, Ho V, et al. Comparative outcome of nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation for patients older than 50 years of age. Blood 2005; 105: 1810-4. [ Links ]
18. Aoudjhane M, Labopin A, Gorin NC, et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT). Leukemia 2005; 19: 2304-12. [ Links ]
19. Koreth J, Aldridge J, Haesook T, et al. Reduced-intensity conditioning hematopoietic stem cell transplantation in patients over 60 years: hematologic malignancy outcomes are not impaired in advanced age. Biol Blood Marrow Transplant 2010; 16: 792-800. [ Links ]
20. Koreth J, Pidala J, Perez W, et al. Role of reduced-intensity conditioning allogeneic hematopoietic stem-cell transplantation in older patients with de novo myelodisplastic syndromes: an international collaborative decision analysis. J C Oncol 2013; 31: 2662-71. [ Links ]
21. Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106: 2912-9. [ Links ]
22. Nakaya A, Mori T, Tanaka M, et al. Does the hematopoietic cell transplantation specific comorbidity index (HCT-CI) predict transplantation outcomes? A prospective multicenter validation study of the Kanto Study Group for Cell Therapy. Biol Blood Marrow Transplant 2014; 20: 1553-9. [ Links ]
23. Barba P, Piňama JL, Martino R, et al. Comparison of two pretransplant predictive model and a flexible HCT-CI using different cut off points to determine low-, intermediate-, and high-risk groups: the flexible HCT-CI Is the best predictor of NRM and OS in a population of patients undergoing allo-RIC. Biol Blood Marrow Transplant 2010; 16: 413-20. [ Links ]
24. Sorror ML. How I assess comorbidities bejore hematopoietic cell transplantation. Blood 2013; 121: 2854-63. [ Links ]
25. Shimoni A, Kroőger N, Zabelina T, et al. Hematopoietic stem-cell transplantation from unrelated donors in elderly patients (age > 55 years) with hematologic malignancies: older age is no longer a contraindication when using reduced intensity conditioning. Leukemia 2005; 19: 7-12. [ Links ]
26. Sorror ML, Sandmaier BM, Storer B, et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA 2011; 306: 1874-83. [ Links ]
27. Schetlig J, Bornhauser M, Schmid C, et al. Matched unrelated or matched sibling donor result in comparable survival after allogeneic stem-cell transplantation in elderly patients with acute myeloid leukemia: a report from the cooperative german transplant study group. J C Oncol 2008; 28: 5183-91. [ Links ]
28. Takasaki H, Tanaka M, Tachibana T, et al. Prognostic factors in patients aged 50 years or older undergoing allogeneic hematopoietic stem cell transplantation for hematological malignancy. Int J Hematol 2012; 95: 291-8. [ Links ]
29. Inamoto Y, Flowers M, Appelbaum F, et al. A retrospective comparison of tacrolimus versus cyclosporine with methotrexate for immunosuppression after allogeneic hematopoietic cell transplantation with mobilized blood cells. Biol Blood Marrow Transplant 2011; 17: 1088-92. [ Links ]
30. Horowitz MM, Przepiorka D, Bartels P, et al. Tacrolimus vs. cyclosporine immunosuppression: results in advanced-stage disease compared with historical controls treated exclusively with cyclosporine. Biol Blood Marrow Transplant 1999; 5: 180-6. [ Links ]
31. Yanada M, Emi N, Naoe T, et al. Tacrolimus instead of cyclosporine used for prophylaxis against graft-versus-host disease improves outcome after hematopoietic stem cell transplantation from unrelated donors, but not from HLA-identical sibling donors: a nationwide survey conducted in Japan. Bone Marrow Transplant 2004; 34: 331-7. [ Links ]
32. Hiraoka A, Ohashi Y, Okamoto S, et al. Phase II study comparing tacrolimus (FK506) with cyclosporine for graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation. Bone Marrow Transplant 2001; 28: 181-5. [ Links ]
33. Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood 2000; 96: 2062-8. [ Links ]
34. Nasu R, Nannya Y, Shinohara A, et al. Faborable outcomes of tacrolimus compared with cyclosporine A for GVHD prophylaxis in HSCT for standard-risk hematological diseases. Ann Hematol 2014; 93: 1215-23. [ Links ]
35. Ratanatharthorn V, Nash R, Przeplorka D, et al. Phase III study comparing methrotrexate and tacrolimus (prograf, FK 506) with methotrexate and cyclosporine for graft-versus-host disease prophilaxis after HLA-identical sibling bone marrow transplantation. Blood 1998; 92: 2303-14. [ Links ]
36. Ram R, Gafter-Gvili A, Yeshurun M, et al. Prophylaxis regimes for GVHD: systematic review and meta-analysis. Bone Marrow Transplantat 2009; 43: 643-83. [ Links ]














