Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Medicina (Buenos Aires)
versión impresa ISSN 0025-7680versión On-line ISSN 1669-9106
Medicina (B. Aires) vol.76 no.4 Ciudad Autónoma de Buenos Aires ago. 2016
BRIEF COMMUNICATION
Possible senescence associated change in the predominant α-Na+/K+ ATP-ase isoform in the renal cortex of the rat
María Constanza Potilinski, Rosalía Moretta, Leonardo Casal, Eduardo García Gras, Carlos E. Amorena
Centro de Estudios en Salud y Medio Ambiente, Escuela de Ciencia y Tecnología, Universidad Nacional de General San Martín, San Martín, Buenos Aires, Argentina
Postal Address: Dr. Carlos E. Amorena, Centro de Estudios en Salud y Medio Ambiente, Escuela de Ciencia y Tecnología, Universidad Nacional de General San Martín, Av. Gral. Paz 5445, ed# 23, 1650 San Martín, Buenos Aires, Argentina
e-mail: carlos.amorena@unsam.edu.ar
Received: 3-III-2016
Accepted: 5-V-2016
Abstract
With aging the kidney exhibits progressive deterioration, with a decrease in renal function. Most of the filtered Na+ is actively reabsorbed in the proximal tubules through different transporters located in apical membrane. This process is possible because basolateral Na+/K+-ATP-ase generates electrochemical conditions necessary for energetically favorable Na+ transport. The α-subunit is the catalytic domain of Na+/K+-ATP-ase. There are three isoforms of the α/subunit present in rat kidney. The present study was undertaken to examine the expression pattern of rat α-Na+/K+-ATP-ase during senescence. We tested the impact of aging on mRNA expression of α-Na+/K+-ATP-ase in cortex and medulla of aged Wistar rats. We observed a significant expression decrease in mRNA levels and a possible change of isoform in the cortex of aged animals. These expression changes observed for αsubunit could be contributing to affect the renal function in conditions of water and salt stress.
Key words: Aging; Kidney; Sodium-potassium-exchanging ATPase.
Resumen
Posible cambio en la isoforma predominante de la α-Na+/K+ATP-asa asociada a la senescencia en corteza renal de rata.
Con el avance de la edad los riñones exhiben un deterioro funcional progresivo con disminución de la función renal. La mayor parte del sodio (Na+) filtrado es reabsorbido activamente en los túbulos proximales a través de diferentes transportadores ubicados en la membrana apical. Este proceso es posible por la existencia de la Na+/K+-ATP-asa basolateral, que genera las condiciones electroquímicas necesarias para que el transporte de Na+ sea energéticamente favorable. La subunidad αde la Na+/K+-ATP-asa es el dominio catalítico de la enzima. Existen tres isoformas de subunidad α, que están presentes en el riñón de la rata. En este trabajo se examinan los patrones de expresión de la α-Na+/K+-ATP-asa durante la senescencia. Se estudió así si el aumento de la edad incidía en la expresión del ARNm de la α-Na+/K+-ATP-asa en corteza y médula renal de ratas Wistar senescentes. Se observó una disminución en la expresión del ARNm de la subunidad αy un posible cambio de isoforma predominante en la corteza de los animales senescentes. Los cambios observados para la expresión de la subunidad αpodrían contribuir a afectar la función renal en condiciones de estrés hídrico y salino.
Palabras clave: Envejecimiento; Riñón; ATPasa-intercambiador sodio potasio
As a consequence of the normal aging process there is a decrease in renal function and an increased susceptibility to develop renal diseases1. As a result, both a decrease in the glomerular filtration rate and renal blood flow as well have been described beside an impairment in the urinary concentrating mechanisms2, 3.
Approximately 70% of the filtered Na+ is actively reabsorbed by an active process in the proximal tubules, along with chloride and water. Net apical reabsorption of NaCl involves both coupling of Na+/H+ exchange with Cl-/base exchange and acid recycling or triple coupling of Na+-sulfate cotransport, sulfate-anion exchange and Cl-/anion exchange4. This system operates in series with the basolateral Na+/K+-ATP-ase, which generates the electrochemical gradient across the cell, providing the energy for Na+ transport.
Acidification of the proximal convoluted tubule (PCT) fluid is also altered with age. In previous studies, we have observed in old rats a decrease in the capacity of proximal tubular H+ secretion, without changes in the acid-base balance5. This reduction is due to a diminished expression of the NHE8 isoform of the Na+/H+ 6.
The Na+/K+-ATP-ase is an heteromeric transmembrane protein composed of an α- and a β-subunit. The α-subunit is the catalytic subunit, responsible for the transport activities of the enzyme, and contains the binding site for Na+, K+, ATP and cardiac glycosides like ouabain, an specific inhibitor of the enzyme7, 8. There are four isoforms of the catalytic α-subunit, each of them with a tissue-specific distribution. The relative distribution of αisoform mRNA in the kidney has been reported to be approximately 70% for α1, 20% for α2 and 10% for α39.
The expression profile of the alpha isoform mRNA is also subjected to a temporal regulation in the kidney and other organs10. However, most of the work in the field has been done over the developmental and growth processes, and not in the age-associated changes occurring during senescence. The aim of this work is to explore the expression pattern of α-Na+/K+-ATP-ase mRNA in young and aged Wistar rats, in order to shed light into the changes that may take place with both aging and decline of renal function.
Twelve Wistar rats were randomly assigned to two groups and sacrificed either at 3 months old (3M, control group) or 18 month old (18M, aged group). The animals were maintained under a cycle of 12 hour light/dark and room temperature at 22 ± 2 °C with food and water ad libitum. They were anaesthetized using 5% Inhelthran® enflurane (Abbott, Italy) by inhalation. Adequate anesthesia was assured by the absence of reflexes prior to cervical dislocation and subsequent sample collection. This protocol was approved by the University of San Martin Ethical Review Committee and conformed to the "Revised guide for the care and use of laboratory animals" of NIH.
Total RNA was isolated from both renal cortex and renal medulla using TRIzol reagent (Invitrogen, USA) and according to the manufacturer's instructions. Reverse transcription was carried out using random primers and RevertAid M-MuLV Reverse Transcriptase (Fermentas, Lithuania), using the standard protocol provided by the manufacturer. Primers were designed to amplify all alpha isoforms (α1: NM_012504.1; α2: NM_012505.2; α3: NM_012506.1; α4: NM_001271030.1) (αFor: 5` CTGACTGCCAAGCGCATGGC 3`; αRev: 5` GGCCACTGTCATGCGGTTC 3`). The housekeeping gene gapdh (NM_008084.2) was amplified with the following primers: gapdhFor 5` TGCATCCTGCACCACCAACT3`; gapdhRev 5` CTTGGCAGCACCAGTGGATG 3`.
The amplification was performed in a Step One (Applied Biosystems, USA) equipment using a final volume of 12.5 μl (FastStart Universal SYBR Green Master (Rox) (Roche, Switzerland) with 0.1 μg cDNA and 300 μM primers) with the following cycling steps: 50 °C for 2', 95 °C for 10' and 40 cycles at 95 °C for 20'', 61 °C for 1', 72 °C for 20''. Transcriptional level was determined by RT-PCR and normalized to the expression of gapdh using the 2-ΔΔCT method, efficiency of each reaction (gapdh and α isoforms) were determined before the correspondent analysis11. PCR products corresponding to different melting temperatures were purified using QIAquick gel extraction kit (QIAGEN, Netherlands) and sequenced in a ABI prism 3100 Genetic Analyzer (Applied Biosystems, USA) with the same primer set used to quantify mRNA. Sequences were exported from chromatograms using Chromas Lite 2.1.1 (Technelysium, Australia), manually curated and aligned with ClustalW (www.expasy.org). Phylogram was performed with ClustalW2-phylogeny (EMBL-EBI).
Results, were expressed as mean ± standard error of the mean (SEM) and analyzed by Student's t-test. Differences were deemed significant at P < 0.05.
The first step of our study was to determine the total expression level o αsubunit of the Na+/K+-ATP-ase. The abundance of specific mRNA was quantified by RT-PCR using primers that allowed the amplification of all αisoforms (α1-4). In the renal cortex, we observed a reduction in the 18M group, compared to the level in 3M rats (p < 0.01; Fig. 1A). There were no significant changes in the expression of α-Na+/K+-ATP-ase in the renal medulla (Fig. 1B).
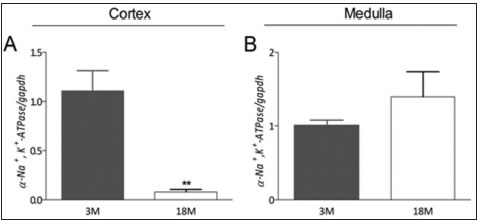
Fig. 1. Transcriptional level and melting curves of total α-Na+/K+-ATP-ase in renal cortex and renal medulla in 3 months old and 18 months old rats. Transcriptional level was determined by RT-PCR and relativized to the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), (A) cortex and (B) medulla. Values were normalized to the 3M control group and reported as means ± SEM. ** p < 0.01.
Analyzing melting curves of PCR products a right-shift in the melting temperature was observed in the renal cortex of the 18M group (Fig. 2A) that was absent in the renal medulla (Fig. 2B). Melting curves show if sequences with different AT/GC contents are present in PCR products. This analysis does not allow knowing the amounts of each sequence.

Fig. 2. Representative melting curves and alignment obtained from amplification and sequencing of total α-Na+/K+-ATP-ase mRNA. (A) Melting curves from renal cortex; (B) Melting curves from renal medulla. (C) Clustal alignment and phylogram of the sequence obtained from 3M PCR product, 18M PCR product, α1 isoform, α2 isoform and α3 isoform. Notice that 3M corresponds to α1 while 18M predominant isoform is α2. 3M sequence show a punctual mutation (T instead C or A).
The targeted region differs in %GC content between α1 (56%) and the other three isoforms (62-63%). Partial DNA sequences obtained from PCR products from both peaks, found in the cortex products, confirmed that the predominant isoform in the 3M group was α1, while the right-shift in the 18M group corresponded to α2 isoform (Fig. 2C).
The present study inspects the changes in the renal expression of the alpha subunit of the Na+/K+-ATP-ase, associated with the senescence process in Wistar rats. In addition, we report a method based on RT-PCR coupled to melting curve analysis that enables both the simultaneous amplification of α1-4 isoforms and the rapid screening of isoform switch from α1 to α2-4.
The drastic fall in total α-Na+/K+-ATP-ase transcription level in the renal cortex was puzzling, given that previous reports performed in F344 x BNF1 and WYK rats have shown no age-specific alteration in its expression12. However, differences in the physiology of aging features among rat strains are well documented and may account for the discrepancy between our results using outbreed Wistar animals and those of former studies13. Defining a suitable animal model to test hypotheses concerning senescence is without doubt an important issue. In this sense, we believe our results provide an alternative perspective for renal aging that differs from that observed in the long-lived F344 x BNF1 and the inbreed WYK strains. Nonetheless, to address the question of which model is readily applicable to study the aging process in humans is beyond the scope of this work.
It was unexpected to find a punctual mutation in 3M sequence; T nucleotide present instead A (in α1 sequence) or C (α2 and α3 sequence). Although transversion mutations are possible (A to T in this case), transitions (C to T) appear more frequent in rat genome14. Thus, it is more likely that the original sequence was a C than A, which corresponds with α2 sequence, and accordingly with the rest of the sequence. When considering the reading frame, this is a conservative mutation located in the third base encoding for a glycine amino acid in all the sequences (α1, α2 and α3), and the mutation is conservative.
It is generally assumed that α1 isoform compromises up to 70% of the total Na+/K+-ATP-ase in the kidney9. A decrease in the mRNA of total α-Na+/K+-ATP-ase may be a consequence of a decrease in the α1 mRNA. This means that without changes or in the increase in α2 transcription level will result in a significant increase in the ratio α2 α1. To the best of our knowledge, this is the first study to report a possible age-associated change of the predominant isoform in the renal cortex. Since α-Na+/K+-ATP-ase isoforms differ in many aspects, such as: substrate affinity (KmNa+), sensitivity to cardiotonic steroids and association with both channels and regulatory proteins, the alterations in the isoform expression profile could impact the functionality of the pump.
Considering that our results involve a decrease in the alpha subunit of the Na+/K+-ATPase, that could be associated with a decrease in proximal sodium reabsorption, we expected a compensatory increase in distal function in order to keep total sodium balance in steady state.
Although there were not statistical differences in Na+/K+-ATPase mRNA expression in renal medulla, there was a tendency to increase in aged animals. Besides, in unpublished observations, we found an increase in Na+/K+-ATP-ase activity in renal medulla. In conclusion, renal aging could be associated to alterations in sodium transporters along the nephron15.
Acknowledgements: María Constanza Potilinski is supported by PhD fellowship from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). Leonardo Casal is supported by PhD fellowship from Universidad Nacional de General San Martín and CONICET. Rosalía Moretta, Eduardo García Gras and Carlos Amorena are members of the Research Career of CONICET.
This work has been supported by CONICET (PIP 730).
Conflict of interests: None to declare
1. Hemmelgarn BR, Zhang J, Manns BJ, et al. Progression of kidney dysfunction in the community-dwelling elderly. Kidney Int 2006; 69: 2155-61. [ Links ]
2. Corman B, Pratz J, Poujeol P. Changes in anatomy, glomerular filtration, and solute excretion in aging rat kidney. Am J Physiol 1985; 248: 282-7. [ Links ]
3. Fiori MC, Ossani GP, Lago NR, Amorena C, Monserrat AJ. Chronic progressive nephropathy: functional, morphological, and morphometrical studies. Ren Fail 2010; 32: 112-8. [ Links ]
4. Aronson PS, Giebisch G. Mechanisms of chloride transport in the proximal tubule. Am J Physiol Renal Physiol 1997; 273: 179-92. [ Links ]
5. Fiori M, Radrizzani M, Díaz-Sylvester P, et al. Relative contribution of V-H+ATPase and Na+/H+ exchanger to bicarbonate reabsorption in proximal convoluted tubules of old rats. Aging 2006; 5: 367-72. [ Links ]
6. Fiori M, Gras EG, Amorena C. Decreased NHE8 isoform expression and defective acidification in proximal convoluted tubules of senile rats. Age (Dordr) 2009; 31: 77-84. [ Links ]
7. Féraille E, Doucet A..Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: hormonal control. Physiol Rev 2001; 81: 345-418. [ Links ]
8. Wallick ET, Schwartz A. Interaction of cardiac glycosides with Na+/K+-ATP-ase. Methods Enzymol 1988; 156: 201-13. [ Links ]
9. Clapp WL, Bowman P, Shaw GS, Patel P, Kone BC. Segmental localization of mRNAs encoding Na(+)-K(+)-ATPase alpha- and beta-subunit isoforms in rat kidney using RT-PCR. Kidney Int 1994; 46: 627-38. [ Links ]
10. Orlowski J, Lingrel JB. Tissue-specific and developmental regulation of rat Na, K-ATPase catalytic alpha isoform and beta subunit mRNAs. J Biol Chem 1988; 263: 10436-42. [ Links ]
11. Livak KJ, SchmittgenTD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta DeltaC(T)) Method. Methods. 2001; 25: 402-8. [ Links ]
12. Eiam-Ong S, Sabatini S. Age-related changes in renal function, membrane protein metabolism, and Na, K-ATPase activity and abundance in hypokalemic F344 x BNF(1) rats. Gerontology 1999; 45: 254-64. [ Links ]
13. Broson RT. Rate of occurrence of lesions in 20 inbred and hybrid genotypes of rats and mice sacrificed at 6-month intervals during the first years of life; in Harrison DE (ed): Genetic Effects of Aging II. New Jersey: Telford Press, 1990, p 279-358. [ Links ]
14. Mei N, Arlt VM, Phillips DH, Heflich RH, Chen T. DNA adduct formation and mutation induction by aristolochic acid in rat kidney and liver. Mutat Res 2006; 602: 83-91. [ Links ]
15. Yang H, Fogo AB. Cell senescence in the aging kidney. J Am Soc Nephrol 2010; 21: 1436-39. [ Links ]














