Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Medicina (Buenos Aires)
Print version ISSN 0025-7680On-line version ISSN 1669-9106
Medicina (B. Aires) vol.77 no.1 Ciudad Autónoma de Buenos Aires Feb. 2017
ARTÍCULO ORIGINAL
Electrical approach to improve left ventricular activation during right ventricle stimulation
María Paula Bonomini1, 3, Daniel F. Ortega2, Luis D. Barja2, Nicolasa Mangani2, Analía Paolucci2, Emilio Logarzo2
1Instituto de Ingeniería Biomédica, Facultad de Ingeniería, Universidad de Buenos Aires (FIUBA),
2Servicio de Electrofisiología, Hospital Universitario Austral, Pilar,
3Instituto Argentino de Matemática Alberto P. Calderón, CONICET, Buenos Aires, Argentina
Postal address: Dra. María Paula Bonomini, IAM. Instituto Argentino de Matemática Alberto Calderon, Saavedra 15 3° piso - C1083ACA Buenos Aires Argentina email: paula.bonomini@conicet.gov.ar
Received: 3-III-2016
Accepted: 4-VII-2016
Abstract
Coronary sinus mapping is commonly used to evaluate left atrial activation. Herein, we propose to use it to assess which right ventricular pacing modality produces the shortest left ventricular activation times (R-LVtime) and the narrowest QRS widths. Three study groups were defined: 54 controls without intraventricular conduction disturbances; 15 patients with left bundle branch block, and other 15 with right bundle branch block. Left ventricular activation times and QRS widths were evaluated among groups under sinus rhythm, right ventricular apex, right ventricular outflow tract and high output septal zone (SEPHO). Left ventricular activation time was measured as the time elapsed from the surface QRS onset to the most distal left ventricular deflection recorded at coronary sinus. During the above stimulation modalities, coronary sinus mapping reproduced electrical differences that followed mechanical differences measured by tissue doppler imaging. Surprisingly, 33% of the patients with left bundle branch block displayed an early left ventricular activation time, suggesting that these patients would not benefit from resynchronization therapy. SEPHO improved QRS widths and left ventricular activation times in all groups, especially in patients with left bundle branch block, in whom these variables became similar to controls. Left ventricular activation time could be useful to search the optimum pacing site and would also enable detection of non-responders to cardiac resynchronization therapy. Finally, SEPHO resulted the best pacing modality, because it narrowed QRS-complexes and shortened left ventricular activations of patients with left bundle branch block and preserved the physiological depolarization of controls.
Key words: Artificial cardiac pacing; Cardiac electrophysiological study; Coronary sinus; Cardiac resynchronization therapy; Left bundle-branch block.
Resumen
Abordaje eléctrico para mejorar la activación ventricular izquierda durante estimulación del ventrículo derecho
El mapeo del seno coronario se utiliza comúnmente para evaluar la activación de la aurícula izquierda. Aquí, investigamos su utilidad para evaluar qué modalidad de estimulación ventricular derecha produce los menores tiempos de activación ventricular izquierda (R-LVtime). Se definieron tres grupos: 54 controles; 15 pacientes con bloqueo de rama izquierda y 15 con bloqueo de rama derecha. El ancho de QRS y los tiempos de activación fueron evaluados en cada grupo bajo las siguientes modalidades: ritmo sinusal, ápex del ventrículo derecho, tracto de salida del ventrículo derecho y alta salida en septum (SEPHO). El R-LVtime se midió como el tiempo transcurrido desde el inicio del QRS de superficie y la deflexión ventricular izquierda más distal del seno coronario. Durante las distintas modalidades de estimulación, el mapeo del seno coronario reprodujo diferencias eléctricas acompañadas por diferencias mecánicas que fueron evaluadas mediante Tissue Doppler Imaging. El 33% de los pacientes con bloqueo de rama izquierda mostró R-LVtime tempranos, lo que sugiere que estos pacientes no se beneficiarían con terapia de resincronización. SEPHO mejoró el ancho de QRS y el R-LVtime de todos los grupos, especialmente en los pacientes con bloqueo de rama izquierda. En conclusión, el R-LVtime serviría para identificar el sitio óptimo de estimulación y permitiría detectar ciertos no respondedores a la terapia de resincronización. Además, el SEPHO resultó la mejor modalidad de estimulación porque estrechó el QRS y acortó el R-LVtime de los pacientes con bloqueo de rama izquierda pero no alteró la despolarización fisiológica de los controles.
Palabras clave: Estimulación cardíaca artificial; Estudio electrofisiológico cardíaco; Seno coronario; Terapia de resincronización cardíaca; Bloqueo de rama izquierda.
Cardiac resynchronization therapy (CRT) has become a widely accepted therapeutic strategy for patients with chronic heart failure and left ventricular conduction disturbances1-3. Recent studies have demonstrated that the initiation of the electrical impulse at right ventricular locations associates with muscle fiber conduction not dependant on His-Purkinje propagation, causing an asynchronous right ventricle and left ventricle contraction4-6. Left bundle branch block (LBBB) and other non-specific intraventricular conduction disturbances also create functional abnormalities because of interventricular asynchrony7, 8.
Conventional ECG is of limited value in the description of complex intraventricular conduction disturbances, as are two-dimensional dual M-mode echocardiograms9 and tissue doppler imaging10. More recently, high density left ventricular endocardial activation maps have been performed using an electroanatomic mapping system (CARTO), and left ventricular conduction abnormalities based on endocardial activation patterns have been successfully analyzed11. However, this system is very expensive and time-consuming, and fails to provide a universal solution to the assessment of intraventricular conduction disturbances.
This study presents a new simple method to invasively analyze left ventricular activation during an electrophysiological study by monitoring deflections from the most distal coronary sinus. Coronary sinus mapping is widely used in electrophysiological studies to evaluate left atrial activation, especially in atrioventricular re-entrant tachycardias12. However, it is rarely used to monitor left ventricular activation and study contraction patterns initiated at different pacing sites.
Materials and methods
This study enrolled 84 consecutive adult patients with indication of an electrophysiological study. Three study groups were conformed. The control group consisted in 54 patients with no intraventricular conduction disturbance (51.9 ± 17.1 years old, 23 women) referred for electrophysiological evaluation. In this group, the indications for electrophysiological study were sick sinus syndrome and supraventricular tachyarrhythmia ablation surgery. The LBBB group included 15 patients (59.2 ± 16.1 years old, 7 women) presenting baseline left branch bundle block. The RBBB group included 15 patients (57.4 ± 20.6 years old, 9 women) with baseline right branch bundle block. This study complies with the Declaration of Helsinki, the research protocol was approved by the San Camilo's Clinic ethics committee and in all cases patients provided written informed consent to the study.
The modalities of stimulation analyzed were the following: right ventricular apex (RVA), right ventricular outflow tract pacing (RVOT), and high output septal stimulation (SEPHO). A Medtronic 5328 device was used for RVA and RVOT with the following parameters: bipolar configuration with double threshold voltage and 0.5 ms pulse width. The device was programmed to VVI mode at a frequency of 10 beats above the basal rhythm to ensure capture. High output parahisianseptal stimulation was accomplished with a 10V biphasic waveform. In the latter case, the stimulation catheter was moved around until a narrow QRS was observed or the QRS width suffered as little as possible.
Pacing was performed with one standard bipolar catheter having an interelectrode distance of 2.5 mm and a 4 mm tip. For epicardial mapping a decapolar catheter with an interelectrode distance of 2.5-5-2.5 mm was inserted via the coronary sinus, considering the latest local bipolar electrogram for the measurement of the left ventricular activation. A third standard bipolar catheter was placed in the right atrium.
The right to left ventricle time (R-LVtime) was measured from the beginning of surface QRS to the most distal left ventricular deflection recorded from coronary sinus in all patients. The terms “left ventricular activation” and “R-LVtime” will be used indistinctively along this work.
The following measurements were performed: (i) QRSw: Surface QRS width, defined as the widest duration among all the leads; and (ii) R-LVtime: conduction time from right to left ventricle measured as the most distal left ventricular deflection recorded from coronary sinus referred to QRS onset. QRSw and R-LVtime were measured at sinus rhythm and under pacing at different sites in all the groups.
Septal-to-posterior wall motion delay (SPWMD) was utilized to measure left ventricular dyssynchrony. Parasternal long-axis M-mode echocardiography and the moving curve of the left ventricular back wall were analyzed. SPWMD was measured as the delay from the peak of ventricular septum contraction either to the peak of left ventricular posterior wall or to the lateral wall contraction, whatever was more delayed,generally not exceeding 130 ms.
Data are presented as mean ± SD. Since data did not pass the D'Agostino & Pearson normality test, results were analyzed by the nonparametric two-tailed Sum Rank Wilcoxon test, against sinus rhythm at the same pacing modality in all the cases.
Results
Physiological left ventricle contraction patterns under sinus rhythm were defined for every group. Table 1 presents mean ± SDR. LV-time and QRSw values for the three groups under study. In spite of the widened QRS complexes, left ventricular activations remained close to normal values in the RBBB group starting approximately at the middle of the QRS complex. Interestingly, 5 out of 15 LBBB patients (33%) presented a physiological R-LVtime, suggesting a normal electrical activation of the left ventricle, in other words, a synchronic ventricular activation.
Table 1. Mean ± SD QRS characteristics under sinus rhythm (SR) for patients with and without intraventricular conduction disturbances. QRSw: QRS width, R-LVtime: right-to-left ventricular time, LBBB: left bundle branch block, RBBB: right bundle branch block. *p < 0.05, against normal QRS

The fact that RBBB patients did not show late left ventricular activations despite their wide QRS complexes suggests that these patients would not benefit from CRT since it is not possible to improve what already is physiologic. The same reasoning holds for 33% of LBBB patients who presented a normal left ventricular activation. These observations would lead to new criteria for the detection of potential non-responders to CRT.
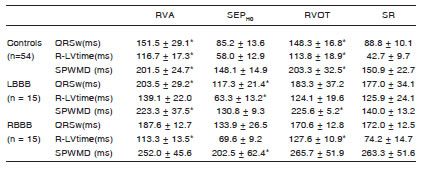
In Table 2, mean ± SD. R-LVtime values produced by different pacing modalities can be found, together with tissue doppler imaging measures and p-values for statistical significance. RVA and RVOT widened QRSw and delayed R-LVtime with respect to sinus rhythm for every group.
Table 2. Mean ± SD QRS widths and activation times for different pacing sites across groups. LBBB: Left bundle branch block, RBBB: Right bundle branch block,QRSw: width of the QRS complex, R-LVtime: right-to-left ventricular time. RVA: Right ventricular apex, RVOT: Right ventricular outflow, SEPHO: High output in septum, SR: sinus rhythm, SPWMD: septal-to-posterior wall motion delay. *p<0.05 vs. SR at the same pacing modality
Accordingly, the mechanical delay between the septum and the free wall of the left ventricle (SPWMD) for the two stimulation modalities was longer than under sinus rhythm. SEPHO remarkably improved the ECG characteristics of LBBB patients and preserved those of controls. As an example, Fig. 1 shows the beneficial effects of SEPHO on a patient with narrow QRS (top), an LBBB patient (middle) and a RBBB patient (bottom). SPWMD values are also displayed at the bottom of each analyzed beat for every group (TE, for Tissue Echo; abbreviation used by the echocardiographist). Note in this case how consistently the mechanical dyssynchrony follows the electrical dyssynchrony. Fig. 2, on the other hand, shows the tissue doppler imaging of a LBBB patient under sinus rhythm (left), RVA pacing (middle) and SEPHO (right). SPWMD shows its minimal value for SEPHO and maximal for sinus rhythm, with an intermediate value for RVA.

Fig. 1. Effect of SEP(HO) on the three groups under study. Paced beats (capture/no capture) start the recording and are preceded by the stimulation spike. Basal measures are done on the second non stimulated beat. QRSw values are marked on top of lead I for each recording and R-LVtime at the bottom, on top of CS67 channel, spanning from the QRS onset to the coronary sinus activation. At the very bottom, the tissue doppler imaging interventricular dyssynchrony is displayed for both paced and sinusal beats.

Fig. 2. Tissue doppler imaging interventricular dyssynchrony for a LBBB patient under SR (left), RVA (middle) and SEP(HO) pacing (right). SPWMD is marked by TE (Tissue Echo, abbreviation of the echocardiographist) at the bottom of each panel.
Fig. 3 illustrates the effects of different pacing sites in a patient without intraventricular conduction disturbances (control). Panel A shows RVA (QRSw: 144 ms, R-LVtime: 100 ms), Panel B displays SEP(HO) showing a QRS close to normal (QRSw: 82 ms, R-LVtime: 58 ms). Panel C illustrates RVOT with an elongation of QRS duration and R-LVtime similar to those produced by RVA (QRSw: 120 ms, R-LVtime: 100 ms) and finally, SR in Panel D presenting the native narrow QRS characteristics (QRSw: 74 ms, R-LVtime: 54 ms).

Fig. 3. Patient with native narrow QRS paced at different sites. A) SR (QRSw = 74 ms; R-LVtime = 54 ms), B) RVA (QRSw = 144 ms; R-LVtime = 100 ms), C) RVOT (QRSw = 120 ms; R-LVtime = 100 ms), and D) SEPHO (QRSw = 82 ms; R-LVtime = 58 ms). SR and SEP(HO) produced similar QRSw and R-LVtime. Meanwhile RVA and RVOT prolonged significantly R-LVtime and widened QRSw.
Discussion
Cardiac depolarization is not a simultaneous phenomenon. Postero-lateral and basal areas of the left ventricle are normally delayed. This phenomenon is further magnified by right ventricle apex stimulation13. The objective of CRT is to restore synchrony in those left ventricular regions which are activated lately14, 15. Our group has long studied the electrical aspects of different pacing sites and observed the left ventricular activation as an important marker of intraventricular synchrony (Ortega DF, Barja L, Amor M, et al. Effect of different right ventricle pacing places on left ventricular electromechanical time. Europace 2007; 9 (Suppl 3): iii69-70. Ortega D, Chirife R, Barja L, et al. Virtual electrode and high output pacing, Effects on QRS duration. PACE 1999; 22(A12):048)16,17. Recently, analogue concepts such as electrical activation were utilized elsewhere for the right ventricle exclusively18.
When used regularly during electrophysiological studies, this simple method proved to be useful to evaluate the depolarization of the delayed portions of the QRS complex, allowing an easy comparison of different conduction patterns during three pacing modalities. It also enables the electrical assessment of patients unlikely to benefit from CRT, contributing to the selection of CRT non-responders, a field that has been taken over by echocardiography19-21. Two different cases of potentially CRT non-responders have been evidenced by means of this simple method; the RBBB patients and the LBBB patients presenting normal R-LVtime. This observation is in line with findings by Emerek et al. on R-LVtime of responders being longer than that of non-responders22, and controverts the view of other authors who argue against the usefulness of electrical assessment of CRT responders23. In the present study, electrical improvements led to mechanical improvements, as shown by tissue doppler imaging measures (see Table 2 and Fig. 2). Furthermore, much evidence is in favour of the electrical assessment of CRT performance.
Unfortunately, these studies involve expensive and time-consuming procedures24, 25.
Silvestrini et al. also studied the potential of coronary sinus mapping to measure left ventricular activation, but without echocardiographical validation26. R-LVtime values assessed in the present work are consistent with Silvestrini's except for septal stimulation, where they failed, and we succeded, to find improved left ventricular activation. This difference might be explained by the improved efficiency of high output stimulation for septal pacing.
In patients with narrow QRS, QRSw and R-LVtime suffered under RVA and RVOT (see Fig. 3). This finding is consistent with reports showing similar deleterious effect of both acute stimulation modalities27. However, the impairment was not as strong as in LBBB patients. This could be due to the fact that normal-ECG patients, unlike baseline LBBB patients, do not present structural myocardiopathy, and therefore, stimulation close to the conduction system would shorten R-LVtime, which explains why not all patients undergoing RVA develop heart failure.
Finally, SEPHO clearly outperformed all the other pacing modalities (Fig. 1 and Table 2). Many reports point to septal pacing as the most physiological pacing modality but fail to agree onthe optimal stimulation site28. We hypothesize that the nature of the high output stimulation would ease the way to the conduction system, making the “sweet spot” concerning septal pacing less critical.
The main limitation of this study is that only acute recordings were carried out for the search of different pacing sites. Recently, Zaho et al. found no echocardiographical differences between RVA and RVO in acute recordings before implantation, but did find a clear SPWMD separation with 1-month and longer follow-ups29. Therefore, to complete the picture, a prospective study should be carried out.
As a conclusion, coronary sinus mapping evidences delayed left ventricle activation in patients with intraventricular conduction disturbances. Thus, it may be useful to evaluate candidates for cardiac resynchronization by detecting potential non-responders, being the cases where left ventricular activation is preserved (not delayed) even though a conduction disturbance is present. Also, this simple method provides a simple, fast and cheap tool for acute testing of alternative pacing sites.
Conflict of interests: None to declare
1. Butter C, Auricchio A, Stellbrink C, et al. Effect of resynchronization therapy stimulation site on the systolic function of heart failure patients. Circulation 2001; 104: 3026-9. [ Links ]
2. Zareba W, Klein H, Cygankiewicz I, et al. Effectiveness of cardiac resynchronization therapy by QRS morphology in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT). Circulation 2011; 123: 1061-72. [ Links ]
3. Boriani G, Biffi M, Martignani C, et al. Is cardiac resynchronization therapy cost-effective? Europace 2009;Suppl 5: 93-7. [ Links ]
4. Samol A, Klotz S, Stypmann J, et al. QRS integral: an electrocardiographic indicator of mechanical interventricular asynchrony. J of Electrocardiol 2010; 43: 242-50. [ Links ]
5. Bedotto JB, Grayburn PA, Black WH, et al. Alterations in left ventricular relaxation during atrioventricular pacing in humans. J Am Coll Cardiol 1990; 15: 658-64. [ Links ]
6. Bank AJ, Gage RM, Burns KV. Right ventricular pacing, mechanical dyssynchrony, and heart failure. Cardiovasc Transl Res 2012; 5: 219-31. [ Links ]
7. Vassallo JA, Cassidy DM, Marchlinski FE, et al. Endocardial activation of left bundle branch block. Circulation 1984; 69: 914-23. [ Links ]
8. Grines CL, Bashore TM, Boudoulas H, Olson S, Shafer P, Wooley CF. Functional abnormalities in isolated left bundle branch block. The effect of interventricular asynchrony. Circulation 1989; 79: 845-53. [ Links ]
9. Auger D BG, Bertini M, Ewe SH, et al. Effect of cardiac resynchronization therapy in patients without left intraventricular dyssynchrony. Eur Heart J 2012; 33: 913-20. [ Links ]
10. Peichl P, Kautzner J, Cihák R, Bytesník J. The spectrum of inter - and intraventricular conduction abnormalities in patients eligible for cardiac resynchronization therapy. Pacing Clin Electrophysiol 2004; 27: 1105-12. [ Links ]
11. Andreu D, Berruezo A, Ortiz-Pérez JT, et al. Integration of 3D electroanatomic maps and magnetic resonance scar characterization into the navigation system to guide ventricular tachycardia ablation. Circ Arrhythm Electrophysiol 2011; 4: 674-83. [ Links ]
12. Amat-y-Leon F, Dhingra RC, Wu D, Denes P, Wyndham C, Rosen KM. Catheter mapping of retrograde atrial activation. Observations during ventricular pacing and AV nodal re-entrant paroxysmal tachycardia. Br Heart J 1976; 38: 355-62. [ Links ]
13. Akerström F, Arias MA, Pachón M, Jiménez-López J, Puchol A, Juliá-Calvo J. The importance of avoiding unnecessary right ventricular pacing in clinical practice. World J Cardiol 2013; 5: 410-9. [ Links ]
14. Yousuf O, Chrispin J, Tomaselli GF, Berger RD. Clinical management and prevention of suddencardiac death.Circ Res 2015; 116: 2020-40. [ Links ]
15. Chatterjee NA, Singh JP. Cardiac resynchronization therapy: past, present, and future. Heart Fail Clin 2015; 11: 287-303. [ Links ]
16. Ortega DF, Salazar AI, Barja LD, Chirife R. Septal His Purkinje ventricular pacing in canines: a new endocardial electrode approach. Pacing Clin Electrophysiol 1993; 16: 1081-3. [ Links ]
17. Ortega DF, Barja LD, Pellegrino GM, et al. Estimulación paraseptal permanente. Indicaciones y seguimiento. Rev argent cardiol 2010; 78: 118-22. [ Links ]
18. Pastore G, Aggio S, Baracca E, et al. A new integrated approach to improve left ventricular electromechanical activation during right ventricular septal pacing. Europace 2012; 14: 92-8. [ Links ]
19. Rouleau F, Merheb M, Geffroy S, et al. Echocardiographic assessment of the interventricular delay of activation and correlation to the QRS width in dilated cardiomyopathy. Pacing Clin Electrophysiol 2001; 24: 1500-6. [ Links ]
20. Breithardt OA, Stellbrink C, Kramer AP, et al. Echocardiographic quantification of left ventricular asynchrony predicts an acute hemodynamic benefit of cardiac resynchronization therapy. J Am Coll Cardiol 2002; 40: 536-45. [ Links ]
21. Verma AJ, Lemler MS, Zeltser IJ, Scott WA. Relation of right ventricular pacing site to left ventricular mechanical synchrony. Am J Cardiol 2010; 106: 806-9. [ Links ]
22. Emerek K, Risum N, Hjortshøj S, et al. New strict left bundle branch block criteria reflect left ventricular activation differences. J Electrocardiol 2015; 48: 758-62. [ Links ]
23. Leclercq C, Faris O, Tunin R, et al. Systolic improvement and mechanical resynchronization does not require electrical synchrony in the dilated failing heart with left bundle-branch block. Circulation 2002; 106: 1760-3. [ Links ]
24. Rad MM, Blaauw Y, Dinh T, et al. Left ventricular lead placement in the latest activated region guided by coronary venous electroanatomic mapping. Europace 2015; 17: 84-93. [ Links ]
25. Yu CM, Fang F, Luo XX, Zhang Q, Azlan H, Razali O. Long-term follow-up results of the pacing to avoid cardiac enlargement (PACE) trial. Eur J Heart Fail 2014; 16: 1016-25. [ Links ]
26. Silvestrini TL, Medeiros de Vasconcelos JT, dos Santos Galvão S, et al. Avaliação do tempo de ativação do ventrículo esquerdo, e da duração do QRS a partir de diferentes sítios. Relampa 2011; 24: 256-63. [ Links ]
27. Miranda RI, Nault M, Simpson CS, et al. The right ventricular septum presents the optimum site for maximal electrical separation during left ventricular pacing. J Cardiovasc Electrophysiol 2012; 23: 370-4. [ Links ]
28. Srivatsa SS. Finding the sweetspot for non-apical RV pacing “Love's labor's lost or much ado about nothing:” A new angiographic technique to accomplish accurate physiological RV septal pacing in under 5 minutes from venous cannulation (or burst). J Invasive Cardiol 2014; 26: 140-7.
29. Zhao Q, Wo J-S, Guo J, Cai S-L. Evaluation of cardiac function by pacing at different right ventricular sites in patients with third-degree atrioventricular block using Doppler ultrasound. Int J Clinl Exp Med 2015; 8: 6822-8. [ Links ]














